R E S E A R C H A R T I C L E
A phytomodulatory hydrogel with enhanced healing effects
Mirele S. Vasconcelos
1|
Tamiris F.G. Souza
2|
Ingrid S. Figueiredo
3|
Emília T. Sousa
4|
Felipe D. Sousa
5|
Renato A. Moreira
5|
Nylane M.N. Alencar
2|
José V. Lima
‐
Filho
6|
Márcio V. Ramos
11Departamento de Bioquímica e Biologia
Molecular, Universidade Federal do Ceará, Campus do Pici, Cx. Postal 6033, CEP, Fortaleza, CE 60451‐970, Brazil
2Departamento de Fisiologia e Farmacologia,
Faculdade de Medicina, Universidade Federal do Ceará, Fortaleza, Ceará, Brazil
3Centro Universitário Estácio, Fortaleza, CE,
Brazil
4Departamento de Patologia, Faculdade de
Medicina, Universidade Federal do Ceará, Fortaleza, Ceará, Brazil
5Núcleo de Biologia Experimental (NUBEX),
Centro de Ciências da Saúde, Universidade de Fortaleza (UNIFOR), Fortaleza, CE, Brazil 6Universidade Federal Rural de Pernambuco,
Recife, PE, Brazil
Correspondence
Márcio V. Ramos, Departamento de Bioquímica e Biologia Molecular, Universidade Federal do Ceará, Campus do Pici, Cx. Postal 6033, CEP, Fortaleza, CE 60451‐970, Brazil. Email: vramos@ufc.br
Funding information
Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: 407690/2013‐1
The healing performance of a hydrogel composed of hemicelluloses extracted from seeds of
Caesalpinia pulcherrima(Fabaceae) and mixed with phytomodulatory proteins obtained from the
latex ofCalotropis procerawas characterized on excisional wounds. The hydrogel did not induce dermal irritability. When topically used on excisional wounds, the hydrogel enhanced healing by wound contraction. Histology and the measurement of inflammatory mediators (myeloperoxidase, interleukin‐1β, and interleukin‐6) suggested that the inflammatory phase of the healing process was intensified, stimulating fibroplasia and neovascularization (proliferative phase) and tissue remodeling by increasing new collagen fiber deposition. In addition, reduction on levels of malondialdehyde in the groups that the hydrogel was applied suggested that the oxidative stress was reduced. The hydrogel performed better than the reference drug used, as revealed by the extended thickness of the remodeled epithelium.
K E Y W O R D S
Calotropis procera, galactomannans, inflammation, latex, proteases
1
|I N T R O D U C T I O N
Polysaccharide‐based hydrogels have emerged as interesting formulations for a variety of uses. In particular, stable associations of polysaccharides with a variety of bioactive compounds for use as drug‐delivery systems are of interest to the pharmaceutical industry. The literature also reports the intrinsic pharmacological and antimicro-bial activities of some polysaccharides, which enhance their potential-ities for pharmacological applications (Li et al., 2017).
In this study, a homogeneous hydrogel composed of hemicellu-loses obtained from the seeds ofCaesalpinia pulcherrimain association with soluble proteins extracted from the latex ofCalotropis procerawas prepared and used topically to enhance experimental wound healing.
The hemicelluloses (galactomannans) ofC. pulcherrimawere previously studied in terms of viscosity, critical concentration (Andrade, Azero, Luciano, & Gonçalves, 1999), rheology (Thombre & Gide, 2013), physico‐chemical properties, and enzymatic hydrolyses (Buriti et al., 2014). Studies of drug delivery using the polysaccharides of
C. pulcherrima have been performed (Jeevanandham,
Dhachinamoorthi, Bannoth, & Sekhar, 2011). The authors reported on the chemical aspects of the controlled release of water insoluble and water soluble chemicals and concluded that the mechanism of release of soluble compounds was anomalous. However, there are no studies regarding the delivery of proteins associated with these galactomannans. Here, we intended to use the galactomannans of
C. pulcherrimaas the support to delivery phytomodulatory proteins
DOI: 10.1002/ptr.6018
and to examine the effectiveness of them in wound experimental wounds.
Water soluble proteins extracted from the latex ofC. proceraAit.
Br. (Asclepiadaceae) have been demonstrated to alleviate arthritis and oxidative stress (Chaudhary et al., 2015; Chaudhary et al., 2016) and prevent oral and intestinal mucositis that result from chemother-apy (Alencar et al., 2017; Freitas et al., 2012). When incorporated into a biomembrane of polyvinyl alcohol, latex proteins have been found to accelerate the healing of incisional and excisional wounds (Figueiredo et al., 2014; Ramos et al., 2016). The underlying mechanisms involve the modulation of pro‐inflammatory cytokine release. Here, after for-mulating the hydrogel, its effectiveness for topical use was evaluated by estimating wound contraction, histology, and biochemical parameters.
2
|E X P E R I M E N T A L
2.1
|Materials
Cetyltrimethylamine bromide; iodoacetamide (IAA); Azocasein; ketamine chloridrate; and xylazine chloridrate were obtained from Sigma‐Aldrich, São Paulo, Brazil. Purilon® Gel was from MCsurgical, São Paulo, Brazil. All other chemicals were of analytical grade.
2.2
|Bio
‐
resources
The latex ofC. procera(Apocynaceae) was extracted from specimens
growing in the surroundings of Fortaleza City, State of Ceará, Brazil. The collections were carried out from January to June 2015. A voucher (sample specimen no. 32663) was left at Prisco Bezerra Herbarium of Federal University of Ceará, Brazil, where the specimens collected were taxonomically validated by a competent taxonomist.
The soluble proteins of the latex, retaining their phytomodulatory activity, were obtained after processing the crude latex according to a previously reported protocol (Alencar et al., 2006). The cleaned protein fraction was named CpLP, indicating latex proteins ofC. procera. This
preparation was further chromatographed on CM‐cellulose (pH 5.2) according to a previously described protocol (Ramos et al., 2009). The resulting protein fractions (CpLP‐PI; CpLP‐PII; and CpLP‐PIII) were recovered, dialyzed, and freeze‐dried. CpLP‐PII, where almost all pro-teolytic activity of CpLP is found, was tested in further experiments. When necessary, CpLP‐PII was treated with 20 mM IAA to irreversibly inhibit its endogenous proteolytic activity (Ramos et al., 2013). More detailed information about the chemical and biological properties of the latex proteins from C. procera is given in the Supporting Information.
C. pulcherrima (Fabaceae) seeds were collected in the State of Ceará, Brazil, and a voucher specimen was registered in the Herbarium Prisco Bezerra‐EAC (UFC), number 56367. Galactomannan and plant hemicellulose extraction was performed as described in detail by Cerqueira et al. (2009). Additional information about the chemical properties of the sample is presented in the Supporting Information.
2.3
|Animals
Adult male Swiss mice, 12 weeks old and weighing 25 ± 3.0 g, were obtained from the Central Animal House of the Universidade Federal do Ceará, Brazil. One hundred sixty‐eight animals were used. As per experimental design given in materials and methods, four groups of 15 mice each (4 × 15 = 60) were included in the study where the best experimental condition and sample concentration were determined. A different set of animals comprising 15 mice per group (6 × 15 = 90) was used for evaluating histological and macroscopic aspects. A third set of animals comprising 6 per group (6 × 3 = 18) was used to evaluate the dermal biocompatibility. The animals were kept in individual plastic cages under standard experimental conditions (12/12 light/dark cycles, temperature of 25 °C, and humidity 55 ± 10%) and fed with commercial ultraviolet sterilized diet and water ad libitum. All animal procedures were according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Before handling the animals, the experimental procedures were approved by the institutional committee for animal use and care and registered under approval number 64/2014.
2.4
|Hydrogel preparation
In order to prepare the hydrogel, CpPII and CpPII‐IAA (inhibited by iodoacetamide) were used at different weight/volume ratios to obtain the formulations tested in this work. The galactomannan gel was used as the vehicle for active substance release. To the galactomannan solu-tions (1.5%,w/v) were added cetyltrimethylamine bromide (0.1% w/v) and Milli‐Q water q.s.p. (enough for 100 ml). The mixture was homog-enized using a Turratec Tecnal TE‐102 agitator at 20,500 rpm for 1 min and centrifuged at 4 °C, 10,000 g for 15 min. After homogenization to obtain the galactomannan gel, the desired latex protein fraction was slowly added. The hydrogels remained under stirring for 2 hr. The final product, obtained by mixing aqueous solutions of latex proteins and the natural polymer based on plant hemicelluloses, was called HgCpPII and HgCpPII‐IAA. These hydrogels were prepared to have a final concentration of laticifer proteins of 0.2% and 0.5% and were used in all experiments.
2.5
|Wound healing assays
Animals were randomly divided into four groups of 15 animals each, as follows: (a) animals subjected only to the surgical procedure that did not receive any treatment (Sham); (b) animals that received the hemicellulose hydrogel without laticifer proteins (Hemi); (c) animals treated with the hydrogel at 0.2% (w/v) laticifer proteins (HgCpPII
0.2%), and (d) animals treated with the hydrogel at 0.5% (w/v) laticifer proteins (HgCpPII 0.5%).
Subsequently, an additional experiment under similar conditions was designed with the hydrogel HgCpPII‐IAA 0.2%; animals treated with the CpPII fraction 0.2% dissolved in water (WCpPII 0.2%) and a commercial hydrogel (Purilon® Gel, MCsurgical) introduced as a control group.
and further prepared for aseptic surgery using 1% iodopovidone followed by 70% ethanol. A single wound, 1 cm2full thickness, was
performed by excising the skin with a punch biopsy instrument on the dorsal region of each mouse.
The hydrogels (100μl) were applied topically onto the wound bed
once daily for 14 days. All mice were housed in their respective cages for 14 consecutive days starting from Day 0. The wounds were left undressed and exposed to the open environment and observed daily until Day 14. On Day 14, the wounds were evaluated through mor-phometry, and samples were taken for histological analyses following the procedure of Figueiredo et al. (2014).
2.6
|Macroscopic analyses
Excisional wounds were evaluated every 48 hr after surgery in terms of edema and hyperemia. Clinical signs of inflammation (edema and hyperemia) were scored as (0): absent, (1): mild, (2): moderate, and (3): intense.
The area of the excisional wounds was measured using a pachometer and was calculated as follows: A =π× R × r, where A, R, and r are area, large ray, and small ray, respectively. Thus, the unhealed wound area and the percentage of wound contraction were calculated and used for statistical analysis. Wound crust fall off was noted whenever a scab was not covering the wound. After the macroscopic study, 4‐mm punch biopsies from neoformed tissue and excisional wounds with adjacent normal skin were removed (n= 5/group) for histological study. Then the animals were sacrificed with an anesthetic overdose.
2.7
|Microscopic analyses
Samples of neoformed tissue were removed from wounds (n= 5) on
Day 14 and fixed for 24 hr in 10% formaldehyde dissolved in phosphate buffered saline 0.01 M at pH 7.2 and later submitted to his-topathology procedures. The sections (5μm) were stained with hema-toxylin–eosin to quantify fibroblasts and estimate re‐epithelialization progress. Masson trichrome dye was used to expose collagen fiber deposition. Toluidine blue was applied to quantify the number degranulated mast cells. The re‐epithelialization of the tissues was qualitatively assessed by light microscopy at a magnification of 100× to 400×. Re‐epithelialization and ulceration of the tissues were scored as present (+) or absent (−). All histological sections were evaluated according to the histological scoring system proposed by Vasconcelos et al. (2015) and expressed as range of scores for epidermal or dermal remodeling (Akkol et al., 2009).
The percentage of degranulated mast cells was estimated using the following formula: percentage of degranulated mast cells (%) = (number of degranulated mast cells/number of total mast cells) × 100. Collagen fiber deposition was quantified in images obtained from 15 random fields at 200× in 24 regions of 374 × 260 pixels (=10 × 7 μm). ImageJ®1.46 software (U.S. National Institutes of Health, USA) was used to count fibroblasts and evaluate collagenesis by using the“cell counter”and“threshold color”plug‐ins, respectively.
The thickness of the newly formed epidermis of excisional wounds was measured using 20 images of 655 × 492μm in wound sections from Day 14 after surgery.
2.8
|Markers and mediators of inflammation
Myeloperoxidase (MPO) activity was measured as previously described (Bradley, Priebat, Christensen, & Rothstein, 1982). Enzyme activity was determined by measuring the absorbance (460 nm) at 0 and 1 min. The results were reported as MPO (units/mg tissue) activity. Nitrite levels in biopsy lysates were determined indirectly as the total content of nitrite and nitrate (NO3−/NO2−) by spectrophotometry
using a method reported for the Griess reaction (Green et al., 1982). Data are expressed as micromoles of nitrite. The levels of interleukin (IL)‐1β and IL‐6 in biopsy lysates, homogenized individually in phosphate buffered saline, pH 7.4, and processed (Safieh‐Garabedian, Kanaan, Jalakhian, Jabbur, & Saadé, 1997), were determined using a sandwich enzyme linked immunosorbent assay. The results are expressed as picograms/milliliter (pg/ml) and reported as mean ± stan-dard error of the mean (SEM).
As an index of lipid peroxidation, the formation thiobarbituric reactive substances was measured through malondialdehyde (MDA) production determined using the spectrophotometer method (532 nm) described by Draper and Hadley (1990). The results are reported as mean ± SEM of MDA (nM/mg tissue) activity.
All analyses were performed in triplicate with samples obtained from three independent experiments and were reproduced without significant differences.
2.9
|Skin irritation model
Skin irritation tests were performed with some modifications (Jia, Zhao, & Jia, 2008). Swiss mice were shaved on the dorsal surface of the body and then were left under observation for 24 hr in order to confirm that there were no abnormal skin responses. The shaved animals were randomly allocated into groups (n = 6/group) that received only hydrogel at 0.2% (w/v) of laticifer proteins (HgCpPII
0.2%) and the vehicle containing only hemicelluloses (control group) applied topically to a shaved skin area of about 1 cm2, either as a
and after application of the treatment, that is, every 24 hr, using a micrometer.
2.10
|Statistical analyses
Statistical significance was assessed by analysis of variance followed by Bonferroni's test for multiple comparisons of the mean or Kruskal–Wallis for the median. All analyses were performed using the
program Graph‐Pad Prism 4.0 and were considered as significant at
p< .05. The results are expressed in mean ± SEM.
3
|R E S U L T S A N D D I S C U S S I O N
The macroscopic analysis of the healing progress in the excisional wounds and the wound contraction scores determined 14 days postsurgery is given in Figure 1. In the wound‐healing model, all groups
FIGURE 1 Macroscopic evaluation of the hydrogel CpPII on wound healing progress. Images (up) were selected from animals belonging to the
corresponding experimental groups. Percentage of wound contraction (down) in the time‐course until 14 days postsurgery. Date are expressed as mean ± standard error of the mean of average wound contraction (ap< .05 indicate statistical differences compared with the sham group and bp< .05 compared with the hemicellulose group (n= 15 animals/group, analysis of variance test followed by Bonferroni's test) [Colour figure can be
showed clean and exudate‐free wound margins during the period of examination. This was indicative that no microbial proliferation occurred. At the peak of the inflammatory period (Day 2), edema and hyperemia were observed in the excisional wounds of all groups, although these phlogistic signs were more intense in the wounds of ani-mals with topically applied HgCpPII 0.2% and HgCpPII 0.5%. Skin edema and hyperemia were more intense in wounds treated with HgCpPII 0.5% as compared with the sham (p< .001) and hemicellulose (p< .05) control groups (Figure 1). The wound scabs on the animals
treated with HgCpPII 0.5% started to fall off earlier, on Day 6, whereas in the other groups, this occurred after Day 8. Even after the scabs fell off, the hyperemic aspect of the wounds in the animals of HgCpPII 0.5% group remained evident until Day 10. This was suggestive of a more intense and prolonged inflammatory process, and this concentra-tion (0.5%) could overcome the safe size range for the cells health and then displaying toxicity. Indeed, although the hydrogel at 0.5% was effective along with the healing process, the healed tissue still exhibited inflammatory aspects. The macroscopic aspect of the wounds treated with hemicelluloses suggested less inflammation and was comparable with the sham group. It must to be noted that the controls (Sham and Hemi) still exhibited scars at Day 14, indicating that the impairment in wounding area was not fully rectified. On the contrary, both experimen-tal groups submitted to treatment with the hydrogel were repaired.
Wound contraction progressed faster in animals treated with HgCpPII 0.2% compared with the control groups. HgCpPII 0.5% enhanced wound closure only at Day 10 (Figure 1). The healing pro-cess of all the groups followed a normal evolution, that is, the unhealed wound area diminished throughout the 14‐day period, consequently increasing the wound contraction process (Figure 1). By the macro-scopic evaluation, it was thought that both hydrogels were efficient at promoting healing, although CpPII at 0.2% performed better.
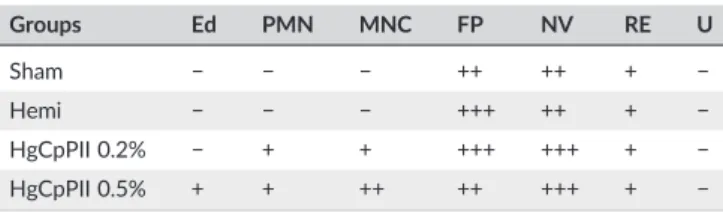
The histological examination of tissue samples from all experimen-tal groups was performed at Day 14 and is given in Figure 2a. On the Day 14, animals treated with HgCpPII 0.2% showed tissues with the discrete presence of remaining inflammatory foci, with a predomi-nance of PMN cells (Figure 2a) and intense fibroblast proliferation (Table 1). This finding is in good agreement with the previous study of Ramos et al. (2016) that reported that 0.2% of latex proteins impreg-nated in a polyvinyl‐alcohol biomembrane promoted healing in excisional wounds even with persistent leukocytes infiltration present until the whole healing. This remaining inflammatory status did not modify the main cellular events occurred posterior to the acute inflam-matory phase (Day 2) in both studies.
In the HgCpPII 0.5% group, only slight edema was seen with the presence of mild inflammation but a predominance of lymphocytes (mononuclear cells) close to hair follicles, although this was weakly significant (Figure 2a). Neovascularization was more pronounced in the groups treated with the hydrogels when compared with the control groups. However, all groups exhibited re‐epithelialization on Day 14, and ulceration was not observed (Table 1). The arrows in Figure 2a indicate the re‐epithelized epidermis with keratin, the formation of new capillary vessels, and the reorganization of collagen fibers and dermal papillae.
An outstanding effect observed with the use of the hydrogels was the increase in epithelial thickness at the end of the remodeling phase
of healing. Figure 2b shows histological slides stained with hematoxylin and eosin to indicate the new epithelium in different groups collected on Day 14 postsurgery. The thickness of the epithelium in the wound beds was greater in the HgCpPII 0.2% group (p< .05). This suggests that a more complete healing process occurred in this group. In addition, it is also seen in Figure 2B3 that the completeness of tissue regeneration was fully reached because total epithelialization, larger epithelial thickness, appearance of dermal papillae, and abundant keratin layer were all present. Thus, the use of the hydrogel reestablished the architecture and shape of the tissue.
The fibroplasia process and collagen deposition are events initiated in the proliferative and remodeling phases of the healing process. These ongoing events were observed on histological slides of the excisional wounds at Day 14 after surgery (Figure 3a, top and bottom sections, respectively). Quantitative evaluation of the sections suggested that HgCpPII 0.2% provided a better stimulus for fibroplasia, as evidenced by a higher number of fibroblasts when compared with the control groups (p< .05). However, collagen deposition on HgCpPII 0.2% was observed to be similar to HgCpPII 0.5% and also higher (p < .0001)
compared with the controls (Figure 3b,c, respectively).
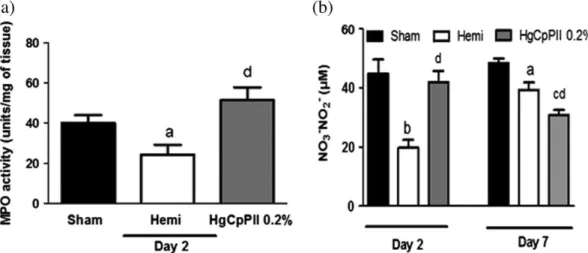
The healing process is initiated by an acute inflammatory phase exhibited as phlogistic signs. In this study, the inflammatory phase was characterized in all groups by increased MPO activity, measured on Day 2, indicating the presence of activated neutrophils in the wounds (Figure 4a). Of note, the hemicelluloses, thought to play a role of inert support, reduced the infiltration of neutrophils into the wounds whereas the latex proteins increased it. The quantification of NO, which is mainly produced by activated neutrophils in the inflam-matory phase, entirely corroborated this observation (Figure 4b). At Day 7, when the peak of inflammation had passed, the level NO was reduced, as it is mainly released by inflammatory cells like mast cells that are attracted by neutrophils.
The number of mast cells in the treated groups increased significantly and was greater in the HgCpPII 0.5% group at Day 14 (Figure S1). Degranulated mast cells are indicative of the inflammatory process and suggest histamine release in the earlier stages of the process and, in the later stages, the production of IL‐1βand IL‐6 that induce fibroblast proliferation and collagen synthesis (Figure 3b,c). Mast cells have been recognized to participate in different aspects of the healing process by acting in the different phases (Wulff & Wilgus, 2013). First, these cells are deeply involved in allergic processes and have been documented to enhance inflammation and contribute to the events of reepithelization and angiogenesis during wound repair. In this context, the measurement of both cytokines (IL‐1βand IL‐6) at Day 2 in tissues treated with HgCpPII 0.2% revealed a nice agreement with this observation (Figure 5a,b). It was interesting to note that the hemicelluloses that were shown to diminish MPO activity (Figure 4a) stimulated the release of IL‐1βand IL‐6 on Day 2 (Figure 5a,b). Therefore, the hemicelluloses conceptually displayed anti‐inflammatory activity at the peak of inflammation of the healing process (probably by reducing neutrophil infiltration) and then signaled for the release of inflammatory mediators (IL‐1βand IL‐6) that are involved in tissue remodeling at the later phase of the healing process, probably via mast cells. It is likely that the hemicelluloses of
phytomodulatory proteins but also contributed to some aspects of the healing process. The literature abundantly reports polysaccharides pro-posed as vehicles for drug delivery for wound repair (Ferreira et al., 2009), exhibiting pharmacological effects (Coura et al., 2017), even associated with wound healing (Ghadi, Jain, Khan, & Domb, 2016).
Although the hemicelluloses ofC. pulcherrimaseem to contribute to
the performance of the hydrogels, further studies will be necessary to better understand what role they play in the formula.
Oxidative stress is frequently a secondary physiological event associated with inflammation. MDA is physiologically formed as a
FIGURE 2 (a) Microscopic analyses of wound re‐epithelialization Sham group (a1); hemicellulose group (a2); HgCpPII 0.2% group (a3) and HgCpPII 0.5% group (a4). The slides were stained hematoxylin and eosin and the corresponding data were recorded at day 14. Arrows: (A) re‐
product of peroxidation of membrane lipids and is an efficient marker of oxidative stress (Yates, Dempster, Murphy, & Moore, 2017). MDA was measured in tissue wounds as reactive against thiobarbituric reactive substances. As reported in Figure S2, MDA was increased on Day 2, at the peak of acute inflammation. However, the hydrogel prevented this increase at Day 2, and oxidative stress was depressed
at Day 7. Therefore, the hydrogel was also efficient in inhibiting oxidative damage, which might contribute to accelerating the healing process. It should be noted that oxidative stress was prevented by the hemicelluloses, and it was not affected when the phytomodulatory proteins were incorporated into the gel.
In an additional set of experiments, the healing effects of CpPII were tested by applying the sample free of the hemicelluloses onto wounds. This would determine whether the proteins are indepen-dently active. Macroscopically, CpPII, dissolved in water (WCpPII 0.2%), efficiently accelerated healing, compared with the sham group and similar to HgCpPII 0.2%. This thinking was confirmed by grading wound contraction. Further, when CpPII was inhibited with IAA and incorporated into the gel, the hydrogel HgCpPII‐IAA 0.2% performed similarly to HgCpPII 0.2%, indicating that the proteolytic activity of the sample was not involved in the improved healing performance. The final analyses of performance involved the direct comparison of HgCpPII 0.2% with a commercial drug (Purilon®), which is a hydrogel for topical use composed of carboxymethylcellulose and calcium alginate. The macroscopic comparison of wounds showed similar aspects among the groups. However, wound contraction was more pronounced in the Purilon® group, suggesting that it would result
TABLE 1 Semi‐quantitative evaluation of histological sections deter-mined at the ending of the healing process
Groups Ed PMN MNC FP NV RE U
Sham − − − ++ ++ + −
Hemi − − − +++ ++ + − HgCpPII 0.2% − + + +++ +++ + −
HgCpPII 0.5% + + ++ ++ +++ + −
Note. Hematoxylin and eosin stained sections at Day 14 postsurgery were scored as mild (+), moderate (++), and severe (+++) for epidermal and/or dermal remodeling. Re‐epithelialization and ulceration were scored as pres-ent (+) and abspres-ent (−). Ed = edema; PMN = polymorphonuclear cells,
MNC = mononuclear cells; FP = fibroblast proliferation; NV = neovasculari-zation; RE = re‐epithelialization; U = ulceration. Sham: received no treat-ment; Hemi: hemicellulose hydrogel; HgCpPII 0.2% and 0.5% (mg/ml of CpPII protein fraction ofCalotropis procerain hemicellulose hydrogel).
FIGURE 3 Laticifer protein hydrogels stimulate the proliferative and remodeling phases of the healing process. (a) Histological views of fibroplasia
and collagen deposition on Day 14 postsurgery (top and bottom sections at 400× and 200× magnification, respectively). (b) Fibroplasia quantification and (c) collagen deposition. Data are mean of three independent experiments and are expressed as mean ± standard error of the mean of fibroblasts number for fibroplasia and percentage of total pixel number for the collagen content.ap< .05 indicates statistical difference
compared with the Sham group.bp< .05 compared with the hemi group andcp< .05 compared with HgCpPII 0.5% (n= 5 animals/group/time
healing first. This was investigated by examining the histological progress of tissue repair.
The thickness of the new epithelium, examined by histology at Day 14 was taken as the reference of completed tissue repair. The data revealed that, despite promoting faster wound contraction, Purilon® did not help to develop new epithelium. It was comparable with the sham group in this regard. The hemicelulloses did not stimulate synthe-sis of the epithelium. Instead, they somehow inhibited it as compared with the sham group. However, all groups treated with the phytomodulatory proteins (WcpPII 0.2%; HgCpPII 0.2%; and HgCpPII‐IAA 0.2%) notably underwent new epithelium synthesis, exhibited by the evident increase in epithelium thickness. Comparative additional data from these assays, such as the time‐course of scab fall and phlogistic signs, are detailed in Table S1. These results point to the efficacy of the phytomodulatory proteins of latex in wound repair as reported before (Ramos et al., 2016). Furthermore, the formula tested in this study performed better in terms of wound repair than the refer-ence drug, specifically considering the completeness of the histological process.
Dermal irritation is a limiting point in the development of formu-las aiming for topical application, and this is especially critical for products to promote wound healing. Dermal irritation was evaluated for seven consecutive days by assessing the presence of edema by a skin challenge with the lowest dose that exhibited pharmacological activity in the previous wound healing model (HgCpPII 0.2%). In the first approach, HgCpPII 0.2% was applied on the skin (shaved) and observed for 6 hr and then daily. The thickness of the skin was the parameter considered. No differences were observed. In the second approach, HgCpPII 0.2% was applied daily until Day 7; no differences were observed among the groups. In the multiple‐
dose test on abraded skin, the HgCpPII 0.2% group presented greater skin thickness when compared with the control groups starting on the first day until Day 3 of the test (p < .05). These results were accompanied by the observation of intense edema and hyperemia, indicating that HgCpPII 0.2% increases the phlogistic signs of inflammation in this first post‐injury period. After Day 4, the thickness remained constant in all groups (p> .05). The primary irritation index (PII) was graded after the evaluation of the HgCpPII
FIGURE 4 Hemicelluloses decrease myeloperoxidase (MPO) activity and nitrite levels. Myeloperoxidase activity (a) and nitrite levels (b) in wound
samples on Days 2 and 7 after surgery. Data are the mean of three independent experiments and are expressed as mean ± standard error of the mean of MPO units/mg of tissue and nitric oxide (NO3/NO2) levels (μM), respectively.ap< .05,bp< .001, andcp< .0001 indicates statistical
differences compared with the sham group anddp< .05 compared with the hemicellulose group (n= 5 animals/group/time point/experiment;
analysis of variance with the Bonferroni post hoc test)
FIGURE 5 Pro‐inflammatory cytokines measured in wounds. On Day 2 after surgery, animals were sacrificed, and tissues in the excisional wounds were removed to determine interleukin (IL)‐1 (a) and IL‐6 (b) cytokines levels by enzyme linked immunosorbent assay. Data are the mean of three independent experiments and are expressed as mean ± standard error of the mean of cytokine/ml from supernatant/mg tissue.ap< .05,bp< .001,
andcp< .0001 indicate statistical differences compared with the sham group (n= 5 animals/group/time point/experiment; analysis of variance with
0.2% hydrogel on intact skin and abraded skin, as shown in Table S2. Treated mice showed no signs of corrosions when compared with the control groups. The total score for both groups, intact and abraded skin, were respectively 0.03 IPP and 0.98 IPP, indicating that HgCpPII at 0.2% was not an irritant and is suitable for dermal use. To finish, the experimental procedure performed in this study did not affect the overall activity of the animals. After surgery, the animals were observed for 2 hr, and no abnormal behavior such as lethargy or agitation, itchiness, or irritability among the groups was documented. Animals fed and drank water normally. At Day 14, before collecting the final data and sacrifice, the animals seemed healthy without any documented adversity, including normal motor capacity.
4
|C O N C L U S I O N
This study was focused on the formulation of a hydrogel composed of hemicelluloses and phytomodulatory proteins extracted from latex. The hydrogel did not induce dermal irritation and efficiently stimulated wound healing by modulating some aspects of the inflammatory phase, enhancing the proliferative phase and mainly improving tissue remod-eling by achieving complete wound repair. The hemicelluloses, initially proposed as the delivery vehicle for the phytomodulatory proteins, were involved, at least in part, in the mechanistic modulation of the process. The study concludes that the proposed basal formula would be interesting for deeper investigations into the ideal composition in terms of skin repair and absorption.
A C K N O WL E D G E M EN TS
Biochemical, functional, and applied studies of latex proteins are supported by grants from the following Brazilian Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP). This study is part of the consortium
“Molecular Biotechnology of Plant Latex.”
C O N F L I C T O F I N T E R ES T
The authors hereby declare no conflicts of interest.
O R C I D
Márcio V. Ramos http://orcid.org/0000-0002-8260-4144
R E F E R EN C E S
Akkol, E. K., Koca, U., Peşin, I., Yilmazer, D., Toker, G., & Yeşilada, E. (2009).
Exploring the wound healing activity of Arnebia densiflora (Nordm.) Ledeb. by in vivo models.Journal of Ethnopharmacology,124, 137–141. Alencar, N. M. N., Bitencourt, F. S., Figueiredo, I. S. T., Luz, P. B., Lima‐Júnior, R. C. P., Aragão, K. S.,…Ramos, M. V. (2017). Side‐effects of irinotecan (CPT‐11), the clinically used drug for colon cancer therapy, are elimi-nated in experimental animals treated with latex proteins from
Calotropis procera(Apocynaceae).Phytotherapy Research,31, 312–320. Alencar, N. M. N., Oliveira, J. S., Mesquita, R. O., Lima, M. W., Vale, M. R., Etchells, J. P., … Ramos, M. V. (2006). Pro‐ and anti‐inflammatory activities of the latex fromCalotropis procera(Ait.) R.Br. are triggered
by compounds fractionated by dialysis. Inflammation Research, 55,
559–564.
Andrade, C. T., Azero, E. G., Luciano, L., & Gonçalves, M. P. (1999). Solution properties of the galactomannans extracted from the seeds of
Caesalpinia pulcherrimaandCassia javanica: Comparison with locust bean gum.International Journal of Biological Macromolecules,26, 181–185. Bradley, P. P., Priebat, D. A., Christensen, R. D., & Rothstein, G. (1982).
Mea-surement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker.The Journal of Investigative Dermatology,78, 206–209.
Buriti, F. C. A., Santos, K. M. O., Sombra, V. G., Maciel, J. S., Teixeira Sá, D. M. A., Salles, H. O.,…Egito, A. S. (2014). Characterization of partially hydrolyzed galactomannan from Caesalpinia pulcherrima seeds as a potential dietary fibre.Food Hydrocolloids,35, 512–521.
Cerqueira, M. A., Pinheiro, A. C., Souza, B. W. S., Lima, Á. M. P., Ribeiro, C., Miranda, C.,… Gonçalves, M. P. (2009). Extraction, purification and characterization of galactomannans from non‐traditional sources.
Carbohydrate Polymers,75, 408–414.
Chaudhary, P., Ramos, M. V., Vasconcelos, M. S., & Kumar, V. L. (2016). Protective effect of high molecular weight protein sub‐fraction of
Calotropis proceralatex in monoarthritic rats.Pharmacog Magazine,12, S147–S151.
Chaudhary, P., Viana, C. A., Ramos, M. V., & Kumar, V. L. (2015). Antiedematogenic and antioxidant properties of high molecular weight protein sub‐fraction ofCalotropis proceralatex in rat.Journal of Basic and Clinical Pharmacy,6, 69–73.
Coura, C. O., Chaves, H. V., Val, D. R., Vieira, L. V., Silveira, F. D., Santos Lopes, F. M. L.,…Benevides, N. M. (2017). Mechanisms involved in antinociception induced by a polysulfated fraction from seaweed
Gracilaria corneain the temporomandibular joint of rats.International Journal of Biological Macromolecules,97, 76–84.
Draize, J. H., Woodard, G., & Calvery, H. O. (1944). Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. The Journal of Pharmacology and Experimental Therapeutics,82, 377–390.
Draper, H. H., & Hadley, M. (1990). Malondialdehyde determination as index of lipid peroxidation.Meth Enzymol,186, 421–431.
Dutok, C. M. S., Berenguer‐Rivas, C. A., Rodríguez‐Leblanch, E., Pérez‐
Jackson, L., & Queiroz, M. (2015). Acute toxicity and dermal and eye irritation of the aqueous and hydroalcoholic extracts of the seeds of“Zapote”Pouteria mammosa(L.) Cronquist.Scientific World Journal, e642906.
Ferreira, L. M., Blanes, L., Gragnani, A., Veiga, D. F., Veiga, F. P., Nery, G. B.,
…Okamoto, R. (2009). Hemicellulose dressing versus rayon dressing in the re‐epithelialization of split‐thickness skin graft donor sites: A multicenter study.Journal of Tissue Viability,18, 88–94.
Figueiredo, I. S. T., Ramos, M. V., Ricardo, N. M. P. S., Gonzaga, M. L. C., Pinheiro, R. S. P., & Alencar, N. M. N. (2014). Efficacy of a membrane composed of polyvinyl alcohol as a vehicle for releasing of wound healing proteins belonging to latex of Calotropis procera. Process Biochemistry,49, 512–519.
Freitas, A. P. F., Bitencourt, F. S., Brito, G. A. C., Alencar, N. M. N., Ribeiro, R. A., Lima‐Júnior, R. C. P., … Vale, M. L. (2012). Protein fraction of
Calotropis procera latex protects against 5‐fluorouracil‐induced oral mucositis associated with downregulation of pivotal pro‐inflammatory mediators. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385,
981–990.
Ghadi, R., Jain, A., Khan, W., & Domb, A. J. (2016). Microparticulate polymers and hydrogels for wound healing.Rapid Communication Mass Spectrometry,2, 203–225.
Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S., & Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids.Analytical Biochemistry,126, 131–138.
Jeevanandham, S., Dhachinamoorthi, D., Bannoth, C., & Sekhar, K. (2011). Drug release studies fromCaesalpinia pulcherrimaseed polysaccharide.
Iranian Journal of Pharmaceutical Research,10, 597–603.
Jia, Y., Zhao, G., & Jia, J. (2008). Preliminary evaluation: The effects of Aloe ferox Miller andAloe arborescensMiller on wound healing.Journal of Ethnopharmacology,120, 181–189.
Li, P., Zhao, J., Chen, Y., Cheng, B., Yu, Z., Zhao, Y., … Jin, S. (2017). Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydrate Polymers,157, 1383–1392.
OECD Guidelines for theTesting of Chemicals (2000). (http://www.oecd.org/ chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm) Ramos, M. V., Alencar, N. M. N., Oliveira, R. S. B., Freitas, L. B. N., Aragão, K.
S., Andrade, T. A. M.,…Figueiredo, I. S. (2016). Wound healing modula-tion by a latex protein‐containing polyvinyl alcohol biomembrane.
Naunyn‐Schmiedeberg's Archives of Pharmacology,389, 747–756.
Ramos, M. V., Araújo, E. S., Jucá, T. L., Monteiro‐Moreira, A. C. O., Vasconcelos, I. M., Moreira, R. A.,…Moreno, F. B. (2013). New insights into the complex mixture of latex cysteine peptidases in Calotropis procera.International Journal of Biological Macromolecules,58, 211–219. Ramos, M. V., Pereira, D. A., Souza, D. P., Araújo, E. S., Freitas, C. D., Cavalheiro, M. G.,…Carvalho, A. F. (2009). Potential of laticifer fluids for inhibiting Aedes aegypti larval development: Evidence for the
involvement of proteolytic activity.Memórias do Instituto Oswaldo Cruz,
104, 805–812.
Safieh‐Garabedian, B., Kanaan, S. A., Jalakhian, R. H., Jabbur, S. J., & Saadé, N. E. (1997). Involvement of interleukin‐1 beta, nerve growth factor, and prostaglandin‐E2 in the hyperalgesia induced by intraplantar injec-tions of low doses of thymulin.Brain Behavior Immun,11, 185–200.
Thombre, N. A., & Gide, P. S. (2013). Rheological characterization of galactomannans extracted from seeds ofCaesalpinia pulcherrima. Carbo-hydrate Polymers,94, 547–554.
Vasconcelos, M. S., Gomes‐Rochette, N. F., Oliveira, M. L. M., Nunes‐Pinheiro, D. C. S., Tomé, A. R., Maia‐Sousa, F. Y.,…Melo, D. F. (2015). Anti‐inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Experimental Biology and Medicine,240, 1648–1655.
Wulff, B. C., & Wilgus, T. A. (2013). Mast cell activity in the healing wound: More than meets the eye?Experimental Dermatology,22, 507–510. Yates, S. A., Dempster, N. M., Murphy, M. F., & Moore, S. A. (2017).
Quantitative analysis of malondialdehyde‐guanine adducts in genomic DNA samples by liquid chromatography tandem mass spectrometry.
Rapid Comm Mass Spectrom. https://doi.org/10.1002/rcm.7843
S U P P O R T I N G I N F O R M A T I O N
Additional Supporting Information may be found online in the supporting information tab for this article.
How to cite this article: Vasconcelos MS, Souza TFG, Figueiredo IS, et al. A phytomodulatory hydrogel with enhanced healing effects. Phytotherapy Research. 2018;32: