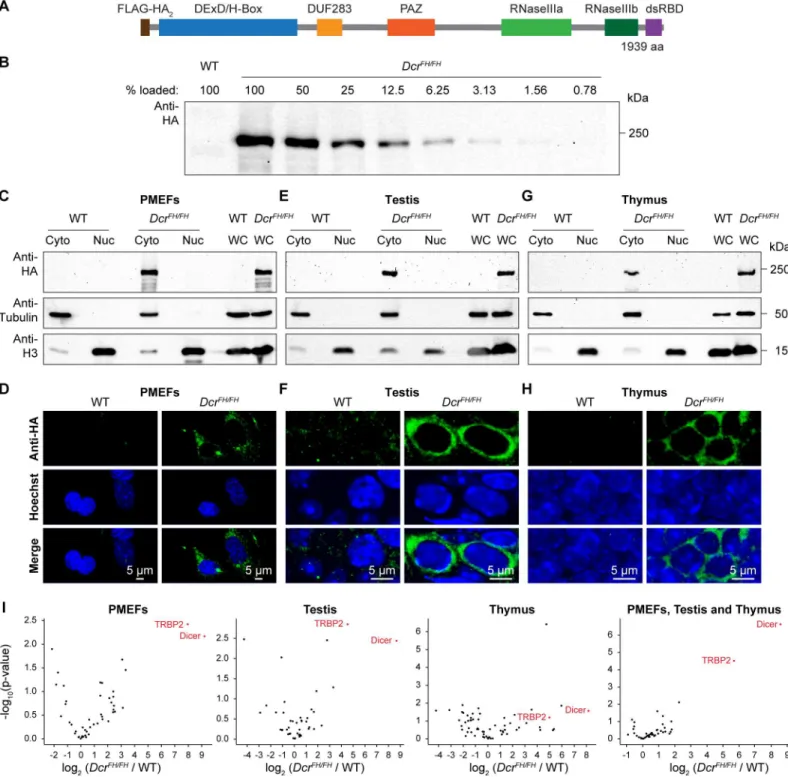
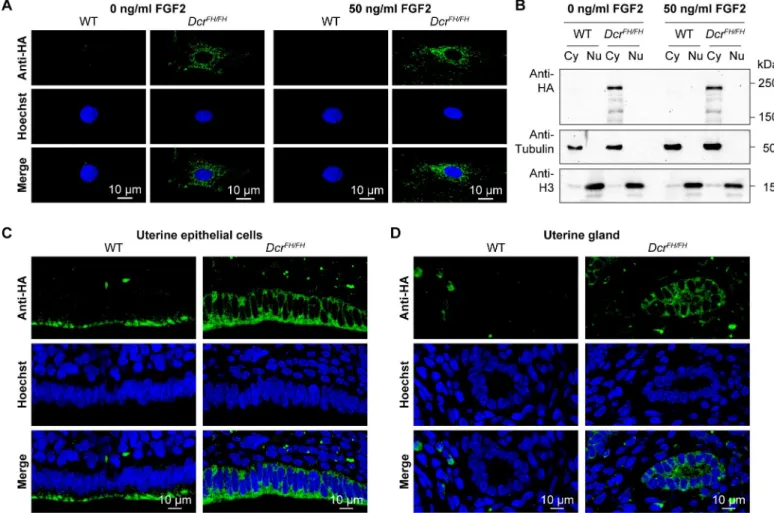
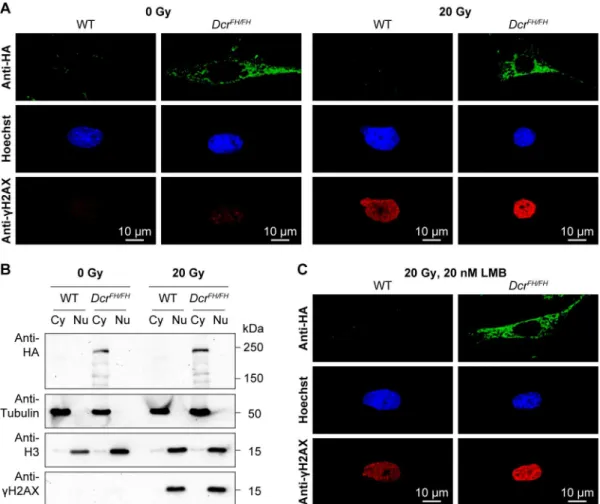
Endogenous Mouse Dicer Is an Exclusively Cytoplasmic Protein.
Texto
Imagem

Documentos relacionados
Resultados: O presente estudo demonstrou que o antagonista do MIF (p425) diminuiu significativamente proteinúria, excreção urinária de GAGs , relação proteína/creatinina na
(A) Dicer-like protein (DCL2, DCL3) processing of transcripts containing inverted repeats (Meister & Tuschl, 2004); (B) Dicer-like protein (DCL2, DCL3) processing of
In the secretory epithelial cell layer, Rab27B expression is concentrated at the luminal side but also a strong cytoplasmic staining is observed.. In the testis Rab27B protein is
Second, we measured their DNMT activity in a rebound DNA methylation assay: DNA methylation was stripped from Cre/ loxP conditionally mutant Dicer1 ES cells using a shRNA
We believe that reprogramming Dicer D / D MEFs was possible only when repro- gramming factors were introduced one day after Cre induction because residual Dicer protein and
Using a previously described mutant mouse that is deficient in ZFP191 protein expression (Zfp191 null ), we demonstrate that key transcripts are reduced in the whole brain as well
We show here that HEN2 is a nucleoplasmic protein that is associated with the Arabidopsis exosome core complex and has a specific role in the exosome-mediated degradation of non-
Here we use a combination of in vitro and in vivo studies to show that maize CENPC has both DNA-binding and RNA-binding capacities, that the DNA/RNA-binding domain is localized to
