Ich danke meiner Familie und meinen Freunden, dass sie bei mir sind und mich jederzeit unterstützen. Die Industrielle Biotechnologie eröffnet neue Perspektiven und Möglichkeiten für die Herstellung von Produkten aus nachwachsenden Rohstoffen. Im zweiten Teil dieser Arbeit wurden zwei Schlüsselenzyme für die Itaconsäure-Biosynthese (cis-Aconitat-Decarboxylase und Aconitase) gezielt in Mitochondrien exprimiert, was zu einer signifikanten Steigerung der Itaconsäure-Produktion führte.
Industrial biotechnology opens up new perspectives and opportunities for the production of biological products from renewable sources. This work focuses on the development of a genetic tool for the filamentous fungus Aspergillus niger, which is an efficient organic acid producer. The first part of this work was devoted to the characterization of new constitutive promoters of A.
Itaconic acid concentrations correlated with the strength of the applied promoters, thus proving their functionality. In the second part of this work, the key enzymes of this metabolic pathway, cis-aconitate decarboxylase and aconitase, were targeted in mitochondria leading to a significant increase in the production of itaconic acid.
INTRODUCTION
- Renewable resources
- Microorganisms
- Filamentous fungi
- Metabolic engineering
- Itaconic acid production with A. niger
- Genetic tool-box
- Genetic manipulations
- Expression systems
- Cellular compartmentalization
The most advanced biorefineries utilize the various components of biomass for the production of several products. 5 biomass as raw material for the production of bioproducts, food, feed and energy as well as limited availability of land (Chen 2012). In this work, we aim at the utilization of the most efficient citric acid producer A.
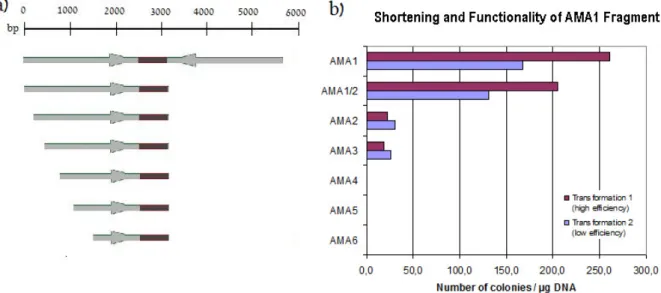
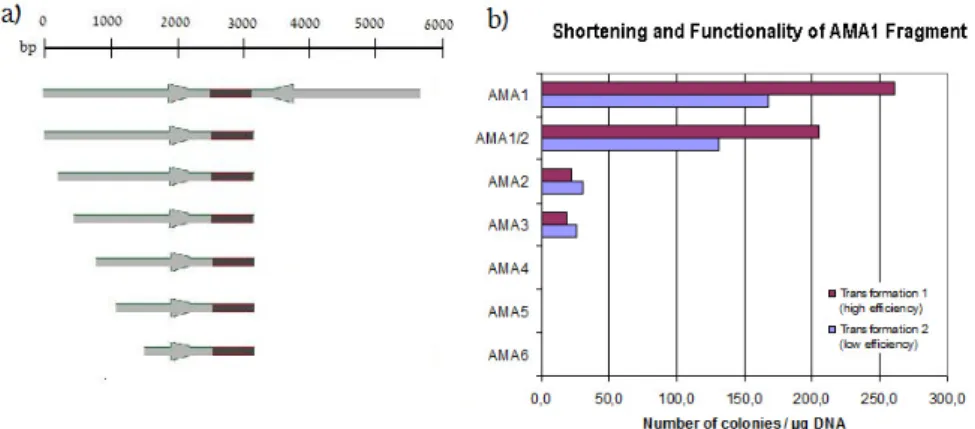
The low-cost production that resulted in increased demand for itaconic acid could significantly broaden the horizons of the market. To overcome this obstacle, a system based on a deletion of the non-homologous end-joining (NHEJ) pathway, previously described for Neurospora crassa, was applied (Ninomiya et al. 2004). In this study, we examined truncated AMA1 fragments lacking either of the two palindromic sequences (see Manuscript C).
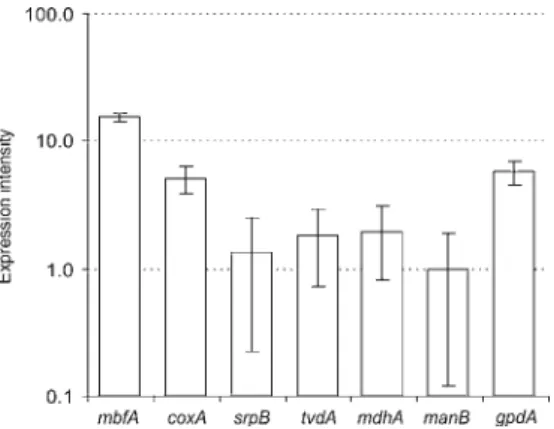
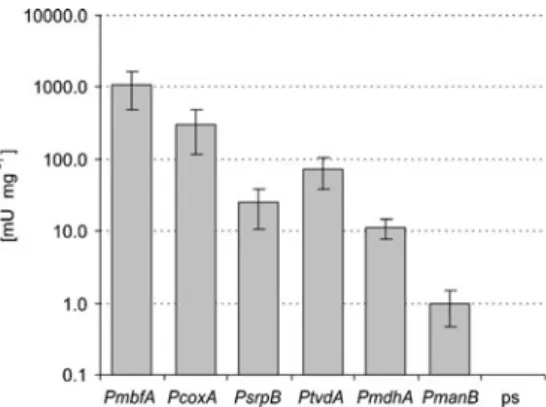
These promoters are one of the easiest and most efficient ways to regulate gene expression. The second group includes promoters that provide strong constitutive gene expression (eg the glyceraldehyde-3-phosphate dehydrogenase promoter PgpdA). To prove the functionality of the promoters, we applied them in the metabolic pathway for itaconic acid production.
Targeting proteins to the mitochondria or other small cell organelles can result in a significant improvement in the production of the final metabolite.
Jarboe LR, Zhang X, Wang X, Moore JC, Shanmugam KT, Ingram LO (2010) Metabolic engineering for the production of renewable fuels and chemicals: contributions of synthetic biology. Karaffa L, Kubicek CP (2003) Aspergillus niger citric acid accumulation: do we understand this well-functioning black box? Kim IK, Roldão A, Siewers V, Nielsen J (2012) A systems-level approach to metabolic engineering of yeast cell factories.
18 Li A, van Luijk N, ter Beek M, Caspers M, Punt P, van der Werf M (2011) A clone-based transcriptomic approach for the identification of genes important for itaconic acid production in Aspergillus. Meyer V, Arentshorst M, El-Ghezal A, Drews AC, Kooistra R, van den Hondel CA, Ram AF (2007) Highly efficient gene targeting in the Aspergillus niger kusA mutant. Meyer V, Wanka F, van Gent J, Arentshorst M, van den Hondel CA, Ram AF (2011) On-demand fungal gene expression: an inducible, tunable, and metabolism-independent expression system for Aspergillus niger.
Mojzita D, Wiebe M, Hilditch S, Boer H, Penttilä M, Richard P (2010) Metabolic engineering of fungal strains to convert D-galacturonate to meso-galactarate. Polack R, Wood S and Smith K (2010) An analysis of fossil fuel dependence in the United States with implications for community social work. Punt PJ, van Biezen N, Conesa A, Albers A, Mangnus J, van den Hondel C (2002) Filamentous fungi as cell factories for heterologous protein production.
Sahasrabudhe NA and Sankpal NV (2001) Production of organic acids and metabolites by fungi for the food industry. Sauer M, Porro D, Mattanovich D and Branduardi P (2010) 16 years of research on lactic acid production by yeast – ready for market. Scott E, Peter F, Sanders J (2007) Biomass in the production of industrial products - the use of proteins and amino acids.
Sharma R, Katoch M, Srivastava PS, and Qazi GN (2009) Approaches to fine-tuning heterologous protein production in filamentous fungi. Somoza AD, Lee KH, Chiang YM, Oakley BR, Wang CC (2012) Redesign of an azaphilone biosynthetic pathway in Aspergillus nidulans to create lipoxygenase inhibitors. Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J (2006) Redirection of cytosolic or plastidic isoprenoid precursors increases terpene production in plants.
AIM OF THE STUDY
OUTLOOK
The overexpression of certain transporters located in the same gene cluster can result in a correct flux of metabolites and significant production improvement.
PUBLICATIONS AND SCIENTIFIC MANUSCRIPTS
Publications of this study
Scientific manuscripts
Other publications
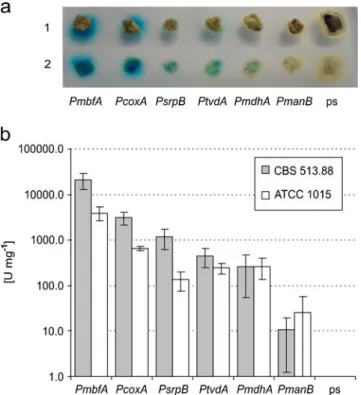
The map of the plasmids is included in the online resource (Fig. S1 in the ESM). For the measurement of the β-glucuronidase (GUS) activity, 10μL of the cell-free extract was mixed in eight different dilutions with 40μL ×2 GUS reaction buffer and diluted with 50μL 4 mM 4-methylumbelliferyl-β-D-glucuronide ( CUP). The linear series of fluorescence increase was used for the calculation of the GUS activity.
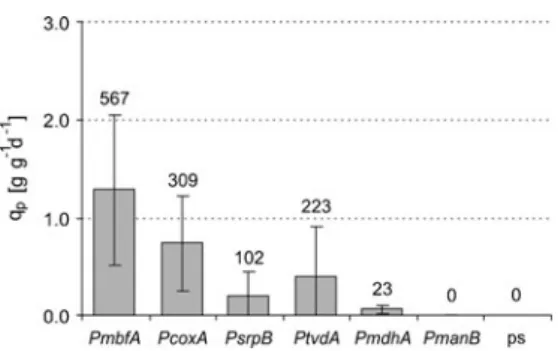
Final concentration of the organic acids produced in the medium and the remaining glucose was determined by HPLC. The ranking of the other 4 promoters is comparable to the results of the GUS activity assay. Itaconic acid was obtained for all cultivars except for the transformants carrying the cad1 gene under the control of the very weak PmanB promoter (Fig.4).
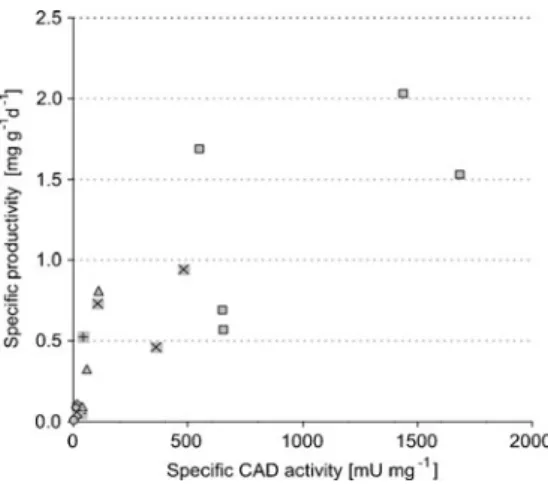
The functionality of the new promoter set for metabolic engineering purposes was demonstrated in a case study using the target metabolite itaconic acid. Intermediate concentrations for the other constructs demonstrated the correlation of the promoter strength with the final titer. However, overexpressing the plasma membrane carrier in a strain already expressing the mitochondrial carrier did not produce a further increase in itacon acid titers (Li et al., 2013).
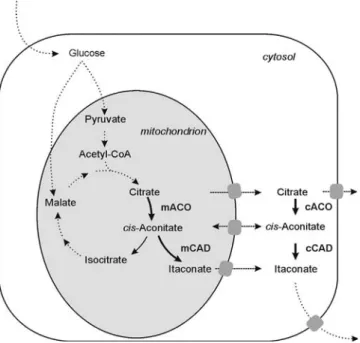
Cloning and isolation of the plasmids were performed according to standard methods (Sambrook and Russel, 2001). Citric acid production and the following reactions in the TCA cycle take place in the mitochondrion. The increase in the itaconic acid titer is not as pronounced as when the CadA protein is located in the same compartment.
A combination of the cytosolic and mitochondrial pathways in one strain leads to an even higher itaconic acid production. Cloning and functional characterization of the cis-aconic acid decarboxylase (CAD) gene from Aspergillus terreus. Today, genetic engineering plays a major role in the improvement of the industrial Aspergillus niger cell factories.
This review describes the current status and recent advances in understanding the molecular processes leading to the biotechnological production of itaconic acid. The activity of the cis-aconitic acid decarboxylase is critical to the performance of the entire itaconic acid biosynthetic pathway. Also the genetic regulation of the itaconic acid pathway in A. terreus requires a thorough analysis. Li et al.
Cloning and functional characterization of the cis-aconic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl. 1981). Production of Itaconic Acid by Fermentation.
CURRICULUM VITAE