Brazilian
Journal
of
OTORHINOLARYNGOLOGY
www.bjorl.org
ORIGINAL
ARTICLE
Spontaneous
healing
of
the
tympanic
membrane
after
traumatic
perforation
in
rats
夽
,
夽夽
Marcos
Miranda
de
Araújo
a,∗,
Adriana
Andrade
Batista
Murashima
a,
Vani
Maria
Alves
b,
Maria
Celia
Jamur
b,
Miguel
Angelo
Hyppolito
aaDepartmentofOphthalmology,OtorhinolaryngologyandHeadandNeckSurgery,FaculdadedeMedicinadeRibeirãoPreto,
UniversidadedeSãoPaulo(USP),RibeirãoPreto,SP,Brazil
bDepartmentofCellandMolecularBiologyandPathogenicBioagents,FaculdadedeMedicinadeRibeirãoPreto,Universidadede
SãoPaulo(USP),RibeirãoPreto,SP,Brazil
Received9August2013;accepted22March2014 Availableonline11June2014
KEYWORDS
Tympanicmembrane
perforation;
Woundhealing;
Histology
Abstract Themostcommonetiologiesoftympanicmembraneperforationareinfectionsand trauma.
Objective:Theobjectiveofthepresentstudywastoassessthehealingoftraumatictympanic membraneperforationinrats.
Methods:ThetympanicmembranefrommaleWistarratswasperforatedintheanteriorand posterior portions tothe handleofthe malleus. Five tympanic membraneswere evaluated 3 days after tympanic perforation; 5after 5days; 5 after 7 days; 3after 10 days; and4 after14days.Thetympanicmembranesweresubmittedtohistopathologicalevaluationafter hematoxylin---eosinstaining.
Results:Tympanicmembraneclosureoccurredatabout7---10daysafterinjuryandthehealing processwas completebyday14.Theproliferativeactivityoftheouterepitheliallayerwas presentclosetothehandleofthemalleusandtothetympanicannulus.
Conclusion:Thespontaneoushealingprocessofthetympanicmembranestartsfromtheouter epitheliallayer,withlaterhealingofthelaminapropriaandthemucosallayer.
© 2014Associac¸ãoBrasileira de Otorrinolaringologiae CirurgiaCérvico-Facial. Publishedby ElsevierEditoraLtda.Allrightsreserved.
夽 Pleasecitethisarticleas:AraújoMM.MurashimaAA,AlvesVM,JamurMC,HyppolitoMA.Spontaneoushealingofthetympanicmembrane
aftertraumaticperforationinrats.BrazJOtorhinolaryngol2014;80:330---8.
夽夽
Institution:DepartmentofOphthalmology,OtorhinolaryngologyandHeadandNeckSurgery,FaculdadedeMedicinadeRibeirãoPreto, UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil.
∗Correspondingauthor.
E-mails:drmarcosorl@usp.br,drmarcosorl@hotmail.com(M.M.Araújo).
http://dx.doi.org/10.1016/j.bjorl.2014.05.014
PALAVRAS-CHAVE
Perfurac¸ãoda
membranatimpânica;
Cicatrizac¸ão;
Histologia
Cicatrizac¸ãoespontâneadamembranatimpânicaapóssuaperfurac¸ãotraumática: estudoexperimentalemratos
Resumo As causas mais comuns de perfurac¸ões de membrana timpânica são infecc¸ões e trauma.
Objetivos: Avaliaroreparocicatricialdeperfurac¸õestraumáticasdamembranatimpânicaem ratos.
Método: AmembranatimpânicaderatosWistarmachos foramperfuradasnasporc¸ões ante-rioreposterioraocabodomartelo.Cincomembranastimpânicasforamavaliadas3diasapós perfurac¸ãotimpânica;5após5dias;5após7dias;3após10dias;e4após14dias.Asmembranas timpânicasforamsubmetidas àavaliac¸ãohistopatológicaapóscolorac¸ão com hematoxilina-eosina.
Resultados: O fechamentoda membrana timpânica ocorreu em torno de 7 a 10 dias após perfurac¸ãotraumática,eoprocessodecicatrizac¸ãoestavacompletono14◦ dia.Aatividade proliferativa dacamadaepitelial externafoi identificadapróxima ao cabodomartelo eao ânulustimpânico.
Conclusão:O processode cicatrizac¸ãoespontânea damembrana timpânica se iniciacom a camadaepitelialexterna,composteriorcicatrizac¸ãodalâminaprópriaedacamadamucosa. ©2014Associac¸ãoBrasileiradeOtorrinolaringologiaeCirurgiaCérvico-Facial.Publicado por ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
The tympanicmembrane (TM) is an anatomical structure
thatseparatestheouterearfromthemiddleear.TheTMis
responsibleforsoundamplificationandtransmissionthrough
theossicularchaintotheovalwindowandvestibularramp,
inadditiontotheprotectionoftheroundwindowandthe
tympanicramp.1
The ultrastructuralanatomyof TMconsistsof 3layers: theouterlayer,ofepithelial(ectodermal)origin;the mid-dlelayerorlaminapropria,ofmesodermalorigin;andthe innerlayer,ofendodermalorigin,comprisingthemiddleear mucosa.2
The outer layer consists of keratinized stratified squa-mousepithelium.3---5
The middle layer or lamina propria consists of loose subepithelial connective tissue, organized dense connec-tive tissueandsubmucosal loose connectivetissue. Loose subepidermalandsubmucosalconnectivetissueconsistsof looselyarrangedcollagenfibers,fibroblasts,nervefibersand capillaries. The dense connective tissue consists of more externallyorganizedradialcollagenfibersandmore inter-nally organized circular collagen fibers.2---5The inner layer
consistsofsimplecolumnarepithelial tissue,whichis con-tinuouswiththemiddleearmucosa.2---6
ThemostcommonetiologiesofTMperforationsareotitis mediaandtrauma.7TraumaticTMperforationshavea78.7%
rateofhealing.8FactorsthatpreventadequaterepairofTM
intheabsenceofinfectionareyettobedefined.7,8
The objective of this study is to develop the two-dimensional studyof scar repair in traumaticTM perfora-tionsinrats.
Method
Theexperimentalstudywascarriedoutwith19maleWistar albinorats(Rattusnorvegicus),weighingonaverage280g
(range270---290g). The study followed the Ethical Princi-plesinAnimalResearchadoptedbytheBrazilianCollegeof AnimalExperimentation(COBEA)andwasapprovedbythe Ethics Committee on Animal Experimentation (CETEA) on 08/27/2007---protocolnumberforuseofanimalsinresearch No.082/2007.
Before the procedure, all animals were anesthetized with intramuscular ketamine hydrochloride (40mg/kg) (Ketamine 50mg/mL, Cristália Laboratory, São Paulo, Brazil)andintramuscularxylazine hydrochloride(5mg/kg) (Dopaser20mg/mL,HertapeCalier, Minas Gerais,Brazil). TheearsofallanimalswereassessedusingaDFVMU-M19 otomicroscope(DFV,RiodeJaneiro,Brazil)beforethe pro-cedure to rule out infection. A total of 19 animals were includedinthestudy,equivalentto23bullaewithnormal TMs.Bullae withinfectionwereexcludedfromthe proce-dure.
Traumatic perforation of the tympanic membrane was performedwitha30mm×0.8mmBDneedle(Becton Dickin-son,NewJersey,USA)anteriorandposteriortothemalleus handle,intheparstensaregionoftheTM(Fig.1).For histo-logicalevaluation,3animalswereeuthanized3daysafter theperforation(5bullae),4animalsafter5days(5bullae), 5animalsafter7days(5bullae),3animalsafter10days(3 bullae)and3animalsafter14days(4bullae).Oneanimal (1bulla)withintactTM wasassessedascontrol.The ani-malswereeuthanizedwithan intraperitonealinjectionof anoverdoseofthiopental(Thionembutal,Abbot,SãoPaulo, Brazil).
0.5 mm
Figure1 Schematicrepresentationoftympanic perforation inthe parstensaregionoftheratTM region,anteriorly and posteriorlytothemalleushandle.
handleasareference.Toallowhistologicalevaluation,the blockswerewornuntiltheyreachedapproximatelythesame perpendicularlevelrelativetothemalleushandle.Samples werestainedwithhematoxylinandeosin.
Histological evaluation was performed with an Olym-pusBX50microscope(OlympusAmerica,Inc.,Pennsylvania, USA),anddigitalhigh-resolutionimageswereacquiredwith aSpotRT3 camera(DiagnosticInstruments,Inc.Michigan, USA).The thicknessesoftheouterepithelial layer,lamina propriaand mucosa were measured using the Image-Pro-Plus® program, release 7.0 (Media Cybernetics Inc., MD,
USA).InTMperforations,thicknessmeasurementswere per-formedatadistanceof50---100mfromtheborderofthe perforation in orderto evaluate thehealing activity near thelesion.9IntheintactTMs,thisassessmentwas
standard-izedatadistanceof450---550mfromthemalleushandle, aregionthatcorrespondstothesiteofthepreviously per-formedTMperforation.
Results
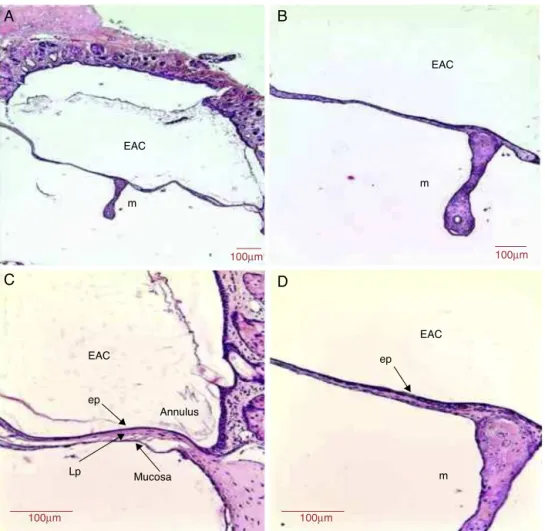
Intacttympanicmembrane---control
ThethicknessoftheTMwasapproximately9.6m,havingas referencethedistanceof500mfromthemalleushandle. At adistance of 250m fromthemalleus handle, theTM thicknesswas 15.8m, andat 250mfrom thetympanic annulus,itsthicknesswas26.6m(Table1).
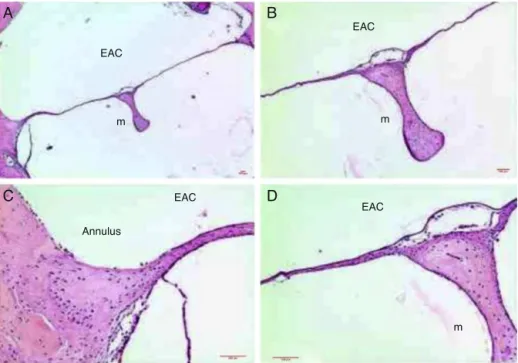
Thestratifiedsquamousepithelialtissueshowedfrom1 to2rowsofflattenedepithelialcellsthroughoutthelength oftheTM(Fig.2).Thethicknessoftheepitheliallayerwas 1.8m at a distance of 500m from the malleus handle (Table1).
This connective tissue present in its middle layer was organized,withoccasional flattenednucleus cells, proba-blyfibroblasts,andorganizedcollagenfibers,withlittleor noresponseofinflammatorycells(Fig.2).
Thecollagenfiberswereconnectedtothemalleus han-dle,andinthetympanicannulusregion,suchfibersformed the fibrocartilaginous ring of the tympanic annulus. Few capillaries without edema or vascular proliferation were locatednearthemalleushandleandwerenotfoundinthe intermediateportionsbetweentheannulusandmalleus han-dle.Thelaminapropriathicknesswas6.3m(Table1).
Themucosallayerhadonerowofflattenedcellsarranged assimplecolumnartissueandexhibitedcontinuitywiththe
EAC
EAC
EAC
EAC
Annulus m
m m
A
C
D
B
Table1 Meanthicknessoftheepithelial,laminapropriaandmucosallayers;andtotalthicknessofthetympanicmembrane (TM)throughoutthestudyondays0(intactTM),3,5,7,10and14.
Timeofeuthanization(days) Epitheliallayer, m(%)
Laminapropria layer,m(%)
Mucosallayer,m (%)
Total,m(100%)
IntactTMa 1.8(18.7%) 6.3(65.6%) 1.5(15.6%) 9.6
IntactTMb 2.6(16.4%) 11.9(75.3%) 1.3(8.2%) 15.8
IntactTMc 2.3(8.6%) 22.3(83.8%) 2(7.5%) 26.6
3daysa 18.9(48.4%) 14.3(36.7%) 5.8(14.9%) 39.0
5daysa 27.1(37.9%) 36.2(50.7%) 8.1(11.3%) 71.4
7daysa 37.5(42.1%) 46.1(51.7%) 5.5(6.1%) 89.1
10daysa 11.2(29.9%) 23.3(62.1%) 3(8.0%) 37.5
14daysa 5.7(27.9%) 12.5(61.3%) 2.2(10.8%) 20.4
a IntheintactTMs,thethicknesswasmeasuredatadistanceof450---550
mfromthemalleushandle.InperforatedTMs,measurements ofthicknesswereperformedatadistanceofbetween50and100mfromtheborderoftheperforation.
b Thethicknessmeasurementswereperformedatadistanceof250
mfromthemalleushandleinanimalswithintactTMs.
c Thethicknessmeasurementswereperformedatadistanceof250
mfromthetympanicannulusinanimalswithintactTMs.
middleearmucosa.Themucosallayerthicknesswas1.5m (Fig.2)(Table1).
Tympanicmembrane3daysaftertraumatic perforation
The mean thickness of TM was approximately 39m (Table1).
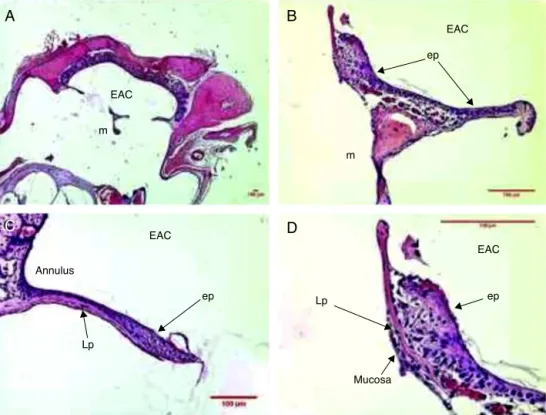
Three days after the traumatic perforation, therewas amoreproliferativeandhyperplasticepitheliallayer,with approximatelythreetofour rowsof epithelial cells, both
nearthemalleushandleandthetympanicannulus(Fig.3). Themeanepitheliallayerthicknesswas18.9m(Table1).
ThemiddlelayeroftheTMshowedthepresenceofcells withbasophilicnucleus,compatiblewithfibroblasts.These occasional disorganized fibroblasts did not overcome the limits of the ruptured collagen fibers in the TM perfora-tion.Therewasedemaintheloosesubepithelialconnective andsubmucosaltissuenearthemalleushandleandinthe tympanicannulus.
Therewasapredominanceofinflammationwith recruit-mentofpolymorphonuclearcellslocatedintheperivascular portion,andloosesubepithelialandsubmucosalconnective
EAC
A
C
B
D
EAC
EAC
EAC m
m
ep
ep ep
Annulus
Mucosa Lp
Lp
Figure3 HistologicalsectionimagesofratTM3daysaftertraumaticperforation,stainedwithHE.EAC,externalauditorycanal; m,malleushandle;ep,epitheliallayer;Lp,laminapropria;mucosa,mucosallayer.Image(A)showsamagnificationof20×;(Band
EAC
A
C
B
EAC
100µm
100µm
100µm
EAC
m
m Lp
Lp
ep
ep
Mucosa
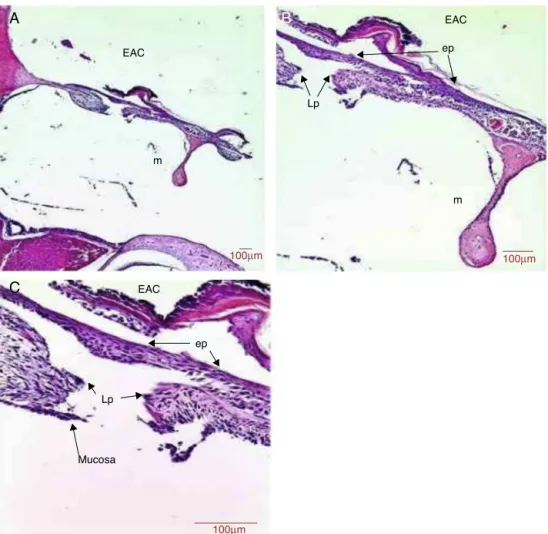
Figure4 HistologicalsectionimagesofratTM5daysaftertraumaticperforation,stainedwithHE.EAC,externalauditorycanal; m,malleushandle;ep,epitheliallayer;Lp,laminapropria;mucosa,mucosallayer.Image(A)showsamagnificationof40×;(B)
100×and(C)400×.
tissue. Blood vessels with plethora or turgescence were present, close to the malleus handle and the tympanic annulusregion(Fig.3).Themeanlaminapropriathickness was14.3m(Table1).
Hyperplasiawasobservedinthemucosaltissuenearthe region of the perforation borders. A row of mucosal tis-suewithhyperplasticcellsandameanthicknessof5.8m (Table1)wasidentified.
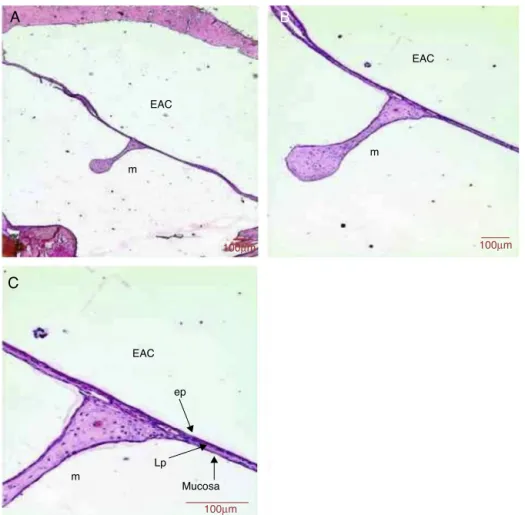
Tympanicmembrane5daysaftertraumatic perforation
Fivedaysaftertheperforation,themeanthicknessofthe TMwasabout71.4m(Table1).
The epithelial layer showed hyperplasia in the region next to the malleus handle and the annulus region. The TMshowed areaswiththreetofiverowsof epithelial tis-sue (Fig.4). There wasformation of an epithelial bridge thatadvancedtowardtheperforationclosureinsomecases (Fig.4). The perforation closure started at the epithelial layer.Themeanthicknessoftheepitheliallayerwas27.1m (Table1).
Inthemiddlelayer,weobservedanincreaseinthe thick-nessoftheTMintheregionadjacenttotheperforationdue
tobasophilicnucleuscells,probablyfibroblasts.Clustersof redbloodcells,indicatingbloodcapillarieswerepresentin theconnectivetissueoftheTM(Fig.4).Themiddle layer thicknesswas36.2m(Table1).
Therewashyperplasiaofmucosaltissuewithtwotothree rowsofmucosalcells(Fig.4).Thethicknessofthemucosal layerwas8.1m(Table1).
Tympanicmembrane7daysaftertraumatic perforation
Seven days after the perforation, the repaired TM had a meanthicknessof89.1m(Table1).
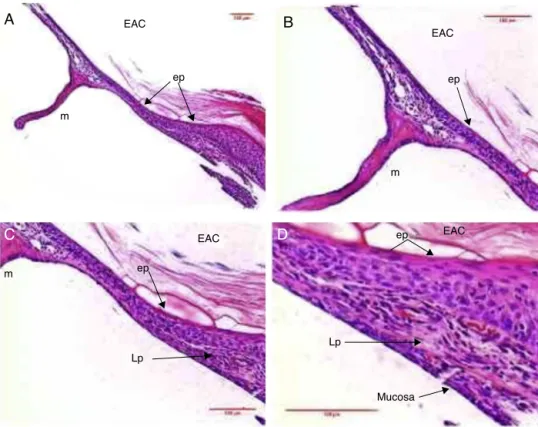
TheepitheliallayeroftheTMshowedhyperplasia,with threetofiverowsthroughout theTMlength. Therewasa moremarkedepithelialreactionintheTMportionmidway betweentheannulusandmalleushandle,attheplacewhere theperforationwasperformed(Fig.5).Themeanthickness oftheepitheliallayerwas37.5m(Table1).
EAC EAC
A
B
C
D
EACEAC
ep
ep ep
ep
Lp m
m
m
Lp
Mucosa
Figure5 HistologicalsectionimagesofratTM7daysaftertraumaticperforation,stainedwithHE.EAC,externalauditorycanal; m,malleushandle;ep,epitheliallayer;Lp,laminapropria;mucosa,mucosallayer.Image(A)showsamagnificationof100×;(B
andC)200×and(D)400×.
asthethickestregionoftheTM,occurredmidwaybetween theannulusandthemalleushandle(Fig.5).Themean thick-nessofthelaminapropriawas46.1m(Table1).
The mucosal layer showed a row of simple hyperpla-sic columnar cells in most of the tympanic membranes (Fig.5).Themeanthicknessofthemucosallayerwas5.5m (Table1).
Tympanicmembrane10daysaftertraumatic perforation
Tendaysaftertheperforation,therepairedtympanic mem-braneshowedthicknessof37.5m(Table1).
Epithelial tissue wasidentified withupto tworowsof cellsinmostoftheTM(Fig.6).Thethicknessofthe epithe-liallayerwas11.2m(Table1).
Thelaminapropriashowedanapparentreductioninthe numberoffibroblastsinallregionsofTM.Suchoccasional fibroblastsshowedamoreorganizedandaggregated distri-bution. There wasa trendof decreased blood capillaries (Fig. 6). The mean thickness of the lamina propria was 23.3m(Table1).
Themucosallayershowedonerowofflattenedmucosal cellsinmostoftheTM(Fig.6).Thethicknessofthemucosal layerwas3m(Table1).
Tympanicmembrane14daysaftertraumatic perforation
Fourteendaysaftertheperforation,therepairedTMhada thicknessof20.4m(Table1).
Theepithelialtissueshoweduptotworowsofflattened epithelialcellsthroughoutthelengthoftheTM(Fig.7).The meanthicknessoftheepitheliallayerwas5.7m(Table1). In the middle layer, basophilic nucleus cells, probably fibroblasts were present, but possiblyat smaller amounts whencomparedtotheshorterperiods (Fig.7). Themean thicknessofthelaminapropriawas12.5m(Table1).
The mucosal layer showed one row of flattened cells throughoutthelengthoftheTM(Fig.7).Themeanthickness ofthemucosallayerwas2.2m(Table1).
Closureofthetympanicperforation
The closure of the tympanic membrane occurred around 7---10daysaftertraumaticperforation,andthehealing pro-cesswascompleteonthe14thday.
Discussion
After the perforation, the healing process of the TM is typically described as occurring in three distinct phases, buttemporallyoverlapping:inflammatory,proliferativeand remodeling.3---7
In experimental skin studies, the inflammatory phase begins immediately after tissue injury and lasts for 4---6 days.10,11 This phase consists of a disarray of blood
EAC
EAC EAC
EAC
m m
m
ep
ep
Lp Mucosa
Annulus
100µm 100µm
100µm
100µm
A
C
B
D
Figure6 HistologicalsectionimagesofratTM10daysaftertraumaticperforation,stainedwithHE.EAC,externalauditorycanal; m,malleushandle;ep,epitheliallayer;Lp,laminapropria;annulus,tympanicannulus;mucosa,mucosallayer.Image(A)showsa magnificationof40×;(B)100×and(CandD)200×.
recruitedtothewound,whilemonocytesarerecruitedafter 48---96h.11
The proliferative phase is classically characterized by epithelialproliferation;proliferationoffibroblastswith col-lagen deposition; and by angiogenesis, with granulation tissueformation.11Theproliferativephaseisusuallypresent
fromday4today14inanexperimentalstudyofskin.11
Onthe3rddayafterthetraumaticperforation,amore proliferative, hyperplastic epithelial layer, with approxi-mately 3---4 rows of epithelial cells, was observed in the TM, both near the malleus handleand close to the tym-panicannulus(Fig.3).After5---7days,theepithelialmitotic activityintensified,withportionsof5rowsofepithelialcells (Figs.4and5).Therewasatendencyforepithelialbridge formationthat advanced inthe direction ofthe tympanic perforationclosure.
Thisproliferativeactivityoftheouterepitheliallayerof TM hasbeen described asoccurring within thefirst hours aftertissueinjury.3,12,13Intheliterature,therearereports
oftheexistenceofepithelialproliferativecentersnearthe malleushandleandthetympanicannulusregion.9,14,15
How-ever,onestudythatdescribedonlyonecenterofepithelial proliferationnearthemalleushandlewasfound.3
In experimental models of skin wound healing, the remodeling phase was described as starting after 8 days
oftissueinjuryandpersistingforafewmonths.Themain molecular events occur in the extracellular matrix of the laminapropria.11
InTM,epithelialremodelingwasobservedfromday10, which remained until the 14th day. The outer epithelial layerbecamethinner,withapproximately2rowsof epithe-lialcells.Inthemiddlelayer,fibroblasts wereobservedin increasingly smaller amounts, showing a flattened shape. Thetendencywasthereductioninthenumberof capillar-iesalongthetympanicmembrane.Themucosallayeralso becamethinner,withonerowofflattenedcellsthroughout thelengthoftheTM.
DuetothemorphologicalcharacteristicsoftheTM,the squamousepitheliumoftheTMouterlayerwouldbeinitially responsiblefortheperforationclosure,withtheformation ofanepithelialbridgeoverthelesionandonlysubsequently therewouldbetherestorationofthefibroustissueofthe middlelayerandmucosallayertissue(Fig.4).7
TheepitheliallayerwasthefirsttoclosetheTM,which doesnotmaketheeventsintheotherlayerslessimportant. At the moment when most cell proliferation and migra-tionoccurred,theepitheliallayershowedhighermetabolic activity,requiringgreatersupplyofoxygenandnutrients.16
EAC
EAC
A
B
C
EAC
m
m
m
ep
Mucosa Lp
100µm
100µm 100µm
Figure7 HistologicalsectionimagesofratTM14daysaftertraumaticperforation,stainedwithHE.EAC,externalauditorycanal; m,malleushandle;ep,epitheliallayer;LP,laminapropria;mucosa,mucosallayer.Image(A)showsamagnificationof40×;(B)
100×and(C)200×.
providingmolecularsupplyrequiredforthehealingevents tosatisfactorilydevelopintheTM.17---19
The lamina propria is responsible for the fibroelastic characteristics of the TM, such as vibration capac-ity for sound transmission and middle ear protection. In some cases, the newly formed TM can heal with only two layers, one outer epithelial and one inner mucosal layer, as it has been described in experimental studies.7
However,itispossible thatsuchneomembranesdonot have the ideal characteristics of sound transmission20 or
thefibroelasticstructuralcharacteristicswhichenable with-standing air pressure variations, such as barotrauma or tubal dysfunctiondue to itsweaker thanusual structure, caused by deficient formation of the fibrous layer.8
Kris-tensenpointedtothepossibilityofatelectasisorretraction pouchesinatrophicneomembranesduetofibrouslayer defi-ciency.
Topreventtheoccurrenceofatrophicmembranesdueto lamina propria deficiency, treatments that increase fibro-blast activity and collagen production may be interesting to provide better organization of the lamina propria. In tympanoplasty surgery, whether with autografts such as temporalismusclefascia ortragal perichondrium,or even withtheuseofallografts,suchasAlloDerm®,21thepurpose
oftheseproceduresistorestoretheTMandthegraftsare
usedtorepairthefibrouslayer,participatinginthe forma-tionofthenewextracellularmatrix.
Aprevious studyreportedthatthe innermucosallayer contributedlittle to the healing process of the TM.17 On
theotherhand, forWeinberger,Hawke andGotlieb,22 the
medialmucosallayerofTMwouldbethefirstofthe struc-turestoundergo perforation closure.It wasnot possible, throughthemethodologyemployedinthisstudy,todefine theroleofthemucosallayerinthehealingprocessofthe TM.Moreover,eventhoughitisalayerwithalesscomplex histologicalarchitecturethantheothers,itisimportantto rememberthathealingeventsinthethreeTM layersmust beharmoniouslyorganized,sothatproperTMintegritycan berestored.
Conclusion
EvaluatingthenormalhealingprocessoftheTMinrats,we concludedthatthespontaneoushealingprocessisinitiated bytheTMouterepitheliallayer,withsubsequentclosureof thelaminapropriaandmucosallayer.
Funding
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Wullstein H.Theoryand practiceoftympanoplasty. Laryngo-scope.1956;66:1076---95.
2.LimDJ.Tympanicmembrane.Electronmicroscopicobservation. I:parstensa.ActaOtolaryngolStock.1968;66:181---98.
3.SantaMariaPL,RedmondSL,AtlasMD,GhassemifarR.Histology ofthehealingtympanicmembranefollowingperforationinrats. Laryngoscope.2010;120:2061---70.
4.StenfeldtK,JohanssonC,HellstromS.Thecollagenstructure ofthetympanicmembrane.ArchOtolaryngolHeadNeckSurg. 2006;132:293---8.
5.WenigBM,MichaelsL.Theearandtemporalbone.In:MillsSE, editor.Histologyforpathologists.3rded.Philadelphia: Lippin-cottWilliams&Wilkins;2007.p.372---400.
6.LimDJ.Structureandfunctionofthetympanicmembrane:a review.ActaOthorhinolaryngolBelg.1995;49:101---15.
7.Gladstone HB, Jackler RK, Varav K. Tympanic membrane wound healing --- an overview. Otolaryngol Clin North Am. 1995;28:913---32.
8.KristensenS.Spontaneoushealingoftraumatictympanic mem-braneperforations.JLaryngolOtol.1992;106:1037---50.
9.GüneriEA,TekinS,YilmazO,ÖzkaraE,ErdagTK,IkizAO,etal. Theeffects ofhyaluronicacid,epidermalgrowth factor,and mitomycininanexperimentalmodelofacutetraumatic tym-panicmembraneperforation.OtolNeurotol.2003;24:371---6.
10.KoopmannCF.Cutaneouswoundhealing.OtolaryngolClinNorth Am.1995;28:835---45.
11.WitteMB,BarbulA.Generalprinciplesofwoundhealing.Surg ClinNorthAm.1997;77:509---28.
12.KobaR.Epidermalcellmigrationandhealingofthetympanic membrane:animmunohistochemicalstudyofcellproliferation
usingbromodeoxyuridinelabelling. AnnOtolRhinolLaryngol. 1995;104:218---25.
13.SpratleyJ,Hellstrom S,Eriksson PO,Pais-Clemente M.Early structural tympanic membrane reactions to myringotomy: a studyinanacuteotitismediamodel.ActaOtolaryngolStock. 2002;122:479---87.
14.KobaR, Yagi M,TabeH, KawabataI. Kineticanalysisofcell proliferationusingbromodeoxyuridine labelingin situ detec-tionofdyingcellsinthetympanicmembraneandmiddleear cholesteatoma.ArchHistolCytol.1996;59:339---46.
15.Somers TH, Houben V, Goovaerts G, Govaerts PJ, Offeciers EE. Histologyof the perforatedtympanic membrane and its muco-epithelialjunction.ClinOtolaryngolAlliedSci.1997;22: 162---6.
16.GilesB.Woundhealinginspontaneousperforationor myringo-tomy and middle ear reconstruction. Ear Nose Throat J. 2007;86:30---2.
17.JohnsonAP, SmallmanLA, Kent SE.The mechanismof heal-ing of tympanic membrane perforations: a two-dimensional histological study in guinea pigs. Acta Otolaryngol Stockh. 1990;109:406---15.
18.Makino K, Amatsu M. Epithelial migration on the tym-panic membrane and external canal. Arch Otorhinolaryngol. 1986;243:39---42.
19.Masutani H, NakaiY, Sugita M,Ohashi K, Moriguchi M, Mat-sunagaK. Microvasculatureofthetympanicmembrane. Acta OtolaryngolSupplStockh.1991;486:99---104.
20.O’Connor KN, Tam M, Blevins NH, Puria S. Tympanic mem-branecollagenfibers:akeytohighfrequencysoundconduction. Laryngoscope.2008;118:483---90.
21.LaiP,PropstEJ,PapsinBC.Lateralgrafttype1tympanoplasty usingAllodermfortympanicmembranereconstructionin chil-dren.IntJPedOtorhinolaryngol.2006;70:1423---9.