These communities drive ecosystem functioning, perform key processes in global nutrient cycles, and ultimately define the world as we know it [Baldrian et al., 2013]. Linking this information to ecosystem function helps us understand complex systems and determine the key players and factors that control these [Song et al., 2014].
Phylogenetic Marker Genes
Currently, the UNITE database contains 442,706 fungal ITS sequences and distinguishes between 53,891 different fungal species based on a 98.5% sequence similarity threshold ([Kõljalg et al., 2013]; https://unite.ut.ee). Moreover, information derived from fungal 18S rRNA sequences can be used to calculate phylogenetic relationships between the organisms in one sample, which cannot be achieved with ITS sequences [Lie et al., 2014].
![Figure 1.1 Secondary structure of SSU rRNA of Escherichia coli [Yarza et al., 2014]. Arrow indicates position of target sequences from the probe used in this study.](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273292.42430/11.892.137.753.152.942/figure-secondary-structure-escherichia-yarza-indicates-position-sequences.webp)
DNA-Hybridization
For fungal communities, the ITS region (Figure 1.2) is also used to determine phylogenetic relationships, providing higher resolution to the species level [Hibbett et al., 2011] [Bellemain et al., 2010]. This very specific property of DNA molecules has been used for many methods established in molecular biology, from blotting DNA onto membranes and staining them to annealing primers in all types of PCR reactions to designing microarray chips, to name but a few [ Amann et al., 1990 ] [He et al., 2007] [Amann et al., 2001].
![Figure 1.3 Schematic overview on the magnetic bead capture approach used in this study (adapted from [Abed, 2012])](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273292.42430/13.892.252.637.435.769/figure-schematic-overview-magnetic-capture-approach-study-adapted.webp)
Stable Isotope Probing (SIP) in Microbial Ecology
Density gradient centrifugation methods were developed to separate the light and heavy fraction of RNA or DNA molecules. Nevertheless, it should be mentioned that the phylogenetic resolution can be better by applying a density gradient approach under certain circumstances. Instead of a density gradient centrifugation step, this approach separates the RNA sample by hybridization with phylogenetic specific probes.
The first steps towards this goal included testing the performance of the probes in pure RNA culture and optimizing the hybridization conditions in the assay. Domain-level partitioning of a specific group of rRNAs has the potential to become a standard method in microbial ecology studies in the future. While nowadays we can separate C13-labeled nucleic acids by density gradient centrifugation and then analyze the communities using a sequencing approach, similar experiments with stable isotopes from different elements are still difficult to perform.
Finally, by further developing this approach to specifically target groups of organisms from lower phylogenetic levels, we could track the fate of elements through microbial communities, which would represent a major step in the development of microbial ecology-based research. .
![Figure 1.4 Overview of SIP-based methods in microbial ecology (adapted from Abraham [Abraham, 2014])](https://thumb-eu.123doks.com/thumbv2/pubdocorg/273292.42430/15.892.186.707.131.450/figure-overview-methods-microbial-ecology-adapted-abraham-abraham.webp)
Fungal RNA Isolation
All measurements of RNA concentrations were performed using Quant-iTT M RiboGreenc for RNA analysis (InvitrogenT M) according to the manual on a microtiter plate reader (Enspire 2300 Multilabel Reader). Avoiding melting of the powder, approximately 200 mg was transferred to a pre-chilled 2.0 mL reaction tube. After incubation at room temperature (RT) for 5 min, 0.2 mL of chloroform was added and the mixture was vortexed.
The RNA in the clear hydrophilic phase above the three-phase mixture was transferred to a new RNase-free reaction tube. For RNA precipitation, 0.5 ml of isopropanol was added, then mixed and incubated on ice for 10 min. To avoid RNA hydrolysis, the enzyme heat inactivation step was replaced by an additional purification step using the RNeasy MiniElute Purification Kit (QUIAGEN).
Quality of the RNA was controlled by gel electrophoresis which resulted in two bands at 1.8 and 3.3 kb representing the small and large subunit ribosomal RNA, respectively, and no smear on the gel.
Bacterial RNA Isolation
Bacterial and Fungal Specific Probe Design
SSU rRNA Capture
Hybridization of Bacterial and Fungal Specific Probes with SSU
Capture of Hybridized Probes with Magnetic Beads
Washed and blocked beads were added to the hybridization mixture and placed back on the thermal shaking block at RT for 2 h. Shaking speed was adjusted so that beads did not settle in the reaction tube (1150 rpm for 75 µL reaction). Reaction tubes were placed back on the magnet and unhybridized RNA was transferred to a new reaction tube.
For further analysis, these unhybridized RNA samples were cleaned of buffer salts and reactants using a size exclusion chromatography step by running the protocol of Chroma Spin™ M 400 mini spin columns (Clonetech). Buffer concentrations that are too high prevent analysis with the electrophoresis-based Agilent Bioanalyzer system due to the increased conductivity of the sample. Beads were washed 3 times with 7.5 x SSC buffer (4 times the volume of beads used) and collected on the magnetic particle collector in between.
After adequate settling of the beads against the magnet, the RNA was transferred together with the clear supernatant to a new reaction tube, preventing uptake of the beads.
Analysis of RNA Samples on Agilent Bioanalyzer System
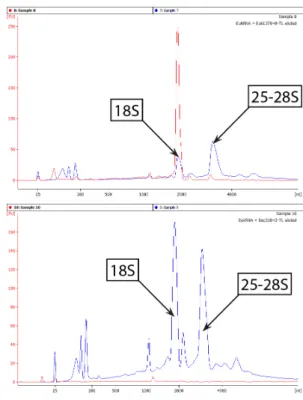
Best results were obtained by adding 3 bp to both ends of the probe, resulting in a recovery of 41.5% of the SSU rRNA initially used in the assay. Although the red line shows a clear elution peak at the position of the SSU rRNA molecule, a decrease in the peak area could also be observed in the unhybridized RNA sample, indicating the specific isolation of the rRNA. Two similar peaks could be observed in the unhybridized RNA sample at the position of the R. terrigena SSU and LSU rRNA, similar to the electropherogram of the RNA initially used in the assay (graph not shown).
Again, a clear elution peak at the position of the fungal SSU rRNA and a decrease in the correlated peak from the unhybridized sample could be observed. 21% of the available bacterial SSU rRNA could be captured, while 37% of the fungal SSU rRNA in the sample could be isolated. Capture of fungal and bacterial SSU rRNA from pure culture RNA Changing the length of the probes showed clear effects for the fungal capture efficiency, whereas the performance of bacterial probes was not affected.
Results using the optimized probes Bac338-TL and Euk1379+6-TL on RNA from pure cultures show the specificity of the two probes in the assays. Differences in the shape of unhybridized samples in the graphs can be explained by the many manipulative steps performed during the protocol. Capture of Fungal and Bacterial SSU RNA from a Soil RNA The unhybridized RNA sample of the sampled soil was completely degraded after multiple hybridization reactions were performed.

Bu ers, Media & Solutions
Reagents & Equipment
Fungal Probes
The continuous addition of bases to both sides of the initially used fungus-specific probe sequence (Euk1379) increased the capture efficiency 6-fold (Figure 4.1). The initial amount of SSU rRNA added from Doratomyces to these assays was 260 to 520 ng (∼1 to 2 µg total RNA). By adding only one base pair to the 3' and 5' end of the probe sequence, we can increase the capture efficiency 3-fold to 26.4%. A further extension of probe length had no effect on capture efficiency, so the optimal probe length under these conditions was 22 bp (Euk1379+6-TL).
Bacterial Probes
Testing of Optimized Probes on Capture Specificity
Tests on Pure-Culture RNA
TL was able to successfully capture SSU rRNA from R.terrigena as shown in the top graph of figure 4.3. Similarly, Euk1379+6-TL was able to specifically capture SSU rRNA molecules from Doratomyces as shown in the top graph of Figure 4.4. No elution peaks were present when Bac338-TL probe and fungal RNA were used in the assay and the unhybridized RNA sample showed a comparable pattern to the RNA initially used in the assay, supporting the result of domain-specific capture , as no fungal RNA hybridized to the bacterial probe.
The blue line indicates the unhybridized RNA sample, the red, green, and turquoise lines indicate the RNA samples eluted from the first, second, and third capture reaction, respectively. An experiment with repeated hybridization reactions was set up to assess the possibility of increasing the capture efficiency of SSU rRNA. The unhybridized sample from the previous reaction was used for the second and third hybridization reactions.
Thus, the actual capture efficiency would be lower than 60%, but an increase in captured SSU rRNA could still be achieved.

Tests on Pooled Pure-Culture RNA
Following this procedure, we can increase the capture e ciency from 41.5% to 60%, although it includes a peak of small RNA fragments eluted from the 2nd hybridization reaction with a size of about 100 bp. At the same time, the targeted peak of the unhybridized RNA sample was reduced in height and peak area (16S peak in figure 4.6; 18S peak in figure 4.7). Moreover, the capture efficiency of the two hybridization reactions was similar to the values obtained with only bacterial or fungal RNA.
Tests on Soil RNA
The improvement in the recovery of fungal SSU rRNA from 6.6 to 41% from the initial added amount is a very promising result regarding the applicability of the method with environmental samples. Differences in peak heights of the SSU and LSU peaks in unhybridized samples can be explained by the differences in RNA losses during the desalting step performed before loading these samples onto the Agilent Bioanalyzer chip. It was therefore possible to identify the origin of the elution peak simply by graphically overlaying the two electropherograms.
This increase is very significant when looking at the applicability of the method for analyzing downstream products in terms of material supply sufficiency. Again, we could observe the degradation of the RNA used in the sample, but the main part of the eluted RNA was found to be the size of the entire rRNA molecule. However, if we only look at the length of the molecules covered in this study, we will not be able to draw scientifically valid conclusions based on our data.
Taxonomic identification will be key in assessing the specificity of the probes in these tests and drive further development.

Statement
Ich erkläre, dass ich die vorliegende Arbeit selbstständig und ohne fremde Hilfe verfasst und keine anderen als die im Literaturverzeichnis angegebenen Quellen genutzt habe. Ich versichere Ihnen, dass ich alle direkten und analogen Übernahmen aus anderen Werken als solche gekennzeichnet habe.
Metagenomic and small-subunit rRNA analyzes reveal the genetic diversity of bacteria, archaea, fungi and viruses in soil. Stable-isotope probing of DNA: insights into the function of uncultured microorganisms from isotope-labeled metagenomes. Dual staining of natural bacterioplankton with 4',6-diamidino-2-phenylindole and uorescent oligonucleotide probes targeting kingdom-level 16S rRNA sequences.
Probing microbial eukaryotic diversity from a global census: insights from a comparison of pyrotag and full-length 18S rRNA gene sequences.