The fourth edition of Modern Practice of Gas Chromatography presents a number of changes from the first three editions. These authors were selected for their expertise and active participation in various fields related to gas chromatography (GC). Second, the content of the various chapters has been revised to be comprehensive.
Third, separate chapters are devoted to gas chromatography/mass spectrometry, sample preparation, rapid gas chromatography, optimization and computer support, and QA/QC validation of gas chromatographic methods.
Introduction
HISTORY AND DEVELOPMENT OF CHROMATOGRAPHY
Introduced paper strip analysis (capillary . analysis) of dyes, hydrocarbons, milk, beer, colloids, drinking and mineral waters, vegetable and animal pigments.

DEFINITIONS AND NOMENCLATURE
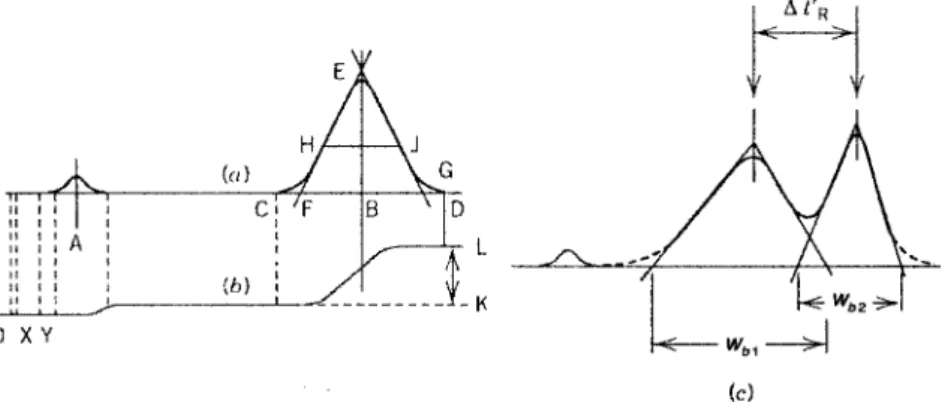
A graph of the detector response (using effluent concentration or another quantity to measure the sample component) versus effluent volume or time. The separation takes place through the partitioning (differences in solubility) of the sample components between the two phases. It is characteristic of the instrument, the mobile phase flow rate and the column used.
The volume of carrier gas (mobile phase) required to fill the injection port of the chromatograph.
SUGGESTED READING ON GAS CHROMATOGRAPHY
B = constant responsible for the effect of molecular diffusion of the vapor in the direction of the column axis. C=constant proportional to the resistance of the column packing to mass transfer of solute through it. This is a PLOT column in which the walls have been etched prior to the deposition of the support.
It is a SCOT column in which the walls are carved before the support is placed.
COMMERCIAL INSTRUMENTATION
Theory and Basics
Theory of Gas Chromatography
CHROMATOGRAPHIC METHODS .1 Classification of Methods
- General Aspects
- Frontal Analysis
- Displacement Development
- Elution Development
- Isotherms
- Process Types in Chromatography
- Linear Ideal Chromatography
- Linear Nonideal Chromatography
- Nonlinear Ideal Chromatography
- Nonlinear Nonideal Chromatography
The result of this interaction is the different distribution of the sample components between the two phases, resulting in the separation of the sample component into zones or bands. Separation of the sample components can be achieved by one of three techniques: frontal analysis, shear development, or elution development. Thus, the bulk of the discussion in the subsequent chapters revolves around elution GC.
The transport of the solute down the column will depend on the distribution constant (partition coefficient) K and the ratio of the amounts of the two phases in the column.
GENERAL ASPECTS OF GAS CHROMATOGRAPHY .1 Applications of Gas Chromatography
- Types of Detection
- Advantages and Limitations
A peak in the chromatogram from the pyrolysis of the unknown may be from the matrix and not the suspected component. Another important application of GC is in the preparation of pure substances or narrow fractions as standards for further studies. The determination of antioxidants and food preservatives is an active part of the gas chromatographic field.
From the limited discussion so far the versatility of the gas chromatographic technique can be visualized.
GAS CHROMATOGRAPHY
- Plate Theory
- Rate Theory
Glueckauf (24) studied the effect of four factors on the chromatographic process: (1) diffusion in the mobile phase perpendicular to the direction of flow, (2) longitudinal diffusion in the mobile phase, (3) diffusion into the mobile phase. particle and (4) size of the particle. The migration is from the higher to the lower concentration range in the axial direction of the column. The velocity of the zone is Ru, where the fraction of dissolved molecules in mobile phase and u is the mobile phase velocity.
The term He is introduced to take into account the characteristics of the equipment used in the system.
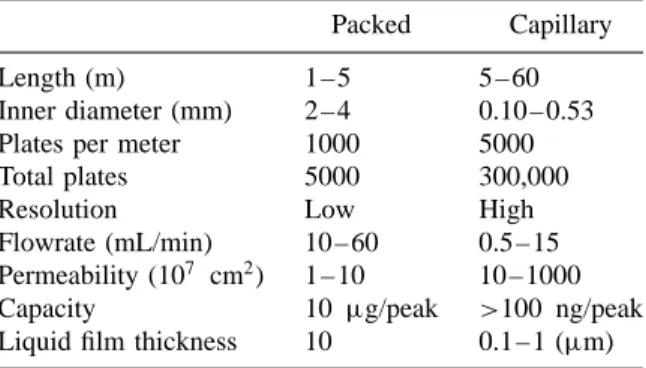
Columns: Packed and Capillary;
Column Selection in Gas Chromatography
OVERVIEW
- CENTRAL ROLE PLAYED BY COLUMN
- JUSTIFICATION FOR COLUMN SELECTION AND CARE
- LITERATURE ON GAS CHROMATOGRAPHIC COLUMNS
- GAS CHROMATOGRAPHIC RESOURCES ON THE INTERNET The World Wide Web (WWW) has provided us with copious amounts of infor-
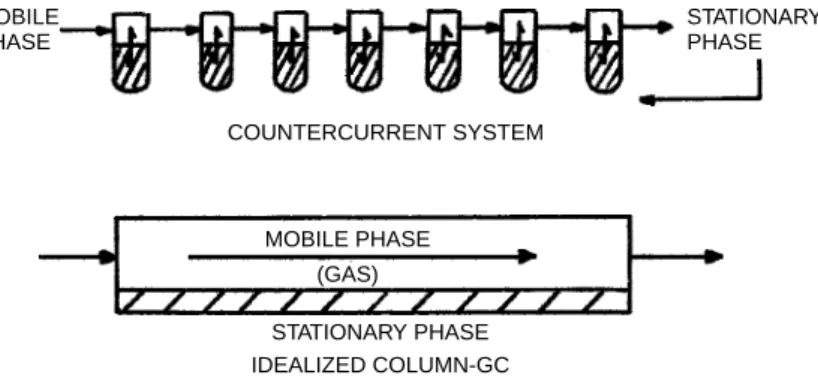
The gas chromatographic column can be considered to be the central element of a gas chromatograph. Since the early 1970s, the nature and design of the column has changed considerably from one containing either a solid adsorbent or a liquid deposited on an inert solid support wrapped in a length of tubing to one containing an immobilized or cross-linked stationary phase bound to inner surface of a much longer length of fused silica tube. Innovations and advances in gas chromatography since the mid-1980s have been made with the advantages of the fused silica column as a focal point and are primarily driven by the environmental, petrochemical, and toxicology fields, as well as by advances in sample preparation and mass spectrometry.
When one also considers the purchase of high-purity gases on a regular basis necessary for the operation of the chromatograph, it quickly becomes clear that a significant investment in capital equipment has been made. Careful attention should also be paid to properly implemented connections of the column to the injector and detector and the presence of high boilers, particles in samples, and other factors. The price of a column can be considered relatively small compared to the initial, the routine and preventive maintenance costs of the instrument.
A further decline in the use of packed columns occurred in 1983 with the advent of the 0.53 mm ID megabore capillary column, which serves as a direct replacement for the packed column. Column manufacturers rely on current literature, the results of their own marketing surveys, the number of clicks on their websites, etc. to keep up to date with the needs of practicing gas chromatographers. The fused silica capillary column has clearly emerged as the column of choice for most gas chromatography applications.
However, the Internet has proven to be the most comprehensive source of chromatographic information, particularly column manufacturer websites, as described in the next section. For example, this author strongly recommends that you find and regularly visit the web addresses of the column manufacturers and bookmark the relevant web pages.
PACKED-COLUMN GAS CHROMATOGRAPHY Packed columns are still utilized for a variety of applications in gas chromatog-
- SOLID SUPPORTS AND ADSORBENTS
- Supports for GLC: Diatomaceous Types, Halocarbons
- Adsorbents for GSC: Porous Polymers, Molecular Sieves, Carbonaceous Materials
- STATIONARY PHASES
- Requirements of a Stationary Phase
- Kovats Retention Indices
- McReynolds Classification of Stationary Phases
- Evaluation of Column Operation
- Optimization of Packed-Column Separations
- COLUMN PREPARATION
- Description of Coating Methods
- Tubing Materials and Dimensions
- Glass Wool Plugs and Column Fittings
- Filling the Column
- Conditioning the Column and Column Care
Lack of adequate packing in a gas chromatographic column is often the source of poor separation. An often overlooked parameter in selecting a packed column is the packing density of the support material. Will there be any irreversible reaction between the liquid phase and the components of the mixture to be separated.
The vapor pressure of the liquid phase should be less than 0.1 Torr at the operating temperature of the column. Apart from its effect on detector noise, liquid phase leakage can interfere with analytical results and determine column lifetime. Two other properties of the liquid phase to consider are viscosity and wettability.
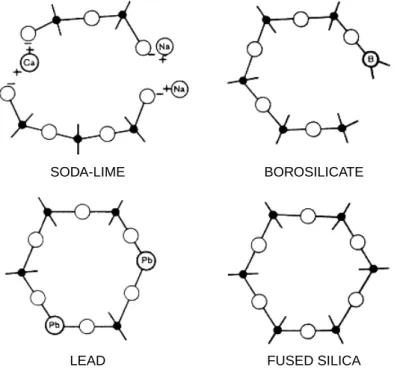
Selectivity, on the other hand, can be considered in terms of the magnitude of the individual energies of interaction. Chemical structures of the more popular polysiloxanes used as stationary phases are illustrated in Figure 3.6. Effects of the various changes in chromatographic parameters on resolution that can be implemented are schematically illustrated in Figure 3.12.
Conditioning a column also helps with the redistribution of the liquid phase onto the solid support. Save the box in which a glass column was shipped for safe storage of the column.

CAPILLARY COLUMN GAS CHROMATOGRAPHY
- INTRODUCTION
- Significance and Impact of Capillary GC
- Chronology of Achievements in Capillary GC
- Comparison between Packed and Capillary Columns
- CAPILLARY COLUMN TECHNOLOGY .1 Capillary Column Materials
- Preparation of a Fused-Silica Capillary Column
- CHROMATOGRAPHIC PERFORMANCE OF CAPILLARY COLUMNS
- Golay Equation versus van Deemter Expression
- Choice of Carrier Gas
- Phase Ratio
- Practical Considerations of Column Diameter, Film Thickness, and Column Length
- Coating Efficiency
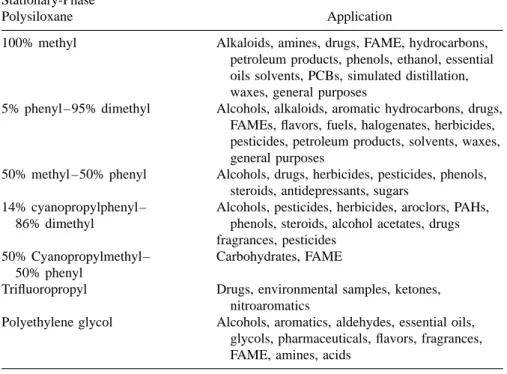
- STATIONARY-PHASE SELECTION FOR CAPILLARY GAS CHROMATOGRAPHY
- Requirements and History
- Cross-Reference of Columns from Manufacturers
- Polysiloxanes
- Polyethylene Glycol Phases
- Crosslinked versus Chemically Bonded Phases
- Specialty Columns .1 EPA Methods
- Capillary Column Care and First Aid .1 Ferrule Materials and Fittings
- Applications
Three stages in the evolution of capillary column technology are shown in Figure 3.15: one compartment packed column and two compartments with a stainless steel and glass capillary column. On the other hand, a capillary column contains a relatively thin immobile film uniformly coated on the inner wall of the tube. The details of the procedure depend on the stationary phase to be coated next, but on deactivation.
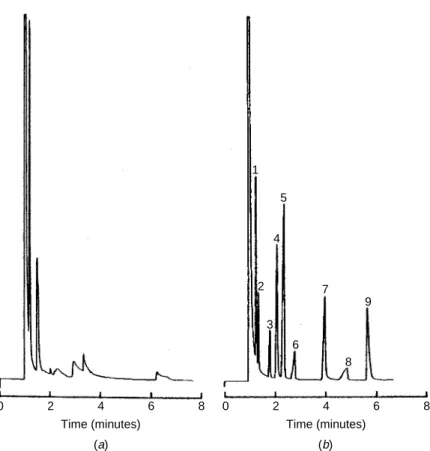
In the test report evaluating the performance of the column, chromatographic data is listed. An example of this situation is the separation of the Grob mixture (Figure 3.26a) which is carried out on. In Figure 3.32 these parameters are put into perspective in a pyramidal format as a function of the inside diameter of a capillary column.
In general, the sample capacity of any capillary column is proportional to the square of the radius of the column. However, with the transition from the packed to the capillary column era, a gradual redefinition of the requirements for the stationary phase took place. Wright and co-workers (103) correlated the viscosity of a stationary phase with the coating efficiency and stability of the coated phase.
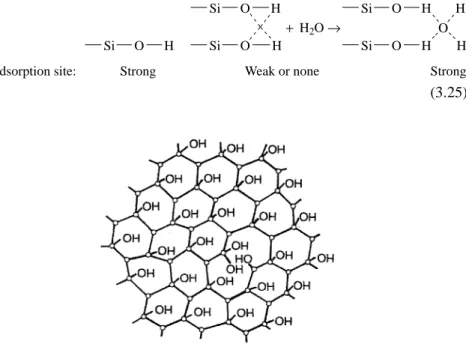
One measure of the polarity of a stationary phase is the cumulative value of the McReynolds constants, as discussed in Section 3.6.3. Using a creasing tool, carefully mark the surface of the column a few centimeters from the end. This procedure eliminates the possibility of fragments of molten silica or polyimide remaining in the end of the column.
Similarly, column bleed increases with increasing film thickness of the stationary phase and with increasing column length.
COLUMN OVEN TEMPERATURE CONTROL
- THERMAL PERFORMANCE VARIABLES AND ELECTRONIC CONSIDERATIONS
- ADVANTAGES OF TEMPERATURE PROGRAMMING OVER ISOTHERMAL OPERATION
- OVEN TEMPERATURE PROFILES FOR PROGRAMMED-TEMPERATURE GC
- CAPILLARY CAGE DESIGN
- SUBAMBIENT OVEN TEMPERATURE CONTROL
Forced air convection is the most popular type of gas chromatographic oven because it provides a uniform temperature in the column oven. Under microprocessor control, a flap or door movement allows mixing of the proper amount of ambient laboratory air with furnace air in controlling furnace temperature. In addition, a cryogenic valve can be opened by a microprocessor for the delivery of carbon dioxide or liquid nitrogen into the column furnace.
The interested reader is urged to consult the classic book Programmed Temperature Gas Chromatography by Harris and Habgood (168) for a detailed treatment of the subject. The initial temperature and holding period are usually determined from a scout run where the elution temperatures are noted; A correct choice of the initial conditions will allow separation of the low-boiling components in the separation, while the chosen final temperature should be sufficient for the elution of the more highly retained components in the sample (taking into account the upper limit of the temperature of the stationary phase) . In this injection mode, a low column temperature is maintained during the introduction of the sample into the retention gap, after which the initial and usually faster rate of rise is initiated.
To relieve stress on the rolled fused silica, the diameter of the cage must be compatible with the inner diameter in the column. In other words, the column cannot be wound too tightly, or tube breakage may occur. The cage also restrains the column so that it does not rotate in the column oven under the influence of forced air convection currents.
Before a column cage is mounted on the suspension bracket in the furnace, the column should be examined to see if the coils are evenly spaced around the width of the cage with minimal overlap, a relevant consideration for uniform heating of the column. It is also important that the ends of the column extending from the cage to connect to the injector and detector do not come into contact with any metal parts of the furnace where wear can erode the protective outer layer of polyimide on the surface of the column.
Optimization of Separations and Computer Assistance
WHY OPTIMIZE?