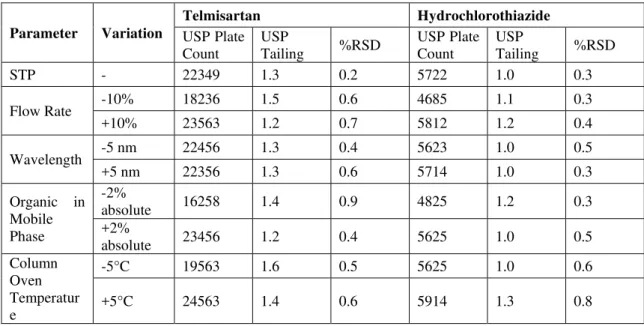
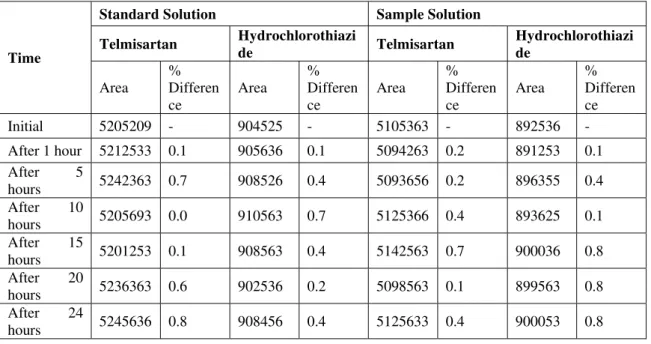
Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Telmisartan and Hydrochlorothiazide in Bulk and Pharmaceutical Dosage Form
Texto
Imagem
Documentos relacionados
Simultaneous estimation and validation of developed method of Nifedipine (NIF) and Atenolol (ATN) in combined dosage form as well as in laboratory mixture
A simple, rapid and reproducible HPLC method was developed for the simultaneous determination of amlodipine and valsartan in their combined dosage forms, and for drug
The objective of the present study was to develop a simple and selective HPLC method for the simultaneous determination of hesperidin (HP), caffeic acid (CA), ferulic acid (FA) and
In the present study, a reversed-phase high-performance liquid chromatographic (RP-HPLC) procedure was developed and validated for the simultaneous determination of seven
The proposed HPLC method is simple, isocratic, rapid, speciic, accurate and precise for determination of GMFX in bulk, pharmaceutical dosage formulation and human serum
Commercial capsule formulation was successfully analyzed using the developed method and the proposed method is applicable to routine analysis of determination of
Development and validation of a stability-indicating HPTLC method for analysis of Nebivolol hydrochloride and Hydrochlorothiazide in the bulk material and in
Preparation of stock solution: The Stock Solution has been prepared by weighing accurately and transferred 10 mg of Prasugral Working standard into a 10 mL volumetric