Keywords
Reference values; walking; exercise.
Mailing address: Victor Zuniga Dourado •
Av. Ana Costa, 95 - 11060-001 - Santos, São Paulo, Brazil E-mail: vzdourado@yahoo.com.br, victor.dourado@unifesp.br Manuscript received October 27, 2009; revised manuscript received February 11, 2010; accepted May 31, 2010.
Victor Zuniga Dourado
Departamento de Ciências da Saúde, Universidade Federal de São Paulo (UNIFESP), Campus Baixada Santista, Santos, São Paulo - Brazil
Abstract
The six-minute walk test (6MWT) has been broadly used in clinical settings. Several reference equations for prediction of the total distance walked during the test (6MWD) are available in literature. The present review aimed to critically discuss studies, published in Portuguese and English (LILACS, SCIELO, MEDLINE, PUBMED), which evaluated normal values and created reference equations for predicting 6MWD in healthy subjects, comparing them with the results that were recently obtained in Brazilian individuals. Age, sex, weight, height and body mass index were the main demographic and anthropometric features more often correlated with 6MWD. The equations derived from these characteristics were able to explain between 25 and 66% of the total variability in the 6MWD. Unfortunately, the foreign equations were not applicable to the Brazilian population. Even when the 6MWT was performed following strict standardization, the difference in 6MWT performance between foreign and Brazilian individuals remains, indicating the necessity of providing specific reference equations for each population and/or ethnic group. Accordingly, these equations developed in Brazil are probably the most appropriate for interpreting 6MWT performance in Brazilian patients with chronic diseases affecting the exercise capacity. Future studies are necessary with larger sample sizes (e.g. multicentric ones) and randomized design for the reference values of the 6MWD to be considered reliable.
Introduction
The use of field walk tests in patients with cardiorespiratory diseases results from the adaptation of the 12-minute run fitness test developed by Cooper1.
This test was developed with the objective of verifying the level of physical fitness of the USA Armed Forces personnel. In its original format, the test consisted in running the longest possible distance in 12 minutes. In the 70s, McGavin et al2 changed Cooper’s run fitness test into a 12-minute walk
test, with the objective of evaluating the exercise tolerance of patients with chronic bronchitis. The 12-minute walk test was adapted to shorter distances (e.g. two and six minutes), mainly because it was strenuous for patients3. On the other hand, the 2-minute test presented limited responsiveness, especially in less debilitated patients4. In this sense, the six-minute walk test (6MWT) became the most popular among the test with controlled duration and it consists in walking as fast as possible for 6 minutes.
The 6MWT was originally developed to evaluate the functional capacity, monitor the effectiveness of several treatments and establish the prognosis of patients with cardiorespiratory diseases5. Patients with such dysfunctions presented exercise intolerance due to malfunctioning respiratory and/or cardiovascular systems and peripheral and respiratory skeletal muscle dysfunction6. However, more recently, the test has been validated in several populations, including patients with fibromyalgia, cerebrovascular accident, amputations, morbid obesity, Down’s syndrome, Alzheimer’s disease and cerebral palsy, among others5,7-12.
In patients with cardiorespiratory diseases, oxygen consumption at the 6MWT does not significantly differ from the maximum oxygen consumption (VO2max) obtained at incremental laboratory tests performed in a cycle ergometer13. Therefore, it is possible to adequately estimate the VO2max by the distance walked at the 6-minute walk test (6MWD). That makes the 6MWT a simple and less expensive tool to assess the cardiorespiratory fitness5. The 6MWT is well-tolerated by patients and it is more representative of the activities of daily living (ADL) in comparison to other walk tests14.
Several demographic, anthropometric and physiological factors can influence the 6MWD in healthy individuals and in patients with chronic diseases. Shorter individuals and women present a shorter step length and, consequently, a shorter 6MWD. Elderly and obese individuals commonly present reduced lean body mass and, consequently, a shorter 6MWD. Demotivated individuals, those presenting cognitive deficit, arthritis and other musculoskeletal disorders also presented reduction in the 6MWD15,16. Muscle strength, symptoms of depression, health-related quality of life impairment, medication use, systemic inflammation and pulmonary function alterations are other factors that can influence the test performance16-18.
the walking velocity during the 6MWT is self-controlled, the 6MWD is extremely variable in healthy individuals5. In fact, the regression equations published in the literature for the 6MWT present great variability in their results. That is probably due to the differences between the assessment protocols, as well as the population-related differences20,21. Although infrequently used in healthy populations22, there are reference values for the 6MWD when applied to children and adolescents23-27, adult individuals28-31 and healthy elderly individuals16, 20, 21, 28, 32-35. Some of these values were obtained before the publication of the consensus of the American Thoracic Society (ATS) for the performance of the 6MWT5, which justifies, in part, the great variability of results.
Among the most popular equations are the ones developed by Enright and Sherrill20 and by Troosters and cols21. These were developed before the publication of the ATS consensus5 and offer considerably different results. In fact, we recently observed that the foreign equations were not adequate for a Brazilian population sample31. Similar results have been described in other populations (e.g., Australians, Arabs, Tunisians and Singaporeans)30,32,33,35. The explanation for the different performances observed among different ethnicities is not a simple one. The level of encouragement, the length and layout of the track and the number of tests performed for familiarization purposes significantly influence the 6MWD. Even when these variables are stringently controlled, the individuals’ motivation during the test has an important contribution, as some studies differ significantly regarding the intensity at which the individuals perform the test, varying from 44.0-81.0% of the estimated maximum heart rate.
Additionally, the demographic, anthropometric and nutritional differences observed among the different ethnicities evaluated must be considered. Greater height and higher amount of lean mass observed in Caucasians have a significant impact on the 6MWD. Therefore, the ATS encourages the scientific community to use the standardization of the 6MWT suggested in its consensus and to develop reference values of the 6MWD for several ethnicities.
The erroneous choice of a reference equation can result in potential mistakes related to the interpretation of the level of physical fitness and the improvement of the 6MWD after interventions in patients with chronic diseases. Therefore, the deeper knowledge of the conditions under which reference equation was obtained is necessary. Hence, the objective of this literature review was to critically discuss the published studies (LILACS, SCIELO, MEDLINE and PUBMED databases) that evaluated the normal values and created reference equations to predict the 6MWD in healthy individuals, comparing them to those obtained in Brazilian individuals23,31.
General characteristics of the individuals
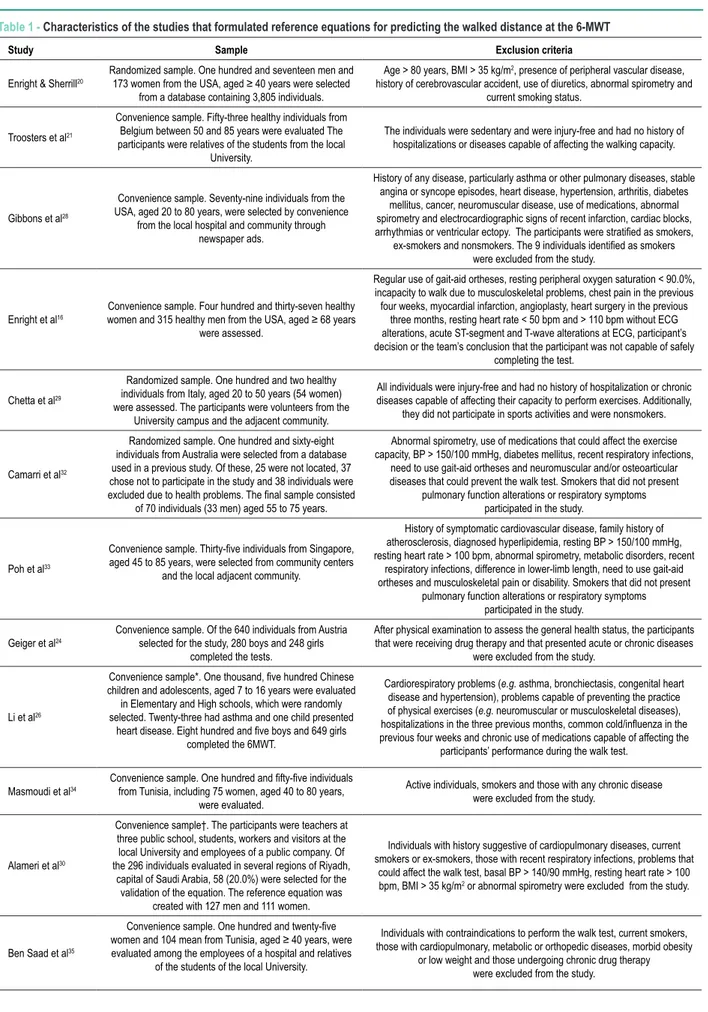
To the best of our knowledge, there have been 19 studies that evaluated the normal values of the 6MWD. Of them, 15 studies evaluated the determinant attributes of the 6MWD through multiple regression and formulated some reference equation (Table 1). Other 4 studies evaluated a reasonable number of individuals, but did not formulate a reference equation25, 36-38.
Steffen et al36, Pires et al38, Roush et al37 and Lammers et al25 evaluated the influence of sex, age and body mass index (BMI) on the 6MWD, respectively in individuals aged 61 to 89 years36, 18 to 80 years38, 7 to 9 years37 and 4 to 11 years25. Of the studies that formulated reference equations, 4 were carried out with children and adolescents23,24,26,27, of which one involved Austrian children and adolescents24, one evaluated Tunisian children and adolescents27, one evaluated Chinese children and adolescents26 and another evaluated Brazilian individuals aged 6 to 12 years23.
Two studies formulated reference equations for Italian29 and Arab adult individuals30. Most studies evaluated
individuals aged ≥ 40 years. These studies were carried out
with individuals from the USA16,20, Belgium21, Singapore33, Australia32 and Tunisia34,35. Two studies evaluated individuals across a wide age range28,31, of which one study involved individuals from the USA28 and another, individuals from two Brazilian towns (Santos and Botucatu, both in the state of Sao Paulo)31.
The several studies published to date have been quite heterogeneous. Some differences in the 6MWD among the studies are due to the test format (i.e., level of encouragement, length and layout of the track, number of test performed for familiarization purposes, etc). However, other population and clinical characteristics must be discussed.
Most of the studies involving adult and elderly participants presented mean BMI values that were representative of overweight. Few studies used BMI > 35 kg/m2 as an exclusion criterion20,21,30. Obesity increases the workload for a given exercise intensity, decreasing the 6MWD. Individuals with BMI > 30 kg/m2 walked approximately 85.0% of the 6MWD6 walked by eutrophic individuals in the study by Enright et al16. Although the BMI is a useful clinical index of obesity, it cannot be considered as the best index to determine the amount of body fat. Additionally, the correlation between body mass and 6MWD is usually more consistent than the correlation between the 6MWD and BMI16,20.
Individuals with other cardiovascular risk factors, such those with as arterial hypertension and smoker, were also included in some studies30-33. Individuals with BP controlled by medication and/or BP < 150/100 mmHg and smokers with no alterations in pulmonary function or respiratory symptoms were included in these studies30-33. Arterial hypertension and smoking have a negative impact on the 6MWD16. Participants that presented cardiovascular risk factors walked significantly less at the 6MWT when compared to individuals without risk factors16,20. The use of medications for cardiovascular diseases can also have a negative influence on the 6MWD39.
Table 1 - Characteristics of the studies that formulated reference equations for predicting the walked distance at the 6-MWT
Study Sample Exclusion criteria
Enright & Sherrill20
Randomized sample. One hundred and seventeen men and
173 women from the USA, aged ≥ 40 years were selected from a database containing 3,805 individuals.
Age > 80 years, BMI > 35 kg/m2, presence of peripheral vascular disease,
history of cerebrovascular accident, use of diuretics, abnormal spirometry and current smoking status.
Troosters et al21
Convenience sample. Fifty-three healthy individuals from Belgium between 50 and 85 years were evaluated The participants were relatives of the students from the local
University.
The individuals were sedentary and were injury-free and had no history of hospitalizations or diseases capable of affecting the walking capacity.
Gibbons et al28
Convenience sample. Seventy-nine individuals from the USA, aged 20 to 80 years, were selected by convenience
from the local hospital and community through
newspaper ads.
History of any disease, particularly asthma or other pulmonary diseases, stable angina or syncope episodes, heart disease, hypertension, arthritis, diabetes
mellitus, cancer, neuromuscular disease, use of medications, abnormal spirometry and electrocardiographic signs of recent infarction, cardiac blocks, arrhythmias or ventricular ectopy. The participants were stratiied as smokers,
ex-smokers and nonsmokers. The 9 individuals identiied as smokers were excluded from the study.
Enright et al16
Convenience sample. Four hundred and thirty-seven healthy women and 315 healthy men from the USA, aged ≥ 68 years
were assessed.
Regular use of gait-aid ortheses, resting peripheral oxygen saturation < 90.0%, incapacity to walk due to musculoskeletal problems, chest pain in the previous four weeks, myocardial infarction, angioplasty, heart surgery in the previous
three months, resting heart rate < 50 bpm and > 110 bpm without ECG alterations, acute ST-segment and T-wave alterations at ECG, participant’s decision or the team’s conclusion that the participant was not capable of safely
completing the test.
Chetta et al29
Randomized sample. One hundred and two healthy individuals from Italy, aged 20 to 50 years (54 women) were assessed. The participants were volunteers from the
University campus and the adjacent community.
All individuals were injury-free and had no history of hospitalization or chronic diseases capable of affecting their capacity to perform exercises. Additionally,
they did not participate in sports activities and were nonsmokers.
Camarri et al32
Randomized sample. One hundred and sixty-eight individuals from Australia were selected from a database used in a previous study. Of these, 25 were not located, 37 chose not to participate in the study and 38 individuals were excluded due to health problems. The inal sample consisted
of 70 individuals (33 men) aged 55 to 75 years.
Abnormal spirometry, use of medications that could affect the exercise capacity, BP > 150/100 mmHg, diabetes mellitus, recent respiratory infections,
need to use gait-aid ortheses and neuromuscular and/or osteoarticular diseases that could prevent the walk test. Smokers that did not present
pulmonary function alterations or respiratory symptoms participated in the study.
Poh et al33
Convenience sample. Thirty-ive individuals from Singapore, aged 45 to 85 years, were selected from community centers
and the local adjacent community.
History of symptomatic cardiovascular disease, family history of atherosclerosis, diagnosed hyperlipidemia, resting BP > 150/100 mmHg, resting heart rate > 100 bpm, abnormal spirometry, metabolic disorders, recent
respiratory infections, difference in lower-limb length, need to use gait-aid ortheses and musculoskeletal pain or disability. Smokers that did not present
pulmonary function alterations or respiratory symptoms participated in the study.
Geiger et al24 Convenience sample. Of the 640 individuals from Austria selected for the study, 280 boys and 248 girls
completed the tests.
After physical examination to assess the general health status, the participants that were receiving drug therapy and that presented acute or chronic diseases
were excluded from the study.
Li et al26
Convenience sample*. One thousand, ive hundred Chinese children and adolescents, aged 7 to 16 years were evaluated in Elementary and High schools, which were randomly selected. Twenty-three had asthma and one child presented
heart disease. Eight hundred and ive boys and 649 girls completed the 6MWT.
Cardiorespiratory problems (e.g. asthma, bronchiectasis, congenital heart
disease and hypertension), problems capable of preventing the practice of physical exercises (e.g. neuromuscular or musculoskeletal diseases),
hospitalizations in the three previous months, common cold/inluenza in the previous four weeks and chronic use of medications capable of affecting the
participants’ performance during the walk test.
Masmoudi et al34 Convenience sample. One hundred and ifty-ive individuals from Tunisia, including 75 women, aged 40 to 80 years, were evaluated.
Active individuals, smokers and those with any chronic disease were excluded from the study.
Alameri et al30
Convenience sample†. The participants were teachers at three public school, students, workers and visitors at the local University and employees of a public company. Of the 296 individuals evaluated in several regions of Riyadh,
capital of Saudi Arabia, 58 (20.0%) were selected for the validation of the equation. The reference equation was
created with 127 men and 111 women.
Individuals with history suggestive of cardiopulmonary diseases, current smokers or ex-smokers, those with recent respiratory infections, problems that
could affect the walk test, basal BP > 140/90 mmHg, resting heart rate > 100 bpm, BMI > 35 kg/m2 or abnormal spirometry were excluded from the study.
Ben Saad et al35
Convenience sample. One hundred and twenty-ive women and 104 mean from Tunisia, aged ≥ 40 years, were evaluated among the employees of a hospital and relatives
of the students of the local University.
Individuals with contraindications to perform the walk test, current smokers, those with cardiopulmonary, metabolic or orthopedic diseases, morbid obesity
Continuation of Table 1 - Characteristics of the studies that formulated reference equations for predicting the walked distance at the 6-MWT
Ben Saad et al27 Convenience sample. Two hundred individuals from Tunisia
(100 males), aged 6 to 16 years, were evaluated.
Resting heart rate ≥ 120 bpm, hypertension, smoking, symptoms or diagnosis of cardiopulmonary, metabolic or orthopedic diseases, previous abdominal or thoracic surgeries, mental disease, incapacity to walk and altered pulmonary
function.
Iwama et al31
Convenience sample. One hundred and thirty-four individuals from Brazil, aged ≥ 13 years, were evaluated. The participants were selected among the students of a local
University and employees of the hospital where the study was carried out, as well as individuals from the adjacent
community.
Abnormal spirometry, diagnosis of cardiovascular and/or pulmonary diseases, any disorder capable of interfering with the capacity of walking (e.g. cognitive
deicit, neuromuscular, metabolic or musculoskeletal diseases, or the necessity to use a gait-aid device) and use of medications for chronic diseases. However, individuals with controlled blood pressure, as well as smokers with
no pulmonary function alterations or respiratory symptoms were included in the study.
Priesnitz et al23
Convenience sample. One hundred and eighty-eight children from Brazil (92 boys and 96 girls), aged 6 to 12 years, were
evaluated among schoolchildren from three local Elementary schools.
Neonatal history of respiratory diseases, chronic cough or wheezing in the previous 12 months, history of asthma medication in the previous 12 months, respiratory symptoms in the previous three weeks, gait limitations or any other
disorder that the researchers deemed signiicant.
* - with randomization of the evaluation sites; † = with randomization of the sample (80.0% of the individuals) in order to create the reference equation.
are known to depend on factors such as: the perception and understanding of the questionnaire, individual characteristics (e.g., age, cultural status, schooling and cognition) and the type of calculation used to estimate the daily energy expenditure41. Although some authors stated that their studies have been carried out in sedentary individuals29,34, the questionnaires have limited capacity to define a sedentary life style in all its domains (e.g., amount and intensity of daily physical activity)41. In fact, our experience with Brazilian participants demonstrated that the correlation between the level of customary physical activity evaluated through Baecke questionnaire42 and the 6MWD, although significant, was weak (r = 0.25; p < 0.01)31. Further studies using movement sensors are necessary for an actually sedentary sample to be analyzed.
In Brazil, the prevalence of overweight was 10.8% among children, 9.9% in adolescents and 28.3% among adults and the prevalence of obesity was 7.3%, 1.8% and 9.7%, respectively. The joint prevalence of overweight and obesity in the Brazilian population is higher in the female sex, with more than 50% of women in the Northeast and Southeast regions of Brazil aged 50 to 69 years presenting overweight and/or obesity43.
Body mass excess is a predisposing factor for hypertension and it can be accountable for 20.0% to 30.0% of cases of arterial hypertension.
Population-based investigations carried out in some cities of Brazil have shown a prevalence of arterial hypertension
(BP ≥ 140/90 mmHg) of 22.3% to 43.9%44. As for smoking,
according to data published in 2004, one-third of the Brazilian adult population smoked, a total of 16.7 million men and 11.2 million women smokers. A prevalence of smoking of 8.9% to 12.1% has been demonstrated among young individuals aged 10 to 19 years45. The prevalence of a sedentary lifestyle is variable and depends on the methodology used to define it and also on the studied sample. A systematic literature review showed that the prevalence of sedentary lifestyle in Brazil is between 26.7% and 78.2%. When only the physical activity carried out during leisure time is considered, this prevalence varies between 55.3% and 96.7%46.
If, on the one hand, the inclusion of participants with the aforementioned cardiovascular risk factors makes the
sample’s healthy profile questionable, on the other hand, the inclusion of individuals with such risk factors make the samples more representative, due to their high prevalence. Additionally, among several demographic, anthropometric, clinical and physiological variables, BMI, hypertension, smoking status and level of customary physical activity were not chosen as determinants of the 6MWD, after the multiple regression analysis16,31.
Other population and clinical characteristics must be considered. The 6MWD was 132-m and 30-m longer in individuals with higher level of schooling and better socioeconomic status, respectively35. After adjusted for age, sex, weight and height, among other factors, the Afro-American individuals from the USA presented a worse performance at the 6MWT, when compared to their Caucasian fellow countrymen and women. Investigators in Japan47, on the other hand, observed that the 6MWD walked by Japanese individuals was similar to the one reported for Caucasian individuals20,21. In this sense, it is logical to speculate that a worse socioeconomic status, lower level of schooling and the degree of miscegenation, commonly found in the Brazilian population, can explain in part the inadequacy of the foreign equations when applied to our population.
Recently, Ben Saad et al35 observed that women that had a higher number of deliveries (7 ± 1) presented a shorter 6MWD, when compared to women that had a lower number of deliveries (3 ± 2)35. Symptoms of depression and lower cognition have also been identified as factors that can negatively influence the 6MWD16. The cognitive-behavioral aspects have not been adequately appraised in most studies that formulated reference equations for the 6MWT.
Considering that Brazil is a country with continental dimensions, it is recommended that clinicians and/or researchers evaluate the usefulness of such equations in other regions of the country.
Associated features
Most studies emphasized the correlations between the 6MWD and age, sex, height, weight and BMI (Table 2). These features were prioritized due to the fact that they are very easy to obtain. In this sense, the reference equations can be applied without the need to use sophisticated equipment. For a better interpretation of the correlations described in the literature, we will use the classification proposed by Lacasse et al48. In brief, coefficients of correlation between 0-0.20 are considered non-significant; those between 0.21-0.35 are weak; those between 0.36-0.50 are moderate and those > 0.50 represent strong correlations.
Age had a significant influence on the 6MWD in several studies. In studies involving healthy adult and elderly individuals, the correlation was a negative one20,21. In studies involving children and adolescents, the correlation was a positive one23,25-27. The correlation between age and 6MWD was not significant only in the study by Camarri et al32, probably due to the narrow age range assessed by the study (55-75 years) and the small sample size (n = 70). In some studies, the correlation was strong21,23,25,27,28 whereas, in others, it was moderate26,29-31,33,35. In some studies, the correlation was significant; however, the authors did not provide the values of the correlation coefficients20,24. The shorter distance walked as age increased can be explained by decreases in muscle mass and strength and the maximum oxygen consumption, inherent
to the aging process49,50. On the other hand, the positive correlation between the 6MWD and age < 20 years is the result of the higher degree of maturation among adolescents, when compared to children.
The correlation observed between the 6MWD and height has been equally consistent. Some studies showed strong correlations21,25,27,32,35, whereas others showed moderate ones23,26,28-31,33. One study reported significant correlations; however, the authors did not provide the values of the correlation coefficients24. Similarly, some authors reported significant correlations between leg length and the 6MWD24,32. Only the study by Enright and Sherrill20 did not show a significant correlation between the 6MWD and height. The consistent correlation between height and the distance walked at the test can be attributed to the longer length of steps in taller individuals. The length of the step is one of the main determinants in gait velocity51.
The correlation between the 6MWD and body weight, on the other hand, showed to be little consistent in many studies20,21,23,28,31,33. Gibbons et al28 observed that age and sex were the determinant features of the 6MWD. In the study by Poh et al33, body weight was not selected as a determinant of the 6MWD. In a Brazilian study31, we observed that age and sex were the determinant features of the 6MWD. Additionally, we found a weak correlation between the 6MWD and BMI (r = -0.27)31. Lammers e al25 observed that age, weight and height were determinants of the 6MWD in children, together explaining 44.0% of the total variability of this variable. Age alone explained 41.0% of these 44.0%25. The authors also observed that the correlation between the 6MWD and body weight presented a linear characteristic only up to 30 kg25. From this weight up, the correlation presented a horizontal behavior. In elderly individuals, such correlation was not linear either, with a point of inflection at approximately 82 kg, after which the 6MWD started to suffer a negative influence of body weight16. When significant, the correlation between the 6MWD and weight is usually a weak or moderate one (r = 0.25)32.
The results described before suggest the little influence of weight on the 6MWD. However, it is more probable that this correlation had not been well-detected in linear regressions due to its curvilinear characteristic.
The influence of sex on 6MWD is a consistent one and has been described in several studies. Among Brazilian adult and elderly individuals, the men walked on average 61.5 m more than the women31. Similar results have broadly presented in the literature (Table 3). On the other hand, some studies in children did not show an influence of sex on the walked distance23,25,27. Such result might be easily explained by the higher number of musculoskeletal similarities observed between the sexes before adolescence.
The influence of sex after the onset of adolescent can be attributed to the higher muscle mass and strength observed in men in absolute values. When the influence of sex is evaluated taking height into account, some reference equations predict similar 6MWD between men and women. For instance, Enright et al16 observed, among asymptomatic elderly individuals, that the 6MWD of elderly men and women were not significantly different when corrected by height. The correlation between Table 2 - Examples of correlations between the distance walked at
6-minute walk test and the main demographic and anthropometric features
Study (years)Age Height(cm) Weight(kg) (kg/mBMI2)
Enright & Sherrill20 * NS * NS
Troosters et al21 r = -0.51 r = 0.54 NS NS
Gibbons et al28 r = -0.50 r = 0.35 NS r = -0.27
Chetta et al29 r = -0.42 r = 0.46 NS NS
Camarri et al32 NS r = 0.56 r = 0.25 NS
Poh et al33 r = -0.36 r = 0.35 NS NS
Geiger et al24 * * * *
Li et al26 r = 0.32 r = 0.32 r = 0.15 r = 0.11
Lammers et al25 r = 0.51 r = 0.65 NS *
Alameri et al30 r = -0.42 r = 0.48 r = 0.21 NS
Ben Saad et al35 r = -0.37 r = 0.70 r = 0.38 r = 0.18
Ben Saad et al27 r = 0.70 r = 0.74 r = 0.69 r = 0.21
Iwama et al31 r = -0.39 r = 0.44 NS r = -0.24
Priesnitz et al23 r = 0.51 r = 0.49 r = 0.29 NS
the 6MWD and height is not a linear one25. Until nonlinear methods are applied to compare the sex-related differences in the 6MWD, it is not possible to affirm that men and women present similar performances at the test.
Reference equations
There are reference equations for predicting the 6MWD in several populations (Table 4). With the exception of 2 studies that were carried out with a randomized sample20,32 and two
other large ones16,26, the remainder vary regarding the number of individuals and analyzed age range; however, they present similar designs. In most studies, the reference equations were obtained by using linear multiple regression models, including demographic and anthropometric features as independent variables. Other variables, such as spirometric indices and heart rate response, were also selected as determinants in some studies26,32,33 (Table 4). In our opinion, these last variables can be considered a problem. It is likely that patients with ventilatory limitations do not present the same association between the forced expiratory volume in the first second and the 6MWD observed in healthy individuals. Another problem is the use of indices that are obtained at the end of the test (e.g. the absolute alteration in heart rate or the percentage of maximum heart rate26,33), considering that patients with cardiopulmonary diseases present abnormal physiological responses during the test. Therefore, the use of such indices can overestimate the exercise capacity of patients.
Peripheral muscle strength has also been identified as a determinant of the 6MWD16. Unfortunately, this index needs specific equipment and careful calibration so that it can be obtained. In this sense, the equations involving only demographic and anthropometric features, such as age, sex, weight and height, seem to be more useful in the clinical setting.
Regrettably, the equations developed in foreign populations were not adequate for Brazilian individuals31. We observed that the equations developed by Troosters et al21, Chetta et al29, Gibbons et al28 and Camarri et al32 overestimated the 6MWD observed in healthy Brazilian individuals (Table 5). The cause for such difference is multifactorial. Both the standardization Table 3 - Distance walked at the 6-Minute Walk test (6MWD) by men
and women in some studies
6MWD (m)
Study Men Women Signiicant Difference
Enright & Sherrill20 576 (399 – 778)* 494 (310 – 664)* Yes
Gibbons et al28 735 ± 98 659 ± 76 Yes
Chetta et al29 638 ± 44 593 ± 57 Yes
Camarri et al32 685 ± 49 628 ± 59 Yes
Poh et al33 586 ± 126 538 ± 82 No
Li et al26 680 ± 65 642 ± 58 Yes
Alameri et al30 429 ± 47 386 ± 45 Yes
Ben Saad et al35 711 ± 81 551 ± 75 Yes
Iwama et al31 622 ± 80 551 ± 71 Yes
* - median (variance); 6MWD - distance walked at the 6-minute walk test.
Table 4 - Reference equations for predicting the distance walked at the 6MWT in healthy foreign individuals
Study Equations
Enright & Sherrill20 ♂: 6MWDm = (7.57 x heightcm) – (5.02 x ageyears) – (1.76 x weightkg) – 309; r2 = 0.42
♀: 6MWDm = (2.11 x heightcm) – (2.29 x weightkg) – (5.78 x ageyears) + 667; r2 = 0.38
Troosters et al21 Both: 6MWD
m = 218 + (5.14 x heightcm – 5.32 x ageyears) – (1.80 x weightkg) + (51.31 x sexmen = 1; women = 0); r2 = 0.66
Gibbons et al28 Both: 6MWDm = 868.8 – (age
years x 2.99) – (sexmen = 0; women = 1 x 74.7); r2 = 0.41
Enright et al16 ♂: 6MWDm = 539 + (6.1 x heightcm) – (0.46 x weightkg) – (5.8 x ageyears); r 2 = 0.20 ♀: 6MWDm = 493 + (2.2 x heightcm) – (0.93 x weightkg) – (5.3 x ageyears); r2 = 0.20
Chetta et al29 Both: 6MWD
m = 518.853 + (1.25 x heightcm) – (2.816 x ageyears) – (39.07 x sexmen = 0; women = 1); r2 = 0.42
Camarri et al32
Both: 6MWDm = 64.69 + (3.12 x heightcm) + (23.29 x VEF1L); r2 = 0.43
Alternative equation. both: 6MWDm = 216.90 + (4.12 x heightcm) – (1.75 x ageyears) – (1.15 x weightkg) – (34.04 x sex
men = 0; women = 1); r2 = 0.36
Poh et al33 Both: 6MWD
m = (5.50 x %HRmax) + (6.94 x heightcm) – (4.49 x ageyears) – (3.51 x weightkg) – 473.27; r2 = 0.78
Geiger et al24 ♂: 6MWDm = 196.78 + (39.81 x ageyears) – (1.36 x age2) + (132.28 x heightcm); r2 = 0.49
♀: 6MWDm = 188.61 + (51.50 x ageyears) – (1.86 x age2) + (86.10 x heightcm); r2 = 0.50
Li et al26 ♂: 6MWDm = 554.16 + (absolute difference in HR x 1.76) + (1.23 x heightcm); r 2 = 0.43
♀: 6MWDm = 526.79 + (absolute difference in HR x 1.66) + (0.66 x heightcm); r2 = 0.37
Masmoudi et al34 Both: 6MWD
m = 299.8 – (4.34 x ageyears) + (342.6 x heightm) – (1.46 x weightkg) + (62.5 x sex men = 1; women = 0); r2 = 0.60
Alameri et al30 Both: 6MWDm = (2.81 x height
cm) + (0.79 x ageyears) – 28.5; r2 = 0.25
Ben Saad et al35 Both: 6MWD
m = 720.50 – (160 x sex men = 0; women = 1) – (5.14 x ageyears) – (2.23 x weightkg) + 2.72 x heightcm); r 2 = 0.77
Ben Saad et al27 Both: 6MWD
m = (4.63 x heightcm) – (3.53 x weightkg) + (10.42 x ageyears) + 56.32 ; r2 = 0.60
♂ - male sex; ♀ - female sex; Both - both sexes; 6MWD - distance walked at the 6-Minute Walk Test; FEV1 - Forced expiratory volume in one second; HR - heart rate;
used in the test (Table 6) and the ethnic-population and clinical differences must be taken into account.
Among the studies, the track length varied from 20 to 50 m, the number of tests used for familiarization purposes varied between one and four21,22,28,32 and, in some studies, the level of encouragement did not follow the recommendations of the ATS5, especially those published before 200220-22,32. On the other hand, we observed that the equation developed by Enright & Sherrill20 underestimated the 6MWD in Brazilian individuals, probably because the authors carried out a single 6MWT (i.e., with no familiarization tests) (Table 6). Chetta et al29 followed the recommendations by the ATS and developed an equation that slightly overestimated (32 ± 71 m) our results in healthy Brazilian individuals31. In this case, the difference is likely due to population differences, which have been previously discussed.
Such difference, even when the 6MWT is performed under strict standardization, emphasizes the need t evaluate the reference values that are specific for each population and/or ethnicity. The population and ethnic profile of our study was quite broad31, which can be attributed to the great internal and external migration, characteristic of the southeast region of Brazil. There are some data in the literature that suggest an influence of ethnicity on the physical fitness test performance52-54. Little is known about the physiological mechanisms of this difference. Predictive studies in populations from the USA and Europe found lower values among minorities, such as African-descendant and Latin populations, although the socioeconomic and nutritional status is a confounding factor in this case55.
The shorter 6MWD observed in the Brazilian population can be attributed, in part, to the multiracial profile of the urban population in our country, and therefore, lower values than those observed in populations that are predominantly Caucasian would be expected. Another noteworthy aspect is the anthropometric characteristic of each population. We observed that a sample of the Brazilian population presented lower height and higher weight (for the women) in comparison to those described for Italian individuals in the study by Chetta et al29. It is possible that the 6MWD also presents diverse values in different regions of Brazil. Tavares et al56 observed significant differences in the anthropometric and nutritional profiles among different regions of Brazil.
Finally, one must consider the level of physical exertion presented by the volunteers at the several studies. The heart
rate attained at the end of the 6MWT varied substantially between moderate and intense levels20,32, suggesting that the exertion was submaximal in some studies, in spite of the initial instruction emphasizing the walking of the longest possible distance within 6 minutes under standardized verbal incentive.
To our knowledge, two studies formulated reference equations to predict the 6MWD in the Brazilian population23,31. Iwama et al31 evaluated 134 healthy Brazilian individuals aged
≥ 13 years (73 women). The mean 6MWD was significantly
higher in men (622 ± 80 vs. 551 ± 71 m, p < 0.05). The 6MWD was significantly correlated (p < 0.05) with age, height, BMI and level of customary physical activity42 (Table 2). Age and sex were selected as determinant variables, together explaining 30.0% of the total variability of the 6MWD. The predictive power described in the study by Iwama et al31 for Brazilian individuals was similar to that described in the literature, using demographic and anthropometric features (r2 = 0.20 to 0.77)16,27.
Eighty-five individuals with characteristics that were similar to those of the initial sample were prospectively evaluated in another Brazilian research center, following the recommendations by the ATS5. The difference between the evaluated 6MWD and the one estimated by the developed equation was not statistically significant (-3 ± 68 m; p = 0.938)31. The assessed 6MWD represented 99.6 ± 11.9% of the predicted values31. Some authors evaluated the applicability of foreign equations and observed very similar results32,33,35. Priesnitz et al23 evaluated 188 healthy children and adolescents aged 6 to 12 years (92 boys), selected by convenience in three Elementary schools in the city of Porto Alegre, state of Rio Grande do Sul, Brazil. Age, height, absolute difference in heart rate before and after the 6MWT (r = 0.30; p < 0.0001) and weight (Table 2) were the features that were significantly correlated with the 6MWD. Such features explained 36.6% of the 6MWD variability. Sex and BMI, however, were not selected as determinants of the 6MWD after multiple regression analysis.
Table 7 presents the reference equations developed in the Brazilian population for children and adolescents23 and for adult and elderly individuals31. These equations can facilitate the interpretation of the exercise capacity of Brazilian patients. The validity of such equations in our population should be investigated in future studies.
Few studies have evaluated the validity of the reference equations that they proposed21,30,31,35. In addition to Table 5 - Comparisons between the walked distance and the distance estimated through foreign equations for the 6MWT in healthy Brazilian individuals aged 13 and 84 years
Used Equations Walked 6MWD (m) Estimated 6MWD (m) Estimated – walked (m) % Predicted
Gibbons et al28 657 (512 – 645) 716 (665 – 773)* 137 ± 74 80 ± 10
Chetta et al29 581 (526 – 648) 624 (594 – 657)* 32 ± 71 94 ± 11
Enright & Sherril20 543 ± 71 506 ± 75* -36 ± 86 109 (95 – 116)
Troosters et al21 534 (482 – 621) 600 (570 – 663)* 71 ± 76 88 (81 – 93)
Camarri et al32 536 (480 – 630) 653 (634 – 702)* 115 ± 67 82 ± 10
Data obtained from the study by Iwama et al31; Data presented as means ± standard-deviation or medians (variance); 6MWD - distance walked at the 6-Minute Walk
Table 6 - Standardization of the 6-Minute Walk Test used in some studies involving healthy individuals
Study Track N. of tests Encouragement Interval Measurements
Enright & Sherrill20 Indoor; straight, 30.48 m
(i.e. 100 feet) One
Standardized each 30 s (e.g. “You are doing
ine .” or “Keep up the good work.”). _
Dyspnea*
SpO2 HR
Troosters et al21 Indoor;
straight, 50 m Two Standardized each 30 s. 2.5 h
Distance
SpO2
HR
Gibbons et al28 straight, 20 mIndoor; Four Standardized each 30 s (ine.” and “Keep up the good work.”).e.g. “You are doing 30 min
Distance
HR RR
BP
Chetta et al29 Indoor; straight, 30 m
Two Standardized each 30 s.
60 min
Distance Dyspnea#
HR HR SpO2
Camarri et al32 Indoor;
straight, 45 m Three
Standardized at each minute (e.g. ‘‘You are doing ine, keep up the
rhythm.’’ and ‘‘Do your best.’’). 20 min
Distance
HR
LL Fatigue*
Poh et al33 Indoor;
straight, 45 m
Three Standardized at each minute (e.g. “You are
doing ine . Keep up the rhythm.” and
“Do your best.”). 30 min
Distance
HR
LL Fatigue*
Geiger et al24 Straight, 20 m One
Standardized at each minute (e.g. “You are
doing ine. Five minutes to go.”, “Keep up the rhythm. Four minutes to go.”, “You are doing ine. You are halfway to the end.”, “Keep up the rhythm. Only two minutes to go.”, “You are
doing ine. Only one minute to go.”).
30 min
Distance
HR
BP
SpO2 Perceived Exertion#
Li et al26 Indoor; straight, 30.48 m (i.e. 100 feet)
One Standardized (e.g. “Keep it up.”, “You are
doing ine.” and “Everything is ine.”). _
Distance
HR
BP
SpO2
Lammers et al25 Straight, 30 to 50 m One Standardized (e.g. ‘‘Keep it up” and “You are
doing ine”). _
Distance
HR SpO2
Alameri et al30 Indoor; straight, 30 m One Standardized at each minute _
Distance
HR SpO2
Dyspnea*
Ben Saad et al35 Indoor; straight, 40 m Two Standardized (only at the 2nd test) ∼ 60 min
Distance
HR
BP
SpO2 Dyspnea#
Iwama et al31 Indoor; straight, 30 m Two
Standardized at each minute (e.g. “You are
doing ine. Five minutes to go.”, “Keep up the rhythm. Four minutes to go.”, “You are doing ine. You are halfway to the end.” “Keep up the rhythm. Only two minutes to go.”, “You are
doing ine. Only one minute to go.”).
∼ 30 min
Distance
HR RR
BP Dyspnea* LL Fatigue*
* - evaluated by Borg scale; # - evaluated by visual analogical scale (VAS); HR - heart rate; RR - respiratory rate; BP - blood pressure; LL - lower-limb; SpO2 - peripheral
oxygen saturation.
aforementioned Brazilian study, developed by Iwama et al31, Troosters et al21 prospectively evaluated 20 individuals with the same demographic and anthropometric characteristics of the sample used in the development of the reference equation. More recently, Ben Saad et al27,35 validated their equations in 30-41 prospectively assessed individuals and Alameri et al30 validated their equation in a similar fashion in 59 individuals. In all cases,
the reliability of the equations was acceptable and the authors recommended their use in similar populations.
Strategies for future studies
References
1. Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968; 203 (3): 201-4.
2. McGavin CR, Artvinli M, Naoe H, McHardy GJ. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. Br Med J. 1978; 2 (6132): 241-3.
3. Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982; 284 (6329): 1607-8.
4. Singh SJ. Walking for the assessment of patients with chronic obstructive pulmonary disease. Eur Respir Mon. 2007; 40 (1): 148-64.
5. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166 (1): 111-7.
6. Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr.
2000; 71 (5): 1033-47.
7. King S, Wessel J, Bhambhani Y, Maikala R, Sholter D, Maksymowych W. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J Rheumatol. 1999; 26 (10): 2233-7.
8. Lin SJ, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil. 2008; 89 (12): 2354-9.
9. Beriault K, Carpentier AC, Gagnon C, Menard J, Baillargeon JP, Ardilouze JL, et al. Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009; 30 (10): 725-7.
10. Vis JC, Thoonsen H, Duffels MG, de Bruin-Bon RA, Huisman SA, van Dijk AP, et al. Six-minute walk test in patients with Down syndrome: validity and reproducibility. Arch Phys Med Rehabil. 2009; 90 (8): 1423-7.
11. Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009; other studies used convenience samples. Li et al26 developed the
largest study to date. In this study, Chinese Elementary and High Schools were randomized for the performance of the evaluations. Alameri et al30 randomized 80.0% of the sample individuals to formulate the reference equation. The remaining 20.0% were enrolled in the cross-validation study of the developed equation. Although the sampling techniques developed by Li et al26 and by Alameri et al30 are capable of reducing the biases that are inherent to convenience sampling, these studies cannot be classified as randomized ones.
Li et al26 randomized the schools; however, inside each school, the selection was carried out by convenience. Similarly, the randomization carried out by Alameri et al30 minimized the sampling biases, but did not entirely work them out.
To date, therefore, only two studies can be considered to have been randomized ones20,32. The practical, operational and economic limitations can explain the lack of multicentric studies of reference values for the 6MWD. This could be important, especially in countries with continental dimensions such as Brazil.
Further studies with considerably larger sample sizes (e.g., multicentric ones) and studies using a randomized sampling technique are necessary, so that the reference values of the 6MWD can be even more representative.
Conclusions
The 6MWD presents great variability in healthy individuals; however, an important part of this variability
has been in general well explained by demographic and anthropometric features. The equations developed in foreign populations were not adequate for the Brazilian population. The equations developed in Brazil23,31 are, probably, the most appropriate to interpret the performance at the 6MWT of our patients with chronic diseases that affect their capacity to perform exercises. However, it is strongly recommended that such equation be validated in other regions of Brazil.
Acknowledgements
To Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support (Process # 2007/08673-3) in the development of the reference values related to the 6MWT and the incremental shuttle walk test in the Brazilian population.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
Table 7 - Reference equations for predicting the distance walked at the 6-minute walk test (6MWD) in healthy Brazilian individuals
Study Age range Equations
Iwama et al31 13 to 84 years Both: 6MWD
m = 622.461 – (1.846 x Ageyears) + (61.503 x sexmales = 1; females = 0); r2= 0.30
Priesnitz et al23 6 to 12 years Both: 6MWD
m = 145.343 + (11.78 x Ageyears) + (292.22 x heightm) + (0.611 x absolute difference in HR) – (2.684 x weightkg); r
2 = 0.36
89 (6): 569-79.
12. Maher CA, Williams MT, Olds TS. The six-minute walk test for children with cerebral palsy. Int J Rehabil Res. 2008; 31 (2): 185-8.
13. Troosters T, Vilaro J, Rabinovich R, Casas A, Barbera JA, Rodriguez-Roisin R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002; 20 (3): 564-9.
14. Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001; 119 (1): 256-70.
15. Enright PL. The six-minute walk test. Respir Care. 2003; 48 (8): 783-5.
16. Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003; 123 (2): 387-98.
17. Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996; 153 (3): 976-80.
18. Dourado VZ, Antunes LC, Tanni SE, de Paiva SA, Padovani CR, Godoy I. Relationship of upper-limb and thoracic muscle strength to 6-min walk distance in COPD patients. Chest. 2006; 129 (3): 551-7.
19. Araujo CO, Makdisse MR, Peres PA, Tebexreni AS, Ramos LR, Matsushita AM, et al. Different patterns for the 6-minute walk test as a test to measure exercise ability in elderly with and without clinically evident cardiopathy. Arq Bras Cardiol. 2006; 86 (3): 198-205.
20. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998; 158 (5 Pt 1): 1384-7.
21. Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999; 14 (2): 270-4.
22. Kervio G, Carre F, Ville NS. Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sports Exerc. 2003; 35 (1): 169-74.
23. Priesnitz CV, Rodrigues GH, Stumpf Cda S, Viapiana G, Cabral CP, Stein RT, et al. Reference values for the 6-min walk test in healthy children aged 6-12 years. Pediatr Pulmonol. 2009; 44 (12): 1174-9.
24. Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007; 150 (4): 395-9, e391-2.
25. Lammers AE, Hislop AA, Flynn Y, Haworth SG. The 6-minute walk test: normal values for children of 4-11 years of age. Arch Dis Child. 2008; 93 (6): 464-8.
26. Li AM, Yin J, Au JT, So HK, Tsang T, Wong E, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med. 2007; 176 (2): 174-80.
27. Ben Saad H, Prefaut C, Missaoui R, Mohamed IH, Tabka Z, Hayot M. Reference equation for 6-min walk distance in healthy North African children 6-16 years old. Pediatr Pulmonol. 2009; 44 (4): 316-24.
28. Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001; 21 (2): 87-93.
29. Chetta A, Zanini A, Pisi G, Aiello M, Tzani P, Neri M, et al. Reference values for the 6-min walk test in healthy subjects 20-50 years old. Respir Med. 2006; 100 (9): 1573-8.
30. Alameri H, Al-Majed S, Al-Howaikan A. Six-min walk test in a healthy adult Arab population. Respir Med. 2009; 103 (7): 1041-6.
31. Iwama AM, Andrade GN, Shima P, Tanni SE, Godoy I, Dourado VZ. The six-minute walk test and body weight-walk distance product in healthy Brazilian subjects. Braz J Med Biol Res. 2009; 42 (11): 1080-5.
32. Camarri B, Eastwood PR, Cecins NM, Thompson PJ, Jenkins S. Six minute walk distance in healthy subjects aged 55-75 years. Respir Med. 2006; 100 (4): 658-65.
33. Poh H, Eastwood PR, Cecins NM, Ho KT, Jenkins SC. Six-minute walk distance in healthy Singaporean adults cannot be predicted using reference equations derived from Caucasian populations. Respirology. 2006; 11 (2): 211-6.
34. Masmoudi K, Aouicha MS, Fki H, Dammak J, Zouari N. The six minute walk test: which predictive values to apply for Tunisian subjects aged between 40 and 80 years?. Tunis Med. 2008; 86 (1): 20-6.
35. Ben Saad H, Prefaut C, Tabka Z, Mtir AH, Chemit M, Hassaoune R, et al. 6-minute walk distance in healthy North Africans older than 40 years: influence of parity. Respir Med. 2009; 103 (1): 74-84.
36. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002; 82 (2): 128-37.
37. Roush J, Guy J, Purvis M. Reference values and relationship of the six minute walk test and body mass index in healthy third grade school children. The Internet Journal of Allied Health Sciences and Practice. 2006; 4 (3): 1-6. [Acessed on 2009 Nov 10]. Available from: http://ijahsp.nova.edu/articles/ vol4num3/rowsh.pdf
38. Pires SR, Oliveira AC, Parreira VF, Britto RR. Teste de caminhada de seis minutos em diferentes faixas etárias e índices de massa corporal. Rev Bras Fisioter. 2007; 11 (2): 147-51.
39. Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed). 1986; 292 (6521): 653-5.
40. Chetta A, Pisi G, Aiello M, Tzani P, Olivieri D. The walking capacity assessment in the respiratory patient. Respiration. 2009; 77 (4): 361-7.
41. Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006; 27 (5): 1040-55.
42. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982; 36 (5): 936-42.
43. Abrantes MM, Lamounier JA, Colosimo EA. Overweight and obesity prevalence in Northeast and Southeast Regions of Brazil. Rev Assoc Med Bras. 2003; 49 (2): 162-6.
44. Mion Jr D, Kohlmann Jr O, Machado CA, Amodeo C, Gomes MAG, Praxedes JN / Sociedade Brasileira de Cardiologia / Sociedade Brasileira de Hipertensão / Sociedade Brasileira de Nefrologia. V Diretrizes brasileiras de hipertensão arterial. Arq Bras Cardiol. 2007; 89 (3): e24-e79.
45. Araujo AJ, Menezes AMB, Dórea AJPS, Torres BS, Viegas CAA, Silva CAR, et al. / Sociedade Brasileira de Pneumologioa e Tisiologia. Diretrizes para cessação do tabagismo. J Bras Pneumol. 2004; 30 (Suppl 2): s1-s76.
46. Hallal PC, Dumith SC, Bastos JP, Reichert FF, Siqueira FV, Azevedo MR. Evolution of the epidemiological research on physical activity in Brazil: a systematic review. Rev Saude Publica. 2007; 41 (3): 453-60.
47. Teramoto S, Ohga E, Ishii T, Yamaguchi Y, Yamamoto H, Mastsuse T. Reference value of six-minute walking distance in healthy middle-aged and older subjects. Eur Respir J. 2000; 15 (6): 1132-3.
48. Lacasse Y, Wong E, Guyatt G. A systematic overview of the measurement properties of the Chronic Respiratory Questionnaire. Can Respir J. 1997; 4 (3): 131-9.
49. Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988; 65 (3): 1147-51.
50. Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993; 123 (2 Suppl): 465-8.
51. Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Sex modifies the relationship between age and gait: a population-based study of older adults. J Gerontol A Biol Sci Med Sci. 2008; 63 (2): 165-70.
52. Miller GJ, Saunders MJ, Gilson RJ, Ashcroft MT. Lung function of healthy boys and girls in Jamaica in relation to ethnic composition, test exercise performance, and habitual physical activity. Thorax. 1977; 32 (4): 486-96.
53. Whitrow MJ, Harding S. Ethnic differences in adolescent lung function: anthropometric, socioeconomic, and psychosocial factors. Am J Respir Crit Care Med. 2008; 177 (11): 1262-7.
55. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991; 144 (5): 1202-18.