FORMULATION AND EVALUATION OF FAST DISSOLVING TABLETS OF CYCLODEXTRIN INCLUSION COMPLEXED WATER INSOLUBLE DRUG: GLIMIPIRIDE
Texto
Imagem

Documentos relacionados
This work aimed to verify the interferences caused by the use of excipients for immediate release tablets based on benznidazole obtained by direct compression and the accomplishment
Different mass transport processes may occur during the drug release from the polymer-based matrix tablets, including water imbibition into the system, polymer
The susceptibility of the matrix tablets to the enzymatic action of colonic bacteria was assessed by continuing the drug release studies in simulated colonic luids prepared using
ranitidine tablets of a reference drug (product A) and a generic (product B) and a similar (product C) drug marketed in Bahia, Brazil using a simple, fast and inexpensive
Taking all of this into account, the aim of the present study was to prepare tablets by direct compression of sodium alendronate-loaded Eudragit ® S100 microparticles using
Tablets containing diferent concentrations of Carbopol (CP), hydroxypropyl methylcellulose (HPMC), or ethyl cellulose (EC) were prepared using direct compression and the drug
The prepared tablets were evaluated for hardness, friability, disintegration time and in vitro drug release.. Furthermore, the interaction of clopidogrel with the
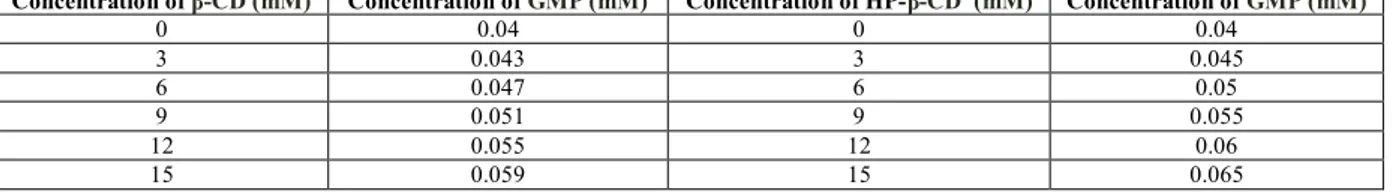
Water solubility and drug dissolution rates were signiicantly increased after the formation of inclusion complexes with the cyclodextrins evaluated in relation to the physical