Rudolf Krska für den herzlichen Empfang am Institut, seine Unterstützung während der Arbeit und die schnelle Korrektur dieser Arbeit. In dieser Arbeit wurden geeignete Haselnusspräparate für die weitere notwendige Charakterisierung intern produzierter Antikörper hergestellt.

Allergy
Food allergy and intolerance
The immune system-mediated food allergy should be distinguished from other types of food sensitivities. The non-toxic reactions are again categorized into immune-mediated, to which food allergy belongs, and non-immune-mediated (better known as food intolerance) [8].
![Table 1: Gell and Coombs [12] classification of allergic reactions types Type Alternative name Mediator Appearance in Clinical symptoms](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273214.42395/11.892.144.798.226.830/classification-allergic-reactions-alternative-mediator-appearance-clinical-symptoms.webp)
Food allergens
- Characterisation of food allergens
- Plant food allergens
- Animal food allergens
- Background of legislative labelling
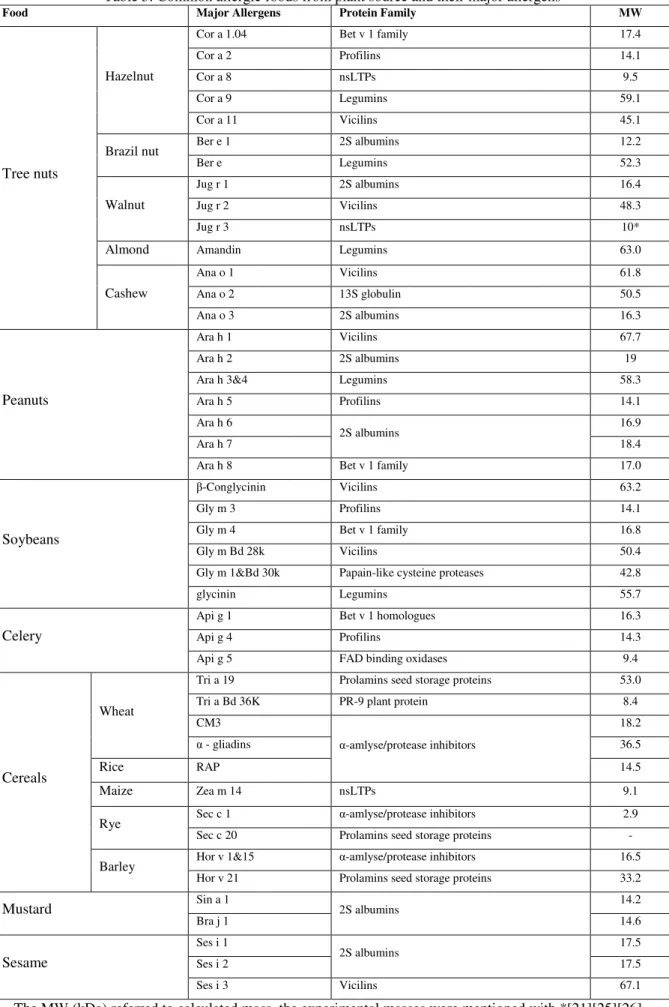
Cross-reactivity between pollen and food allergens from this family has rarely been observed [22]. From this date, ten food allergens (Fig 2) will be subject to the labeling requirement to provide information to the consumer [54].
![Table 2: Classification of food allergens according to their origin [14] [22]](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273214.42395/15.892.150.791.506.931/table-classification-food-allergens-according-origin.webp)
Methods for the detection of allergens in food products
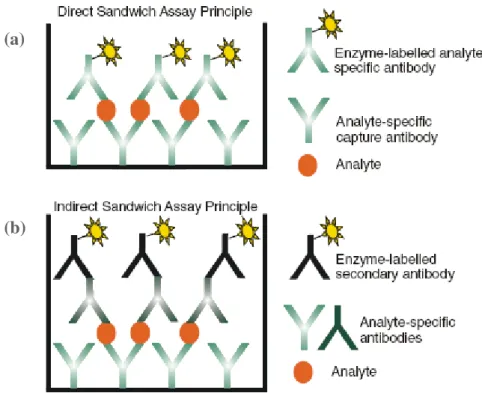
ELISA
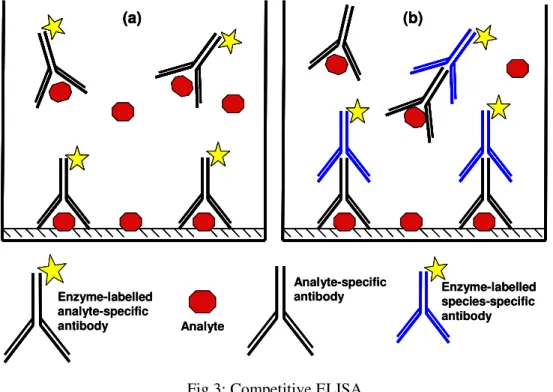
- Competitive ELISA
- Sandwich ELISA
In case of unavailability of enzyme-labeled analyte-specific antibodies, indirect detection can be used (Fig. 3 b). -labeled antibody specific for the analyte. a) Direct detection using analyte-specific labeled antibodies (b) Indirect detection with labeled species-specific antibody [2].
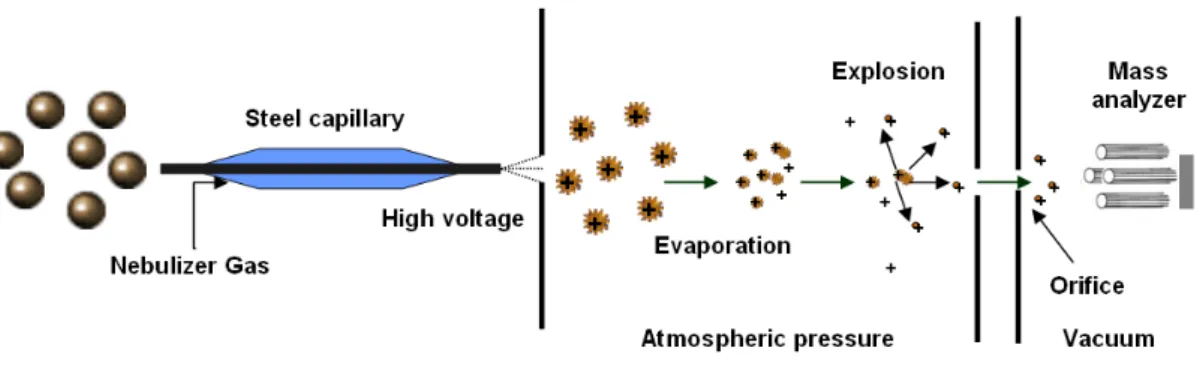
Mass spectrometry
- Liquid chromatography
- Electrospray ionisation
- Quadrupole mass analyser
- Tandem mass spectrometry
- Quantification
- State of the art: Bottom-up approaches for food allergen detection
Depending on the polarity adjustment of the instrument, both positively and negatively charged ions can be detected [58]. In the other technique (tandem-in-time), all the processes are performed sequentially in the same region.
Commercialized rapid immunoanalytical tests for determination of allergenic
The methods currently used for the detection of potential allergens in foods focus on the allergen itself or on a marker that indicates the presence of the food in question. In all three cases, the antigen concentration present in the sample is indirectly proportional to the absorbance of the colored substrate product (Fig.2). Currently, there are at least fourteen LFAs and dipstick test kits on the market, which allow the detection of the following food allergens with LODs between 1 and 25 mg of allergen (food allergen) per kg of food: nuts (almond, hazelnut), shellfish (shellfish), gluten (gliadin), peanut, milk (casein), soybean and egg (Table 1).
A capture antibody is immobilized on the membrane at the tip of the test strip. In such “sandwich” assays, the absorbance of the colored product is directly proportional to the concentration of the analyte present in the sample. In 2006, molluscs were included in the list of allergens of the EU Food Labeling Directive [6].
A surface plasmon resonance (SPR) biosensor has been developed for the detection of the major fish allergen, parvalbumin. Buckwheat is not on the food allergen list of the EU Food Labeling Directive. The demand for fast and reliable analytical tools for the detection of food allergens in foods has increased since the implementation of the EU Food Labeling Directive [4-6] in the legislation of many countries.
Effectiveness of natural and synthetic blocking reagents and their application
Anti-α-casein antibody was coated on the microtiter plate for sandwich ELISA: IgY-anti-α-casein or rabbit anti-α-casein was diluted with coating buffer up to a final concentration of 1 µg ml-1. Three hundred microliters of 1% PVA in 10 mM PBS buffer or 1% Ficoll in coating buffer was used for each well of the microtiter plate as blocking reagent for the sandwich assay. Blocking and washing were performed as described for the sandwich ELISA for Ficoll and PVA.
Therefore, 1% PVA could be considered for the blocking in ELISA assays at pH 7.6. Again, only one curve is shown for the blocking capacity of the two blocking reagents. Rabbit anti-α-casein with PVA blocking (black triangles), rabbit anti-α-casein with BSA blocking (grey triangles), and rabbit anti-α-casein with Ficoll blocking (white triangles).
IgY-anti-α-casein with PVA blocking (black squares), IgY-anti-α-casein with BSA blocking (gray squares) and IgY-anti-α-casein with Ficoll blocking (white squares) were diluted 1 :1,000. PVA blocking in the sandwich format with rabbit anti-α-casein coating is shown (multiplication sign). PVA blocking in the sandwich format with rabbit anti-α-casein coating is shown (multiplication sign).
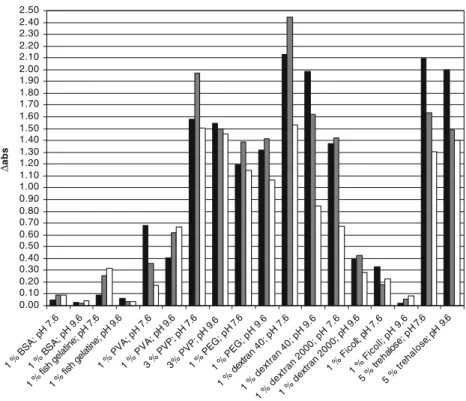
Determination of anti hazelnut antibodies specificities to different hazelnut
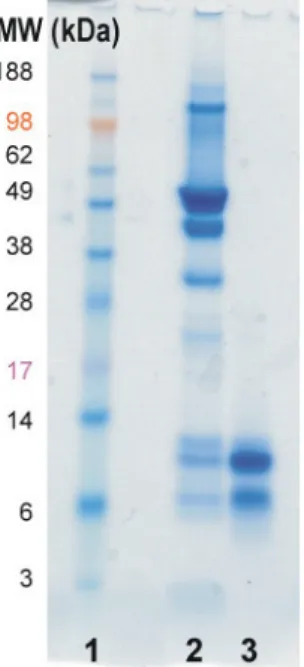
Gel electrophoresis was used to first control the protein profiles before characterizing them via LC-ESI-TOF/MS. This protein can be used as a marker to detect hazelnut components in food samples. The first and most important step in the development of such immunoassays is the preparation and characterization of the appropriate antibodies (AB).
Especially in the branch of allergen detection, it is always discussed which immunogens should be used to obtain different antibodies and what can be done for characterization of the developed antibodies. These preparations were then used for Western blot characterization of internally produced antibodies. The mass spectrometric measurements were performed for the structural characterization of proteins in pooled fractions.
Preparation and subsequent characterization of suitable antibodies is the first and most important step in the development of immunoassays. For characterization of six in-house produced monoclonal and polyclonal anti-hazelnut antibodies, different HN preparations were produced. The gel electrophoresis followed by western blot was the first method for the characterization of ABs.
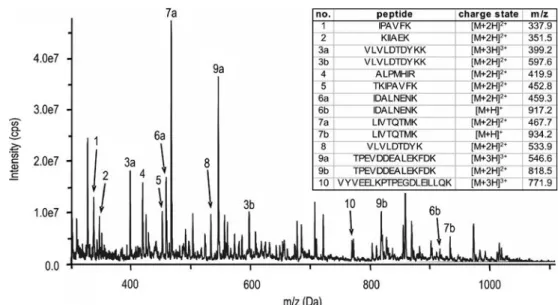
Selection of possible marker peptides for the detection of major ruminant milk
Seven of these peptides were synthesized and used for calibration of the LC-MS/MS system. Most of the publications dealing with the detection of milk allergens analyzed intact milk proteins [e.g. 8]. The precursor ion charge states of the intact tryptic peptides were confirmed by measurements in enhanced resolution (ER) scanning mode, thus scanning a range of 15 Da around the precursors (precursor m/zof ±15 Da).
Calibration of the LC-MS/MS method in SRM mode To determine the concentration of peptides in food samples by tryptic digestion, the LC-MS/MS method in SRM mode was calibrated by analyzing eight dilutions. In addition, MS analysis of metabolites of other milk proteins yielded several peptide ions. Then, the charge states of the peptides were confirmed by individual measurements of the respective ions in enhanced resolution (ER) scanning mode (data not shown).
This method was extended to a multianalyte method by including the SRM parameters for the individual tryptic peptides of the milk allergens listed in Table 1 . The charge states of the peptides were confirmed by MS measurements in the enhanced resolution (ER) scanning mode. All the selected peptides could be detected and measured with a concentration of up to 1 ng/.
Another washing step (3×10 min with PBST) followed before incubation with anti-species secondary antibodies, which were conjugated to horseradish peroxidase (HRP) and used for colorimetric protein detection. For the determination of peptide concentrations in tryptic-digested samples by the LC-MS/MS method in.
Extraction, characterisation and purification of the allergens
Various chromatographic methods have been used for the isolation and purification of dietary proteins from extracts, such as size exclusion chromatography (SEC), ion exchange chromatography (IEC), affinity chromatography, immunoaffinity chromatography and reverse phase chromatography (RPC); and to obtain the best results, the combination of these methods was also tested. For example, purification protocols were drawn up and the preparations obtained were used for the characterization of in-house produced antibodies. The molecular weight of these purified fractions was determined with a time-of-flight mass spectrometry (HPLC-ESI-TOF/MS).
These preparations were used for the characterization of six internally produced monoclonal and polyclonal anti-hazelnut antibodies. Unfortunately, the amount of preparations obtained was only sufficient for some western blot experiments and characterization by HPLC-ESI-TOF/MS. During this work, the extracts of various nuts, listed in EC Directive 2007/86/EC as allergens, were also used to check the cross-reactivity of anti-hazelnut antibodies produced in-house by western blot.
Cross-reactivity of the monoclonal antibody was more limited in the molecular weight range where 7S storage proteins can be found. The polyclonal antibody showed cross-reactivity to proteins in the low molecular weight range, where pollen allergens are also found. Since almost all nut food allergens belong to the family of seed storage proteins and appear in several botanical families of rosids, they have homologous structure and it is difficult to prevent this cross-reactivity between different nuts [4].
Development of mass spectrometric methods
Seven peptides (chain length: 5-12 amino acids) were chosen for the detection of milk, derived from tryptic digestion of four allergenic milk proteins (α- and β-casein, ALA and BLG). According to BLAST search, only one (LIVTQTMK from BLG, m/z 467.6, lot 2+) is specific to the bovine milk; two other peptides, . The LC-MS/MS multi-analyte method, developed for the simultaneous determination of these seven peptides, used them as a standard for the quantification of detected peptides in the food samples.
For the development of the hazelnut detection method, the hazelnut was first extracted, digested and measured with MS/MS by direct infusion. Eight tryptic peptides from three major hazelnut allergens Cor a 8, Cor a 9 and Cor a 11, which showed the highest MS signal, were selected and used as markers for the development of an LC-MS/MS method in SRM mode. Since almost all food allergens derived from nuts belong to the seed storage protein family, they have a similar sequence and homologous structure, which can be a problem for both immuno-based and LC-MS/MS methods developed for the detection of hazelnut in different food samples .
The next step will be to use this method for the determination of trace amounts of hazelnut allergens in processed food samples, leading to the establishment of a reference method [4]. 3] Ansari P, Stoppacher N, Rudolf J, Schuhmacher R, Baumgartner S (2011) Selection of possible marker peptides for the detection of large ruminant milk proteins in foods by liquid chromatography-tandem mass spectrometry. 4] Ansari P, Stoppacher N, Baumgartner S (2011) Marker peptide selection for determination of hazelnut by LC-MS/MS and occurrence in other nuts.
![Fig 1: Classification of adverse reactions to food according to EAACI [15]](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273214.42395/12.892.188.744.281.622/fig-classification-adverse-reactions-food-according-eaaci.webp)
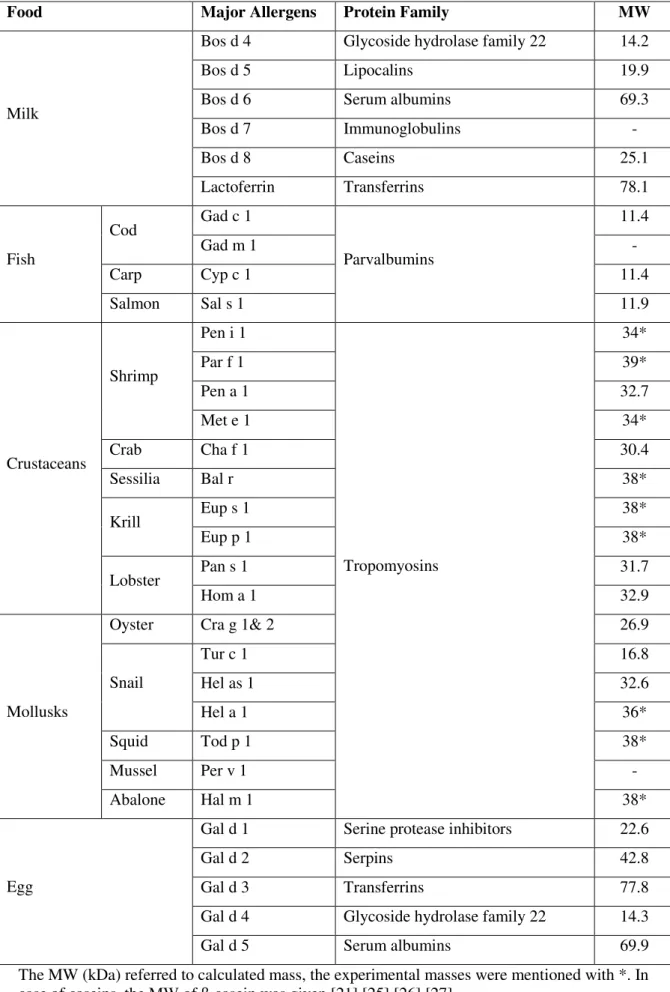
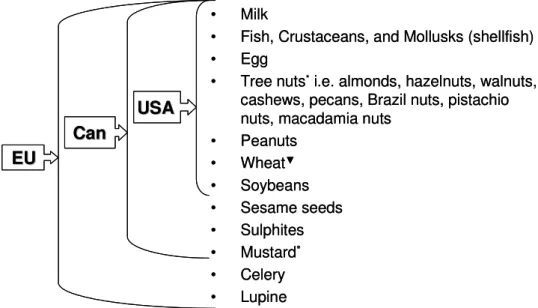
![Table 5: The action levels of major food allergens [mg/kg food] [52]](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273214.42395/24.892.196.746.287.949/table-action-levels-major-food-allergens-mg-food.webp)