I would also like to thank Katarina Hemelikova (Lichnerova) and Kristyna Rehakova (Skrenkova) for their help and support from the very beginning of my work. Hyperactivity or hypoactivity of NMDARs can lead to a wide spectrum of pathological conditions and psychiatric disorders, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, epilepsy, schizophrenia. Different subtypes of NMDARs may have different effects on disease pathogenesis, and therefore it is crucial to investigate the specific role of each subunit in the regulation of normal NMDAR function.
The regulation of NMDARs occurs at different levels, from early processing, including synthesis, assembly, quality control in the endoplasmic reticulum (ER), trafficking to the cell surface, to internalization, recycling and degradation. In this thesis we mainly focused on determining the roles of extracellular and transmembrane regions of different subtypes of NMDARs in the regulation of their function. In particular, using electrophysiology and microscopy methods on HEK293 cells and cultured hippocampal neurons, we investigated: (i) the impact of N-glycosylation and different lectins on the regulation of the functional properties of GluN1/GluN3 -receptors; (ii) the effects of pathogenic mutations in the transmembrane domain (TMD) of the GluN1 subunit on surface delivery, and the functional and pharmacological properties of conventional and non-conventional di-heteromeric NMDARs; (iii) the role of glycine-binding site integrity of the GluN1 and GluN3A subunits in the trafficking and functional properties of NMDARs.
Introduction
Literature review
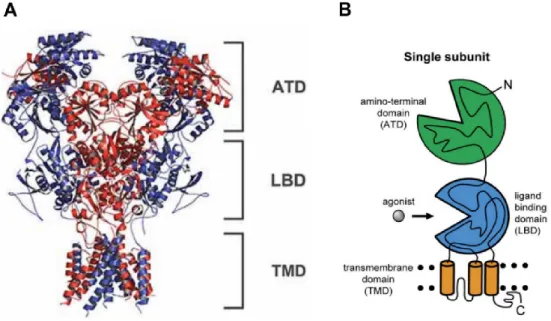
NMDAR structure and function
- NMDAR subunits and their expression
- Organization of NMDAR domains
- Activation and gating of NMDARs
The GluN2A subunit expression begins a few days after birth and the subunit is highly expressed in almost all regions of the CNS (Hansen et al., 2021). The expression of the GluN3B subunit is observed in the pons, midbrain, medulla oblongata and the spinal cord, with a peak around P14 and the expression remains high through adulthood (Wee et al., 2008). Evidence also suggests that the ATD mediates the assembly of the initial GluN subunit dimers in the ER ( Meddows et al., 2001 ).
The LBD of GluN1 and GluN3 subunits contains a glycine binding site while the LBD of GluN2 subunits contains a glutamate binding site (S. F. Traynelis et al., 2010). Other endogenous agonists of the GluN2 subunit include D- and L-aspartates, homocysteine, cysteinesulfinate, and cyclic analogues with conformationally constrained rings (Hansen et al., 2021). From the D state the receptor cannot go directly to the open state, it must be resensitized first (Cais et al., 2008; Lester and Jahr, 1992).
NMDAR pharmacology
- NMDAR competitive antagonists
- NMDAR allosteric modulators
- NMDAR open channel blockers
The GluN2 subunit LBD is also a target for several competitive antagonists, such as D-(-)-2-amino-5-phosphonopentanoic acid (D-AP5), 2-Amino-7-phosphonoheptanoic acid (AP7), 1-( phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA), or 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) (Heckman et al., 1967; Jespersen et al., 2014) . Thus, the high selectivity of CGP-78608 for the GluN1 subunit compared to the GluN3A subunit prevents glycine binding to the GluN1 subunit, but not to the GluN3 subunit, and abolishes GluN1-mediated desensitization (Grand et al., 2018 ). The pH-sensitive regions were found within the M3 helix in the conserved SYTANLAAF motif, as well as in the M3-S2 linker region of the GluN1 subunit (Low et al., 2003).
Voltage-dependent inhibition causes a low-affinity blockade, which occurs in the vestibule of the ion channel (Mayer et al., 1989; Paoletti et al., 1997). The third mechanism is called “foot in the door” when the blocker prevents the ion channel from closing and dissociates the agonist (Blanpied et al., 1997; Hansen et al., 2018). Moreover, a tryptophan residue in the M2 loop of GluN2 subunits ( Williams et al., 1998 ) also appears essential for Mg 2+ block, in a subunit-specific manner.
NMDAR assembly and trafficking
- Processing of NMDARs in the endoplasmic reticulum
- Trafficking of NMDARs from the ER to the plasma membrane
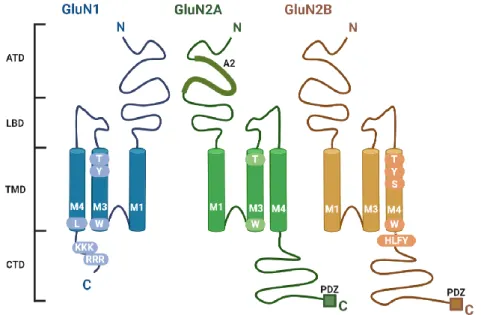
Similarly, amino acid residue L830 in the second half of the M4 domain of the GluN1 subunit is involved in masking retention motifs in the ER, allowing transport of functional NMDARs to the cell surface (Kaniakova, et al., 2012). GluN2B subunit - amino acid residues within the M3 domain, the HLFY motif in the proximal part of the CTD, the PDZ-binding motif in the distal part of the CTD (Horak et al., 2014). In the non-canonical pathway, NMDARs bypass the somatic Golgi apparatus (GA), and instead fuse with dendritic Golgi (dGolgi) outposts through dendritic ER (dER) compartments (Jeyifous et al., 2009) and are then delivered in vesicles to the cell surface via exocytosis (Gu and Huganir, 2016).
The balance between receptor exocytosis and internalization is important in regulating the number and type of NMDARs at the cell surface ( Horak et al., 2014 ; Wenthold et al., 2003 ). In the clathrin-dependent mechanism, an AP-2 interaction motif in the CTD of NMDARs interacts with the AP-2 complex (made of adaptins that bind to clathrin) ( Petralia et al., 2003 ). In the non-clathrin-dependent endocytosis mechanism, NMDAR CTDs interact with flotillin-1, a lipid raft-associated protein ( Swanwick et al., 2009 ).
Glycosylation of NMDARs
- N-glycosylation
- N-glycosylation of NMDARs
The identity of the 'X' can affect glycosylation efficiency, e.g. efficiency is increased when Phe is in the adjacent inverted turn or decreased when acidic residues (Asp or Glu) are present. Further maturation of N-glycans involves sugar additions to the N-glycan core or branching of the GlcNAc residues. In the trans-Golgi, three galactose residues are added and N-acetylneuraminic acid residue is linked to each of the galactose residues (Stanley et al., 2017).
It has been shown that a glycan at the N440 residue of GluN1 affects the conformation of the LBD and increases the EC50 value for glycine by approx. 50% (Sinitskiy et al., 2017). Several types of plant lectins have been shown to bind to NMDARs and affect the functional properties of the receptors. The approximate location of the consensus N-glycosylation sites (N-X-S/T) in the different GluN subunits is shown.

Objectives
Methods
GluN1-A645S/GluN3A receptor glycine EC50 was similar to WT GluN1/GluN3A, but significantly increased for GluN1-M641I/GluN3A receptors. We could not calculate τrepair for GluN1-A645S/GluN2A receptors due to the relatively weak memantine-induced SSI. These data indicate that specific pathogenic mutations in the M3 domain of the GluN1 subunit differentially affect the pharmacological sensitivity of NMDARs in hippocampal neurons.
We found that structural changes in glycine binding sites, such as disruption of the S1 segment of GluN1 and GluN3A subunits, correlate with changes in the surface expression of NMDARs. We observed that the GluN1-S688Y mutation decreases NMDA-induced excitotoxicity in cultured rat hippocampal neurons. In Skrenkova et al., 2020, the author of this paper performed whole-cell patch-clamp recordings from primary hippocampal neurons expressing YFP-hGluN1-1a or YFP-hGluN1-1a-S688Y.
Discussion
- The presence of putative N-glycosylation sites and their interaction with specific
- Three pathogenic mutations in the M3 domain of the GluN1 subunit regulate the
- Effect of glycine-binding site structural features of GluN1 and GluN3 on the surface
- The pathogenic S688Y mutation in the ligand‑binding domain of the GluN1 subunit
Three pathogenic mutations in the M3 domain of the GluN1 subunit regulate the surface delivery and pharmacological sensitivity of NMDARs the surface delivery and pharmacological sensitivity of NMDARs. Our group previously showed that specific amino acid residues in the M3 domain of GluN1 and GluN2 (GluN1-W636, GluN2A-W634, GluN2B-W635) contribute to the regulation of NMDAR trafficking to the cell surface (Kaniakova et al., 2012). However, in our study we did not observe changes in the Mg2+ sensitivity of GluN1/GluN2 receptors with any of the investigated mutations.
Nevertheless, we observed that the GluN1-A645S mutation reduces the sensitivity of GluN1/GluN2 receptors to memantine in the absence of extracellular Mg2+, which is consistent with previous results obtained with Xenopus oocytes expressing GluN1/GluN2B receptors (Kashiwagi et al.), 2002. . Taken together, our data suggest that specific pathogenic mutations in the M3 domain of the GluN1 subunit differentially affect the surface delivery and pharmacological properties of NMDARs. In Skrenkova et al., 2019, we studied the role of the glycine binding sites of GluN1 and GluN3A subunits in the regulation of NMDAR surface expression.
Furthermore, we did not observe differences in the τw of desensitization between mutated and WT GluN1/GluN3A receptors, consistent with the idea that the desensitization of GluN1/GluN3A receptors is regulated by the glycine binding site in the GluN1 subunit. Taken together, our results reveal new information about the role of glycine binding sites in the GluN1 and GluN3A subunits in regulating function and delivery of GluN1/GluN3A receptors. The pathogenic S688Y mutation in the ligand-binding domain of the GluN1 subunit regulates the properties of NMDARs subunit regulates the properties of NMDARs.
In Skrenkova et al., 2020, we investigated how the pathogenic S688Y missense mutation in the S2 GluN1 LBD lobe associated with severe early-onset infantile encephalopathy affects NMDAR function and trafficking. Furthermore, we found that GluN1-S688Y/GluN3A receptors increased the τw of desensitization compared to WT GluN1/GluN3A receptors, which is consistent with previous reports that structural changes in the GluN1 LBD of GluN1 alter the desensitization properties of GluN1/GluN3A receptors (Awobuluyi et al. ., 2007; Kvist et al., 2013). Taken together, our electrophysiological data are consistent with our in-silico modeling and show that the S688Y mutation in the LBD of the GluN1 subunit alters agonist binding.
For example, the pathogenic E413G mutation in the GluN2B subunit has significantly reduced the release of surface NMDARs.
Conclusion
Overall, our results provide additional information about the role of LBD in the regulation of surface delivery and function of NMDARs.
Regulation of the NMDA receptor by its cytoplasmic domains: how the tail wags the dog. Biochemical and electrophysiological characterization of N-glycans on NMDA receptor subunits. residue in the M4 domain of GluN1 subunit regulates the surface delivery of NMDA receptors. GluN3A: an NMDA receptor subunit with excellent properties and functions. 7-Chlorokynureic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex.
Mutational analysis of the glycine binding site of the NMDA receptor: Structural similarity to bacterial amino acid binding proteins. Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. The developmental switch of NMDA receptor composition proceeds independently of GluN2 subunit-specific GluN2 C-terminal sequences.
Alternative splicing of the C-terminal domain regulates cell surface, expression of the NMDA receptor NR1. Post-translational modification of NMDA receptor GluN2B subunit and its roles in chronic pain and memory. The C-terminal domains of the NMDA receptor: how intrinsically disordered tails affect signaling, plasticity, and disease.
Immunolocalization of the NMDA receptor subunit NR3B in selected structures in the rat forebrain, cerebellum, and lumbar spinal cord.
List of publications
Publications in extenso, related to this thesis


