Transgenic Soybean Production of Bioactive
Human Epidermal Growth Factor (EGF)
Yonghua He1, Monica A. Schmidt1, Christopher Erwin2, Jun Guo2, Raphael Sun2, Ken Pendarvis3, Brad W. Warner2, Eliot M. Herman1*
1School of Plant Sciences, University of Arizona, Tucson, Arizona, United States of America,2St. Louis Children's Hospital and Washington University School of Medicine, St. Louis, Missouri, United States of America,3School of Animal & Comparative Biomedical Sciences, University of Arizona, Tucson, Arizona, United States of America
*emherman@email.arizona.edu
Abstract
Necrotizing enterocolitis (NEC) is a devastating condition of premature infants that results from the gut microbiome invading immature intestinal tissues. This results in a life-threaten-ing disease that is frequently treated with the surgical removal of diseased and dead tis-sues. Epidermal growth factor (EGF), typically found in bodily fluids, such as amniotic fluid, salvia and mother’s breast milk, is an intestinotrophic growth factor and may reduce the onset of NEC in premature infants. We have produced human EGF in soybean seeds to lev-els biologically relevant and demonstrated its comparable activity to commercially available EGF. Transgenic soybean seeds expressing a seed-specific codon optimized gene encod-ing of the human EGF protein with an added ER signal tag at the N’terminal were produced. Seven independent lines were grown to homozygous and found to accumulate a range of 6.7 +/- 3.1 to 129.0 +/- 36.7μg EGF/g of dry soybean seed. Proteomic and immunoblot anal-ysis indicates that the inserted EGF is the same as the human EGF protein. Phosphoryla-tion and immunohistochemical assays on the EGF receptor in HeLa cells indicate the EGF protein produced in soybean seed is bioactive and comparable to commercially available human EGF. This work demonstrates the feasibility of using soybean seeds as a biofactory to produce therapeutic agents in a soymilk delivery platform.
Introduction
Each year in the United States, more than 530,000 babies, approximately 12% of total births, are born before 37 full weeks of gestation [1]. As a growing health issue the rate of premature birth has increased by 36 percent since the early 1980s. One of the major problems associated with prematurity is the development of a condition known as neonatal necrotizing enterocolitis (NEC) [2]. This is observed clinically as the abrupt development of bloody diarrhea, abdominal swelling, and tenderness in a premature infant who is otherwise doing well [3]. Current treat-ment often requires surgical removal of the damaged and dead intestine, often resulting in mortality (about 40%) or, if the infant survives, to manifest significant resulting lifetime prob-lems [3–5]. Although the direct cause of NEC is not known, the most significant contributing
a11111
OPEN ACCESS
Citation:He Y, Schmidt MA, Erwin C, Guo J, Sun R, Pendarvis K, et al. (2016) Transgenic Soybean Production of Bioactive Human Epidermal Growth Factor (EGF). PLoS ONE 11(6): e0157034. doi:10.1371/journal.pone.0157034
Editor:Valli De Re, Centro di Riferimento Oncologico, IRCCS National Cancer Institute, ITALY
Received:January 14, 2016
Accepted:May 24, 2016
Published:June 17, 2016
Copyright:© 2016 He et al. This is an open access article distributed under the terms of theCreative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement:All relevant data are within the paper and its supporting information files, and in protein database PRIDE partner repository with the dataset identifier PXD003326 and 10.6019/ PXD003326.
factor is premature birth. Post-partum establishment of an abnormal gut microbiome creates the opportunity for bacterial invasion into gut due to immature intracellular junctions of the intestinal mucosa [6,7]. Experimental and clinical evidence suggest that prematurity and NEC is associated with deficient endogenous production of epidermal growth factor (EGF), which is necessary for normal intestinal development and repair [8,9]. EGF is a critical growth factor found in multiple fluids that bathe the developing intestine including amniotic fluid, fetal urine, breast milk, bile, and saliva [2,10,11]. In the amniotic fluid, there is an increasing con-centration of EGF as gestation progresses [12]. EGF amounts in mother’s milk is highest first days after parturition with mothers of extreme pre-term neonates having 50–80% higher than mother’s milk of full term infants [13]. Human studies have demonstrated that EGF is resistant to proteolytic degradation across a range of gastric pH [14]. While EGF is produced to some extent in duodenal Brunner’s glands and kidney, the vast majority of EGF is produced in the salivary glands [15]. Exogenous infusion of EGFin uterohas been shown to accelerate the mat-uration of intestinal enzyme activity as well as stimulate intestinal growth [16,17]. The impor-tance of EGF to gut development is highlighted by the fact that knockout of the EGF receptor in some mice strains results in death due to a bloody diarrhea that is remarkably similar to human NEC [18]. Transgenic mice directed to intestinally overexpress EGF displayed a num-ber of beneficial effects, including increased body weight and villus height, after a small bowel resection compared to nontransgenic mice [19]. Conversely, inhibition of EGF receptors impairs intestinal adaption following a small bowel resection [20].
A prospective, multi-center trial demonstrated that infants fed regular formula (not con-taining growth factors) were 6 to 10 times more likely to develop NEC than infants fed breast milk [21]. While a large number of biologically active peptides and growth factors have been identified in breast milk, EGF is one of the major peptides present in significant concentrations [22]. The concentration of EGF in milk is found to be inversely proportional to the gestational age of the infant, therefore, the more premature the infant, the more EGF is present in the breast milk [13]. This may be a compensatory response to the premature removal of the fetus from the EGF-rich amniotic fluid. It has been demonstrated in several animal models of NEC that administration of exogenous EGF has been shown to significantly reduce the severity of intestinal injury [23,24]. The proactive treatment of infants at NEC risk with EGF supplemen-tation could therefore accelerate intestinal maturation, thus preventing the development of NEC.
If the proactive EGF feeding strategy is effective to induce the maturation of the neonate intestinal tract then this simple approach may mitigate the development of NEC with its result-ing high costs in medical resources, pain and possible life-long debilitation and for the 40% infants with NEC that proves fatal [25–27]. To accomplish such a proactive therapeutic approach adapting infant formula for EGF delivery would be simple and economic and mimic the delivery of EGF in mother’s milk. The need and potential delivery makes infant formula containing EGF a good model for food-sourced plant biotechnology. Soybean-derived formula encompasses a significant fraction of the total infant formula market. Soybean milk and derived products are a potential food-therapy delivery platform that could include a variety of medically-necessary products including drugs such as EGF but might also include oral vaccines [28,29]. The economy of production and simple conversion into therapeutic materials that has long-shelf life makes soybean biotech products a potentially desirable commodity for use in cost-sensitive scaled applications. Addressing the devastating disease of NEC through a simple proactive treatment protocol is an excellent platform to explore the potential of soybean-pro-duced therapeutics. Here we report the accumulation of human EGF (hEGF) in genetically-engineered soybean seeds and show that the recombinant EGF is indistinguishable from authentic human EGF and is bioactive at stimulating EGF receptor (EGFR) activity. Competing Interests:The authors have declared
Methods
Transgenic EGF soybean seeds
Epidermal growth factor protein from humans was produced in soybean seeds by constructing a plant gene expression cassette that involved a synthetic codon optimized EGF nucleotide sequence (protein sequence from Genbank accession CCQ43157). This 162 bp open reading frame was placed in-frame behind a 20-amino acid endoplasmic reticulum (ER) signal sequence from theArabidopsischitinase gene [30,31]. The ER-directed EGF encoding open reading frame was developmentally regulated by the strong seed-specific storage protein glyci-nin regulatory elements [31]. The entire seed specific cassette to direct EGF production was placed in a vector containing the hygromycin resistance gene under the strong constitutive expression of the potato ubiquitin 3 regulatory elements as previously described [31–33]. The result plasmid pGLY::ShEGF was sequenced using a glycinin promoter primer (5’TCATTCAC CTTCCTCTCTTC 3’) to ensure the EGF open reading frame was placed correctly between the regulatory elements. Somatic soybean (Glycine max L. Merrill cv Jack (wild type)) embryos were transformed via biolistics using 30 mg/L hygromycin B selection and regenerated as pre-viously described [34]. Embryos from resistant lines were analyzed by genomic PCR to confirm the presence of inserted hygromycin cassette using primers specific to the hygromycin gene (HygF 5’CTCACTATTCCTTTGCCCTC3’and HygR 5’CTGACCTATTGCATCTCCCG3’), cetyl trimethyl ammonium bromide (CTAB) extraction genomic DNA isolation and the fol-lowing amplification conditions: 150 ng genomic DNA in 25μl total reaction containing 200 nM primers and 3 U Taq polymerase (NEB) and the following cycling parameters (initial 95°C 4 min then 45 cycles of 95°C 30 s, 55°C 45 s, 72°C 90s; followed by a final extension of 72°C 7 min). Dry seeds from two successive generations of PCR positive plants were analyzed by ELISA for the expression of EGF protein until all 7 lines were confirmed to be homozygous. EGF transgenic soybean plants along with nontransgenic control wild type cultivar plants were grown side by side in a greenhouse at 25°C under 16 h daylight with 1000μm-2/s.
EGF detection via Immunoblot
Total soluble protein was extracted from dry seeds of two homozygous EGF lines and a non-transgenic control by repeated acetone washes followed by acetone precipitation with the pro-tein pellet dissolved in water. Propro-teins with molecular weight 10 kDa and under were isolated by separately passing each extract through an Amicon Ultra centrifugal filter (Merck, Kenil-worth NJ). The samples were each suspended in sample buffer (50mM Tris HCL, pH6.8 2% SDS (w/v), 0.7 Mβ-mercaptoethanol, 0.1% (w/v) bromphenol blue and 10% (v/v) glycerol) and
EGF quantification
Total soluble protein was extracted from dry soybean seeds as described previously [31,32] from all 7 lines of pGLY::ShEGF transgenic plants along with nontransgenic seeds as a negative control. EGF was quantitated by commercially available human EGF ELISA assay (Quantikine ELISA kit from R&D systems, Minneapolis MN) according to the manufacturer’s instructions. The provided positive control was used to create a standard curve in order to determine the amount of EGF in each soybean protein extract. Each homozygote EGF transgenic line was assayed with three biological replicates and results displayed as mean +/- standard error.
Seed Proteome Composition Analysis
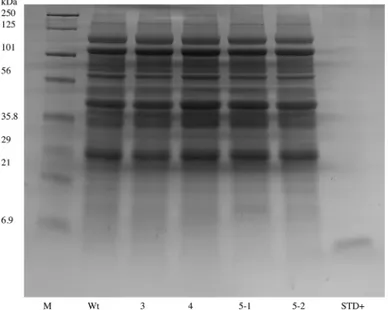
Total soluble proteins were extracted, quantitated and suspended in sample loading buffer as previously described [31,32]. Approximately 30μg of protein extract from dry seeds of 4 homozygous EGF lines were separated on a 4–20% gradient SDS-PAGE gel (BioRad, Hercules CA) along with extract from a nontransgenic seed. The gel was subsequently stained with 0.1% (w/v) Coomassie Brilliant Blue R250 in 40% (v/v) methanol, 10% (v/v) acetic acid overnight and then destained for approximately 3 hrs in 40% methanol, 10% acetic acid with frequent solution changes.
Mass Spectrometry analysis to detect EGF in soybean samples
library [36] X!tandem 2013.09.01.1 [37] and OMSSA [38] algorithms were employed via the University of Arizona High Performance Computing Center to perform spectrum matching. Precursor and fragment mass tolerance were set to 0.2 Daltons for both OMSSA and X!tandem. Trypsin cleavage rules were used for both algorithms with up to 2 missed cleavages. Amino acid modifications search consisted of single and double oxidation of methionine, oxidation of proline, N-terminal acetylation, carbamidomethylation of cysteine, deamidation of asparagine and glutamine and phosphorylation of serine, threonine, and tyrosine. X!tandem xml and OMSSA xml results were filtered using Perl to remove any peptide matches with an E-value>0.05 as well as proteins identified by a single peptide sequence. The protein fasta data-base forGlycine maxwas downloaded on August 5, 2015 from NCBI RefSeq with the addition of the EGF amino acid sequence. A randomized version of theGlycine maxfasta was
concatenated to the original as a way to assess dataset quality. The mass spectrometry proteo-mics data have been deposited to the ProteomeXchange Constortium (http://proteomecentral. proteomexchange.org) via the PRIDE partner repository [39] with the dataset identifier PXD003326 and 10.6019/PXD003326.
Cell Culture, Western Blotting and Immunocytochemistry
Hela cells (obtained from American Tissue Culture Collection) were cultured in Minimum Essential Media (MEM) complemented with 10% Fetal Bovine Serum (FBS), 100 units/ml pen-icillin, and 100μg/ml streptomycin. For western blotting assay, cells grown in 6-well plate were kept in serum free MEM media for 24 hours. Cells were then either kept in serum free medium (control) or stimulated with soy milk alone, soy EGF or commercial recombined human EGF for different time period as indicated. Cells were lysed by directly adding 1× SDS sample buffer (50 mM Tris–HCl, pH 6.8, 10% glycerol, 2% SDS and 5%β-ME) to the cells after washing 3
times with 1X PBS. EGF bio-activity was determined via EGFR phosphorylation and down-stream AKT phosphorylation. Total EGFR was also measured since EGFR is known to undergo internalization when stimulated with EGF. Antibodies used in western blot are anti-p-EGFR (Tyr1068) (#2234, cell signaling Technology), anti-total EGFR (#06–847, Millipore), anti-p-AKT (#4060, cell signaling Technology) and anti-Lamin B1 (# 13435, cell signaling technology) [40]. For immunocytochemistry assay, cells were grown on coverslip in 6-well plate and kept in serum free media for 24 hours before stimulation, cells were then either kept in serum free media (control) or stimulated with human or soy EGF for 6 hours. Cells were washed with PBS and fixed with 4% formalin. EGFR was labeled using anti-EGFR antibody (#4267, cell signaling technology) and detected with Alexa Fluor 594 Goat anti-rabbit IgG (#A11012, life technology). The cell nucleus were shown using mounting medium with DAPI (#H-1200, Vectorshield).
Results
Recombinant hEGF is expressed in both cotyledonary-stage embryos
regenerated in primary transformation events and subsequently in
homozygous soybean seeds
plant signal sequence was added so that the hEGF synthesized would be as a pre-hEGF. The Gly::ShEGF construct was used for biolistic transformation of soybean somatic embryo cells as outlined in [31–34]. Embryos were selected in liquid culture by hygromycin B and individual regenerated lines were separated, propagated, and induced to form cotyledonary embryos. The cotyledonary embryos were evaluated for hEGF production using EGF-specific ELISA that indicated a variation of heterologous protein production (data not shown). The most promis-ing EGF expresspromis-ing lines were moved forward for regeneration by desiccatpromis-ing and subsequent germination. The initial T0generation EGF transgenic plants were grown in the greenhouse
and further selected by genomic PCR for an additional 2–3 generations. Additionally, each gen-eration of seeds produced by the selected lines were assayed for hEGF content by ELISA. The hEGF content of each line in seeds representative of the homozygous population is shown in
Fig 1B. The lines varied in hEGF content but seeds within each line had a narrow range of hEGF accumulation. The EGF transgenic Line 5 produced in excess of 100μg hEGF per gm dry seed weight, a level calculated to be much in excess of potential therapeutic requirements. By comparison, yeast stains have been used as an expression system for both human EGF [41] and mouse EGF [42] with the highest levels produced being from a multicopy insertPichia pas-torisclone secreting 49μg EGF/ml. In both the mouse and human EGF yeast production sys-tems, truncated versions of the EGF were detected.
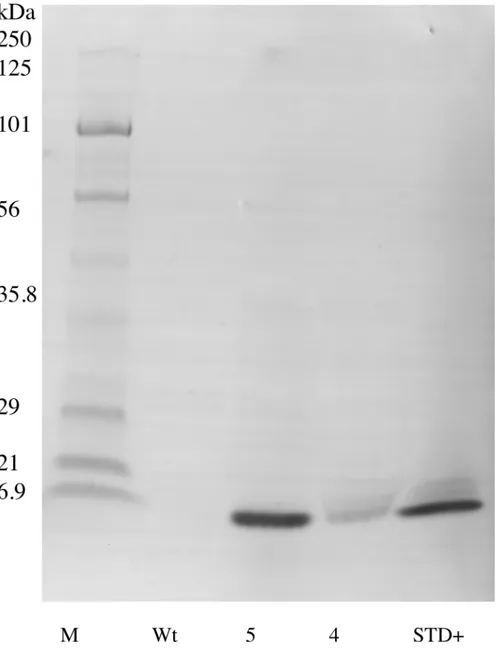
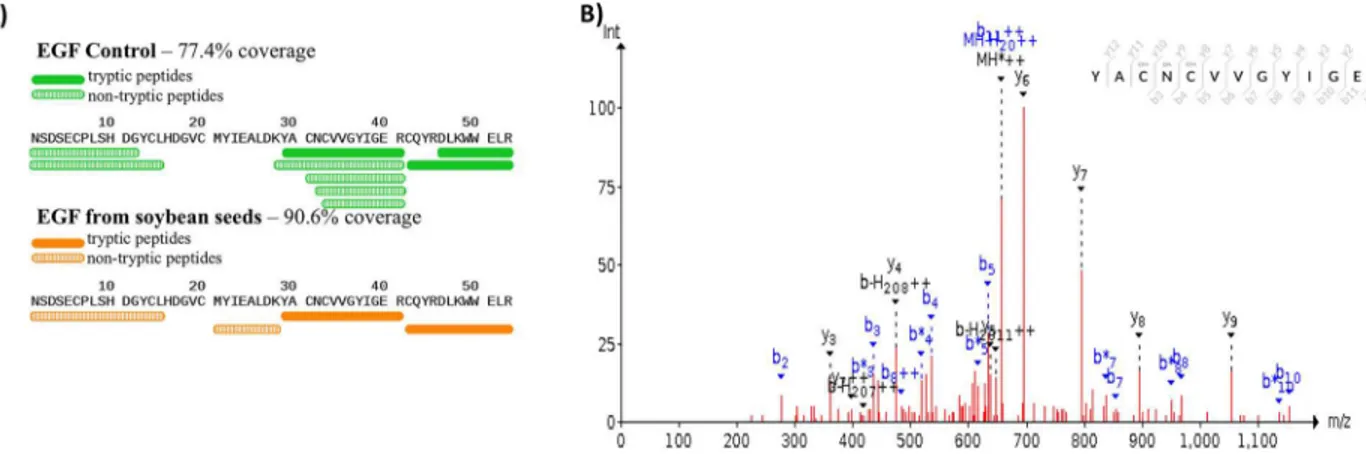
The hEGF soybeans and nontransgenic soybeans were evaluated to determine the biochemi-cal authenticity of the soybean-produced EGF protein. Using 1D SDS/PAGE and parallel immunoblots probed with anti-EGF, the soluble low molecular weight (<10 kDa) seed proteins and the Mr of the soybean-produced hEGF was evaluated. The total protein polypeptide of the hEGF expressing lines appeared to be identical to the standard parental control (Fig 2). Immu-noblots of the 1D SDS/PAGE probed with anti-EGF showed a lack of an immunoreactive band in the nontransgenic soybean seed control and recognized a 6 kDa Mr band in the hEGF expressing Lines 5 and 4. The soybean-produced hEGF has the same apparent Mr as authentic recombinant hEGF fractioned in an adjacent lane (Fig 3). To further assess the soybean-syn-thesized hEGF the seed lysates were enriched in low Mr total proteins and concentrated. The crude low Mr proteins were reduced, alkylated, and cleaved with trypsin prior to analysis by mass spectrometry. The resulting data was queried with the hEGF sequence and exact matches for peptides encompassing the majority of the sequence of the complete mature hEGF protein were obtained (Fig 4). Together the data shows that transgenic soybeans successfully produced and accumulated hEGF that is the correct Mr, is immunoreactive with antibodies directed at
Fig 1. (A) Schematic diagram of seed-specific gene expression cassette to direct ShEGF. Synthetically produced codon-optimized hEGF gene with an ER signal added to the amino-terminus driven by glycinin regulatory elements was transformed via biolistics into somatic soybean embryos. (B) ELISA quantification for both the detection and amount of hEGF in total soluble dry seed protein extract from 7 ShEGF transgenic soybean lines. Independent homozygous lines, 1, 3, 4, 5, 6, 11, 13 were detected to contain hEGF up to 129μg EGF/g seed compared to undetectable amounts in nontransgenic control (Wt). Values shown are mean +/- standard error (n = 3).
authentic EGF in both ELISA and immunoblot assay, and that a majority mass spectrometry of fragments of the soybean-produced hEGF match the human EGF sequence.
Soybean-milk is compatible with EGF bioactivity
The delivery of any biopharma product in the context of compositionally complex food pres-ents the potential that the componpres-ents of the food may act to modulate bioactivity. Plant-source foods in particular pose problems because plant tissues often possess a wide range of intrinsic biologically active components including proteins and natural products. The natural products of food could mask or enhance the effects of an expressed biopharma product. To evaluate the potential of EGF activity in soymilk delivery commercial recombinant human EGF (rhEGF) was added as a supplement to soymilk and the intrinsic activity of the EGF was tested with a HeLa cell assay.Fig 5shows the effects of soymilk on the display of the EGF recep-tor (EGFR) on Hela cells and the effect of commercial rhEGF supplement to soymilk. Soymilk does not modify the display of EGFR on Hela cells showing that soymilk alone is biologically inactive. The binding of EGF to EGFR results in the decrease of displayed EGFR as it is inter-nalized into the HeLa cells. Hela cells treated with commercially available recombinant rhEGF-supplemented soymilk display the same decrease in EGFR as cells treated with rhEGF in media without soymilk. Parallel time-course experiments show that the effect of rhEGF binding to EFGR is rapid with a reduction of displayed EFGR occuring within 5 min of treatment and continuing out to at least 30 min (data not shown). Together these assays show that soymilk has no apparent negative bioactivity with respect to both the binding of commercial rhEGF to the HeLa cell EGFR or the viability of the HeLa cells over the course of the assay.
Soybean-synthesized hEGF is bioactive
To assess the bioactivity of soybean-produced hEGF, samples were prepared from both ShEGF transgenic soybean lines and nontransgenic controls that were used to stimulate HeLa cells to
Fig 2. Analysis of total soluble protein by one-dimensional gel electrophoresis of hEGF expressing transgenic soybean seeds.Proteins from 3 independent homozygous EGF transgenic soybean lines (3, 4, 5) were extracted and compared to seed extracts from nontransgenic (Wt) and commercially available hEGF standard (STD+). M marker, kDa kilobases.
induce EGFR internalization, degradation and phosphorylation. In results shown inFig 5, soy-bean-produced hEGF induces the internalization, degradation and phosphorylation of EGFR that is indistinguishable from the bioactivity of commercial rhEGF delivered in control sam-ples. In contrast, samples prepared from control nontransgenic soybeans exhibited no apparent bioactivity showing the degradation and phosphorylation of EGFR is the result of EGF binding of either commercial rhEGF added to the media or from the hEGF produced by the transgenic soybeans. Together these results show that nontransgenic soybean seeds have no intrinsic EGF-mimic activity able to induce EGFR degradation or phosphorylation, while soybeans pro-ducing hEGF have identical activity in comparison to commercial rhEGF.
Fig 3. Immunoblot of enriched small molecular weight soluble protein extracted from dry transgenic ShEGF soybean seeds.Protein extracts from two independent homozygous lines (5 and 4) are compared to both nontransgenic (Wt) and commercially available EGF standard (STD +). EGF was detected using an EGF specific antibody and indirect secondary antibody coupled to alkaline phosphatase. M marker; kDa kilodalton.
Fig 4. Mass spectroscopy data to detect the presence of EGF peptides in transgenic EGF soybean seeds.(A) Coverage of peptides detected in both commercially available EGF (green) and from transgenic soybean seeds (orange) using both trypsin (solid) and non-trypsin peptides (hatched). (B) Raw spectra data depicting the amino acid sequence CNCVVGYUGER detected from a low molecular weight enriched soluble dry seed protein extract from EGF transgenic soybean.
doi:10.1371/journal.pone.0157034.g004
Fig 5. Soybean produced EGF displayed comparable bioactivity to commercially available EGF.Panel A. Soybean produced hEGF induces a rapid phosphorylation of Hela cell EGFR. Serum free media (SF) and SF media with soymilk alone does not induce EGFR phosphorylation and degradation. Soymilk from seeds producing ShEGF added at different concentrations (0.1, 0.05, 0.025μg/ml) induced concentration-dependent EGFR degradation comparable to the effect of rhEGF. Serum free media and serum free media with nontransgenic soybean soymilk (negative controls) showed no effect on inducing pEGFR. In contrast soymilk from ShEGF soybeans given at different concentrations (0.1, 0.05, 0.025μg/ml) induced pEGFR comparable to control rhEGF. pAKT indicates the functional activation of EGFR. Lamin B1 was used as a loading control. Panel B. Exogenous commercial rhEGF and ShEGF induces an internalization and degradation of EGFR in Hela cells shown as a decrease in abundance assayed by immunoblot. The results shown demonstrate that soymilk alone has no intrinsic bioactivity with respect to EGFR abundance. The rhEGF is not degraded in soymilk over 24 hours having the same bioactivity as control recombinant rhEGF.— Ctrl- SF media alone. Soy EGF and rhEGF are at 0.1μg/ml. Lamin B1 was used as a loading control. Panel C. Shown is an immunohistochemical assay of Hela cells showing that ShEGF induces internalization of the EGFR comparable to that from control rhEGF. In C, the cells were first treated with soy/EGF or human EGF for 6 hours, fixed and then immunostained with EGFR antibody overnight. EGFR shows red staining while nucleus was stained by DAPI and shows blue staining.
Synthesis of hEGF does not affect overt soybean seed composition
In developing a food-based delivery platform for biopharma it is important to address the question of whether there are significant collateral consequences in seed composition resulting from the genetic modification. Ideally a consumption plant biotechnology platform, such as soymilk, should be fully equivalent to the standard type other than the intended modification. Seeds in general, including soybeans, possess an inventory of bioactive proteins and small mol-ecules that will affect the metabolism of consumers in both advantageous and disadvantageous manner. For soybeans some of the relevant molecules are allergens, anti-metabolite proteins, and small molecules especially isoflavones. To test for potential collateral composition in the hEGF-producing soybeans, the ShEGF transgenic and nontransgenic control soybeans were analyzed by non-targeted proteomics and metabolomics. Among the significant proteins iden-tified include various well-documented allergens and anti-metabolite proteins. A comparison of standard soybeans with hEGF-producing soybean lines showed that there was no significant difference (p = .01) between nontransgenic control and ShEGF transgenic soybeans aside from the targeted production of hEGF for any other proteins of concern. This data is available in PRIDE partner repository with the dataset identifier PXD003326 and 10.6019/PXD003326. Non-targeted small molecule metabolomics was used to conduct a parallel analysis of the nontransgenic and hEGF soybeans. Again there were insignificant differences between non-transgenic soybean seeds and the ShEGF non-transgenic seeds (Fig 6) with one notable exception. Soybean highly regulates sulfur availability and its allocation into protein. From a nutritional perspective soybean is considered a somewhat sulfur deficient crop. There have been a number of biotechnology experiments to increase sulfur content be either modifying assimilation and biosynthesis pathways leading to methionine or over-expressing high-methionine proteins such as Maize zeins. Modifying sulfur by pathway or competition has an effect on sulfur-responsive proteins including the Bowman-Birk trypsin inhibitor (BBI) and beta chain of the storage protein conglycinin. EGF is a high sulfur content protein that broadly mimics BBI as a small globular protein synthesized by the ER and presumptively competing for sulfur amino acid charge tRNA. Expressing hEGF in soybean has an effect on metabolites involved in sulfur amino acid metabolism that is consistent with producing a protein of EGF’s composition. A complete dataset of all metabolite abundance of the standard and hEGF-expressing lines is available as an on-line spreadsheet (S1 Table). Among the assayed molecules of particular note is the soybean molecule Genistein, an isoflavone that has been shown to affect the activity of tyrosine phosphatase in the signal cascade associated with EGF signaling [43–46]. Genistein levels were determined to be the same in both the nontransgenic and hEGF-expressing soybean lines. This too demonstrates that the expression of hEGF in soybeans does not produce any incidental collateral consequences of concern for its potential therapeutic use.
Discussion
Soybean seed platform for biopharma
Fig 6. Relative proportion of non-targeted metabolites detected in soybean seeds shown as amount in EGF transgenic compared to nontransgenic (Wt).Complete list of non targeted metabolites quantitated in samples inS1 Table.
is to develop a biopharma platform that is broadly acceptable for food and feed delivery but can be lightly processed to preserve bioactivity and can be massively scaled to maximize the distribution potential of the product. Soybean is a potentially useful biopharma platform that could have broad application in both food and feed end uses [29,30,56,57]. Soybean has been demonstrated as a platform to produce heterologous proteins at a standard that far exceeds the levels typically needed for biopharma [31]. Soybeans can be used to produce both soymilk and formula for potential delivery to human infants or children as well as for production animals such as swine and calves. Soybean can also be used to produce protein concentrates for inclu-sion in industrial food and feed or more simply as protein aggregates as tofu. Soybean produc-tion is efficient and economic that can be massively scaled if needed. Recently developed technology makes it feasible to increase the amount of recombinant protein product by silenc-ing and exchange with a storage protein(s) [31,58,59]. As a platform, soybean is an industrial crop with vast majority of its total production being directed toward products including pro-cessed food, protein used as animal feed, and its oil for food, feed, fuel, and chemical feedstock uses. Many of the goals of further enhancing and modifying soybeans are largely directed at improving its utilization for industry products rather than direct food use. As a biopharma platform to produce soymilk derived products soybean seeds can be stored for years anticipat-ing future needs while retainanticipat-ing the potential to be rapidly processed into formula/milk or tofu using adaptations of traditional technology in use for over a millennium.
Soybeans function as a bioreactor to produce hEGF at a potentially
therapeutic level
Soybeans like many other seeds produce an array of intrinsic small globular proteins with second-ary disulfide bonds accumulated at relatively high levels (>1% of total proteins). Soybean in partic-ular accumulates the Bowman-Birk trypsin inhibitor that is 8.5 kDa with 3 intra-chain disulfide bonds [60]. This suggests that soybean seeds are optimized as a potential bioreactor to produce and store proteins like EGF, a 6.9 kDa protein with 3 intra chain disulfide bonds paralleling intrinsic seed proteins. In a predecessor experiment a mutant inactive BBI was expressed in transgenic soy-beans showing that alternate small proteins can be expressed in soybean [60]. Expression of a con-struct encoding ShEGF regulated by the soybean seed storage protein promoter results in the accumulation of hEGF at>100μg /gm of dry soybean seed, a level to be many fold over the esti-mated therapeutic requirements of 50μg/kg weight of treated individual [61]. Soybean-produced hEGF appears to be completely comparable to authentic hEGF in its Mr, immunoreactivity with specific antibodies, correspondence of fragment sequence in mass spectrometry assay, and in bioac-tivity inducing the internalization, degradation and phosphorylation of EFGR. Together the results shown demonstrate that soybean seeds will produce hEGF at proto-therapeutic levels and the derived hEGF from these seeds are bioactive for EGF activity in a model HeLa cell assay.
The expression of hEGF in soybean has little collateral impact on seed
composition
regulated in the US under FALPA (the 2004 Food and Allergen Labeling Protection Act) and unintended alterations of any of the known seed allergens or anti-nutritional proteins can be of concern. Similarly the non-targeted metabolomics of the soybean seeds showed a significant lack of alteration of the small molecule profile in response to hEGF accumulation. Among the molecules assessed the lack of change in Genistein is among the most significant as this isofla-vonoid has been shown to have activity with tyrosine phosphatase that is in the signal cascade of animal and human cells that includes EGF/EGFR signaling [43,44,70]. In the HeLa cell assessments there was no synergistic effect of standard soybean milk and authentic EGF on EGFR activity indicating that the identical genistein concentration in the standard and hEGF expressing soybeans is below the threshold of effect in the assays conducted. The one signifi-cant alteration in the small molecule profile was in methionine-related metabolism. EGF is a sulfur rich protein containing three disulfide bonds that has some general resemblance to the soybean Bowman-Birk inhibitor. Soybean is a relatively sulfur deficient crop and much effort has been made to increase its sulfur amino acid content by either the co-expression of sulfur-rich proteins such as zeins [71] or by increasing the sulfur flux by altering the sulfur amino acid pathways [72–74]. These studies have shown that within limits the increase of a sulfur sink such as expressing a high-sulfur content protein will collaterally induce modest increases in sulfur amino acid source. The results of increases in sulfur amino acid metabolites accompa-nying hEGF expression in soybean is in accord with these prior experiments. Together the results of the non-targeted proteome and metabolome assessments show that converting soy-bean into a prototype biopharma delivery platform of hEGF does not result in any adverse alterations of the soybean seed’s composition.
Soybean-sourced formula could address NEC and other
epidermal-related disorders
Soybeans could be used to produce biopharma products that might be delivered as milk or for-mula. As a test of this concept human epidermal growth factor (hEGF) has been produced in soy-beans to potentially address the devastating disease of neonatal necrotizing enterocolitis. This is a disease of premature infants of low birth weight. These infants have underdeveloped organs including the intestinal tract. The resulting gangrenous infection is treated by emergency surgery to remove dead portions of the intestinal tract that even under most optimistic situations has a high mortality rate and high cost of treatment. An alternative approach is to proactively treat infants at risk immediately post-partum to attempt to improve the integrity and maturity of the lining epithelial cells. The bioactivity results with model HeLa cells shows that hEGF can be pro-duced and accumulated in soybean seeds and as crude soy-milk lysate is capable of stimulating a response from the EGF receptor (EGFR) that occurs on epidermal surfaces such as the intestinal tract. Soybean-produced hEGF has potential other applications in cosmetics, burn and injury treatment, stimulating improved adaptation of the bowel to massive intestinal loss.
Supporting Information
S1 Table. Non-targeted metabolome set.
(XLSX)
Author Contributions
References
1. Martin J, Hamilton B, Ventura S, Osterman JK, Wilson EC, Mathews TJ. Births: final data for 2010. Natl Vital Stat Rep 2012; 61: 1–100.
2. Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’risk of necrotizing enterocolitis or death. J Perinatology. 2009; 29: 57–62.
3. Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996; 43: 409–432. PMID:8614608
4. Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine year experience: II Outcome assessment. Am J Dis Child; 1981; 135:608–611. PMID:7246487
5. Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999; 117: 1043–1050. PMID:10535866
6. Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009; 3: 944– 954. doi:10.1038/ismej.2009.37PMID:19369970
7. Mai V, Young CM, Ukhanova M. Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing entercolitis. PLoS One. 2011; 6: e20647. doi:10.1371/journal.pone.0020647PMID: 21674011
8. Alderberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009; 98: 229–238. doi:10.1111/j.1651-2227.2008.01060.xPMID:19143664
9. Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev. 2012; 88: S41–S49. doi:10.1016/j.earlhumdev.2011.12.027PMID:22284985
10. Carpenter G. Epidermal growth factor: biology and mechanism of action. Birth Defects Orig Artic Ser. 1980; 16: 61–72. PMID:6260263
11. Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O-Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007; 27: 428– 433. PMID:17443195
12. Hofmann GE, Abramowicz JS. Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta obstetricia et gynecologica Scandinavica 69.3 1990; 217–221.
13. Dvorak B, Fituch CC, Williams CS, Hurst NM, Schanler RJ. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr Res. 2003; 54: 15–19. PMID: 12646719
14. Cohen S, Carpenter G. Human epidermal growth factor: isolation and characterization and biological properties. PNAS. 1975; 72: 1317–1321. PMID:1055407
15. Kasselberg AG, Orth DN, Gray ME, Stahlman MT. Immunocytochemical localization of human epider-mal growth factor/urogastrone in several human tissues. J Histochem Cytochem. 1985; 33: 315–322. PMID:3884705
16. Konturek SJ, Brzozowski T, Majka J, Dembinski A, Slomiany A, Slomiany BL. Transforming growth fac-tor alpha and epidermal growth facfac-tor in protection and healing of gastric mucosal injury. Scandinavian J Gastroenterology. 1992; 27: 649–655.
17. Chaet MS, Arya G, Ziegler MM, Warner BW. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg. 1994; 29: 1035–1039. PMID:7965502
18. Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995; 376: 337–341. PMID:7630400
19. Erwin CR, Helmrath MA, Shin CE, Falcone RA, Jr., Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol. 1999; 277: G533–G540. PMID:10484377
20. O'Brien DP, Nelson LA, Williams JL, Kemp CJ, Erwin CR, Warner BW. Selective inhibition of the epider-mal growth factor receptor impairs intestinal adaptation after sepider-mall bowel resection. J Surg Res. 2002; 105: 25–30. PMID:12069497
21. Lucas A, Cole TJ. Breast milk and neonatal necrotizing enterocolitis. Lancet. 1990; 336:1519–1523. PMID:1979363
23. Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliams DL, Dominquez JA, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002; 282: G156–G164. PMID:11751169
24. Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastro-intestinal Liver Physiol 2006; 291; G938–G949.
25. Collins JB, Georgeson KE, Vicente Y, Kelly DR, Figuerona R. Short bowel syndrome. Semin Pediat Surg. 1995; 4: 60–72; discussion 72–3.
26. Warner BW, Ziegler MM. Management of the short bowel syndrome in the pediatric population. Pediatr Clin N Am. 1993; 40: 1335–1350.
27. Spencer AU, Kovacevich D, McKinney-Barnett M, Hair D, Canham J, Maksym C, et al. Pediatric short-bowel syndrome: the cost of comprehensive care. Am J Clin Nutr. 2008; 88: 1552–1559. doi:10.3945/ ajcn.2008.26007PMID:19064515
28. Arntzen C. Plant made pharmaceuticals: from edible vaccines to Ebola therapeutics. Plant Biotechnol J. 2015; 13: 1013–1016. doi:10.1111/pbi.12460PMID:26345276
29. Herman EM, Schmidt MA. Towards using biotechnology to modify soybean seeds as protein bioreac-tors. In, Recent Advancements in Plant Expression in Crop Plants. Eds Azhakanandam, Silverstone, Daniell and Davey. 2015. pp 193–212.
30. Moravec T, Schmidt MA, Herman EM, Woodford-Thomas T. Production ofEscherichia coliheat labile toxin (LT) B subunit in soybean seed and analysis of its immunogenicity as an oral vaccine. Vaccine. 2007; 25: 1647–1657. doi:10.1016/j.vaccine.2006.11.010PMID:17188785
31. Schmidt MA, Herman EM. The Collateral Protein Compensation Mechanism Can Be Exploited To Enhance Foreign Protein Accumulation In Soybean Seeds. Plant Biotechnol J. 2008; 6: 832–842. doi: 10.1111/j.1467-7652.2008.00364.xPMID:18694455
32. Schmidt MA, Herman EM. A RNAi knockdown of soybean 24 kda oleosin results in the formation of micro-oil bodies that aggregate to form large complexes of oil bodies and ER containing caleosin. Mol Plant. 2008; 1: 910–924. doi:10.1093/mp/ssn049PMID:19825592
33. Schmidt MA, Parrott WA, Hildebrand DF, Berg RH, Cooksey A, Pendarvis K, et al. Transgenic soybean seeds accumulatingβ-carotene exhibit the collateral enhancements of high oleate and high protein content traits. Plant Biotechnol J. 2015; 13: 590–600. doi:10.1111/pbi.12286PMID:25400247
34. Schmidt MA, Tucker DM, Cahoon EB, Parrott WA. Towards normalization of soybean somatic embryo maturation. Plant Cell Rep. 2004; 24: 383–391.
35. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utiliz-ing the principle of protein dye bindutiliz-ing. Anal Biochem. 1976; 72: 248–254. PMID:942051
36. Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008; 24: 2534–2536. doi:10.1093/bioinformatics/ btn323PMID:18606607
37. Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004; 20: 1466–1467. PMID:14976030
38. Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, et al. Open mass spectrometry search algorithm. J Proteome Res. 2004; 3: 958–964. PMID:15473683
39. Guo J, Longshore S, Nair R, Warner BW. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem. 2009; 284:134–40. doi:10.1074/jbc.M806133200PMID:18981186
40. Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, et al. The Proteomics Identifica-tions (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013; 41(D1): D1063–9.
41. George-Nascimento C, Gyenes A, Halloran SH, Merryweather J, Valenzuela P, Steimer KS, et al. Characterization of recombinant human epidermal growth factor produced in yeast. Biochemistry. 1988; 27: 797–802. PMID:3280026
42. Clare JJ, Romanos MA, Rayment FB, Rowedder JE, Smith MA, Payne MM, et al. Production of mouse epidermal growth factor in yeast: high level secretion using Pichia pastoris strains containing multiple gene copies. Gene. 1991; 105: 205–212. PMID:1937016
43. Yang EB, Wang DF, Mack P, Cheng LY. Genistein, a tyrosine kinase inhibitor, reduces EGF-induced EGF receptor internalization and degradation in human hepatoma HepG2 cells. Biochem Biophys Res Com. 1996; 224: 309–317. PMID:8702388
45. Lin AHY, Leung GPH, Leung SWE, Vanhoutte PM, Mon PYK. Genistein enhances relaxation of the spontaneously hypertensive rat aorta by transactivation of epidermal growth factor receptor following binding to membrane estrogen receptors-αand activation of G protein-coupled, endothelial nitric acid synthase-dependent pathway. Pharm Res. 2011; 63: 181–189.
46. Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR, Wang Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. Plos One. 2011; 6(5): e20034. doi:10.1371/journal.pone.0020034PMID:21603581
47. Ma JK, Drake PM, Christou P. The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet. 2003; 4: 794–805. PMID:14526375
48. Stoger E, Ma JK, Fischer R, Christou P. Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotech. 2005; 16: 167–173. PMID:15831382
49. Vitale A, Pedrazzini E. Recombinant Pharmaceuticals from Plants: The Plant Endomembrane System as Bioreactor. Mol Interv. 2005; 5: 216–225. PMID:16123536
50. Howard JA. Commercialization of biopharmaceutical and bioindustrial proteins from plants. Crop Sci. 2005; 45: 468–472.
51. Arakawa T, Chong DK, Langridge WH. Efficacy of a food plant-based oral cholera toxinβsubunit vac-cine. Nat Biotechnol. 1998; 16: 292–297. PMID:9528012
52. Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitisβ surface antigen expressed in transgenic plants. Proc Nat Acad Sci USA. 2001; 98: 11539–11544. PMID:11553782
53. Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine. 2004; 22: 4385–4389. PMID:15474732
54. Elkholy SF, Ismail RM, Bahieldin A, Sadik AS, Madkour MA. Expression of hepatitis B surface antigen (HBsAg) gene in transgenic banana (Musa Sp). Arab J Biotechnol. 2009; 12: 291–302.
55. Pogrebnyak N, Golovkin M, Andranov V, Spitsin S, Smirnov Y, Egolf R, et al. Severe acute respiratory syndrome (SARS) S protein production in plants: development of recombinant vaccine. Proc Nat Acad Sci USA. 2005; 102: 9062–9067. PMID:15956182
56. Piller KJ, Clemente TE, Jun SM, Petty CC, Sato S, Pascual DW, et al. Expression and immunogenicity of an Escherichia coli K99 fimbriae subunit antigen in soybean. Planta. 2005; 222: 6–18. PMID: 15609046
57. Bost K, Piller K. Protein expression systems: why soybean seeds?”in Soybean—Molecular Aspects of BreedingISBN: 978-953-307-240-1, ed Sudaric A. ( Rijeka: InTech). 2011; pp. 1–18. doi:10.5772/ 15376
58. Kinney AJ, Jung R, Herman EM. Cosuppression of theα-subunits ofβ-conglycinin in transgenic soy-bean seeds induces the formation of endoplasmic reticulum-derived protein bodies. Plant Cell. 2001; 13: 1165–1178. PMID:11340189
59. Schmidt MA, Barbazuk WB, Stanford M, May G, Song Z, Hong W, et al. Silencing of soybean seed stor-age proteins results in a rebalanced protein composition preserving seed protein content without major collateral changes in the metabolome and transcriptome. Plant Physiol. 2011; 156: 330–345. doi:10. 1104/pp.111.173807PMID:21398260
60. Livingston D, Beilinson V, Kalayaeva M, Schmidt MA, Herman EM, Nielson NC. Reduction of protease inhibitor activity by expression of a mutant Bowman-Birk gene in soybean seed. Plant Mol Biol. 2007; 64:397–408. PMID:17429741
61. Shin CE, Helmrath MA, Falcone R Jr., Fox JW, Duane KR, Erwin CR, et al. Epidermal growth factor augments adaptation following small bowel resection: optimal dosage, route, and timing of administra-tion. J Surg Res. 1998; 77: 11–16. PMID:9698525
62. Cunha NB, Murad AM, Ciprano TM, Araujo ACG, Aragao FJL, Leite A, et al. Expression of functional recombinant human growth hormone in transgenic soybean seeds. Transgenic Res 2011; 20: 811– 826. doi:10.1007/s11248-010-9460-zPMID:21069461
63. Phillip R, Darnowski DW, Maughan PJ, Vodkin LO. Processing and localization of bovineβ-casein expressed in transgenic soybean seeds under control of a soybean lectin expression cassette. Plant Sci. 2001; 161: 323–335. PMID:11448763
64. Shi J, Wang H, Schellin K, Li B, Faller M, Stoop JM, et al. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol. 2007; 25: 930–937. PMID: 17676037
66. Venegas-Caleron M, Sayanova O, Napier JA. An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega 3 long chain polyunsaturated fatty acids. Prog Lipid Res. 2010; 49: 108– 119. doi:10.1016/j.plipres.2009.10.001PMID:19857520
67. Cunha NB, Araujo ACG, Leite A, Murad AM, Vianna GR, Rech E L. Correct targeting of proinsulin in protein storage vacuoles of transgenic soybean seeds. Genet. Mol. Res. 2010; 9: 1163–1170. doi:10. 4238/vol9-2gmr849PMID:20589613
68. Ding SH, Huang LY, Wang YD, Sun HC, Xiang ZH. High-level expression of basic fibroblast growth fac-tor in transgenic soybean seeds and characterization of its biological activity. Biotechnol. Lett. 2006; 28: 869–875. doi:10.1007/s10529-006-9018-6PMID:16786271
69. Leite A, Kemper EL, da Silva MJ, Luchessi AD, Siloto RMP, Bonaccorsi ED, et al. Expression of cor-rectly processed human growth hormone in seeds of transgenic tobacco plants. Mol Breeding. 2000; 6: 47–53.
70. Croisy-Delcey M, Croisy A, Mousset S, Letourneur M, Bisagni E, Jacquemin-Sablon A, et al. Genistein analogues: effects on epidermal growth factor receptor tyrosine kinase and on stress-activated path-ways. Biomed Pharmacother. 1997; 51:286–294. PMID:9309250
71. Dinkins RD, Reddy R, Meurer CA, Yan B, Trick H, Thibaud-Nissen F, et al. Increased sulfur amino acids in soybean plants overexpressing the maize 15 kD zein protein. In Vitro Cell. Dev. Biol. Plant. 2001; 37: 742–747. doi:10.1007/s11627-001-0123-x
72. Avraham T, Badani H, Galili S, Amir R. Enhanced levels of methionine and cysteine in transgenic alfalfa (Medicago sativaL.) plants over-expressing theArabidopsiscystathionineγ-synthase gene. Plant Bio-tech. J. 2005; 3: 71–79. doi:10.1111/j.1467-7652.2004.00102.x
73. Kim WS, Chronis D, Juergens M, Schroeder AC, Hyun SW, Jez JM, et al. Transgenic soybean plants overexpressingO-acetylserine sulfhydrylase accumulate enhanced levels of cysteine and Bowman-Birk protease inhibitor in seeds. Planta. 2012; 235: 13–23. doi:10.1007/s00425-011-1487-8PMID: 21805150