w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Laboratory
evaluation
of
Clusia
fluminensis
extracts
and
their
isolated
compounds
against
Dysdercus
peruvianus
and
Oncopeltus
fasciatus
Rodrigo
C.
Duprat
a,b,
Maria
C.
Anholeti
c,d,
Bruno
P.
de
Sousa
a,
João
P.F.
Pacheco
a,b,
Maria
R.
Figueiredo
e,
Maria
A.C.
Kaplan
f,
Marcelo
Guerra
Santos
g,
Marcelo
S.
Gonzalez
a,
Norman
A.
Ratcliffe
a,h,
Cicero
B.
Mello
a,
Selma
R.
Paiva
d,
Denise
Feder
a,∗aLaboratóriodeBiologiadeInsetos,UniversidadeFederalFluminense,CampusValonguinho,Niterói,RJ,Brazil
bProgramadePós-graduac¸ãoemCiênciaseBiotecnologia,InstitutodeBiologia,UniversidadeFederalFluminense,Niterói,RJ,Brazil
cProgramadePós-graduac¸ãoemQuímicadeProdutosNaturais,InstitutodePesquisasdeProdutosNaturais,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil dLaboratóriodeBotânicaEstruturaleFuncional,InstitutodeBiologia,UniversidadeFederalFluminense,Niterói,RJ,Brazil
eLaboratóriodeQuímicadeProdutosNaturais,Far-Manguinhos,Fiocruz,RiodeJaneiro,RJ,Brazil fInstitutodePesquisasdeProdutosNaturais,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
gDepartamentodeCiências,FaculdadedeFormac¸ãodeProfessores,UniversidadedoEstadodoRiodeJaneiro,RiodeJaneiro,RJ,Brazil hDepartmentofBiosciences,CollegeofScience,SwanseaUniversity,SingletonPark,Swansea,UnitedKingdom
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received11May2016 Accepted1August2016
Availableonline23September2016
Keywords: Clusianone Lanosterol Oncopeltusfasciatus Dysdercusperuvianus Developmentalinhibition Mortality
a
b
s
t
r
a
c
t
TheeffectsofthehexanicextractsofthefruitsandflowersofClusiafluminensisPlanch.&Triana, Clu-siaceae,aswellastheirmainconstituents,thetriterpenelanosterolandthebenzophenoneclusianone, wereevaluatedonhemipteransDysdercusperuvianusandOncopeltusfasciatus.Thetopicaltreatments ofinsectswiththehexanicextractssignificantlyaffectedthesurvivalofO.fasciatus,butnotthatofD. peruvianus.Concomitantly,extractsdelayedthedevelopmentofbothhemipterans.Moreover,isolated lanosterolsignificantlyreducedboththesurvivalanddevelopmentofO.fasciatusandD.peruvianus,while clusianoneonlyreducethesurvivalofD.peruvianusandmarginallyinhibitedthedevelopmentofboth insects.Theresultsshowthespecificactivityoflanosterolandclusianoneagainstthetwoevaluated insectspeciesandindicatethepotentialofcompoundsderivedfromC.fluminensisforthedevelopment ofspecificbiopesticidesforthecontrolofagriculturalpests.Subsequentworkwillexaminethemodeof actionoflanosterolandclusianoneisolatesfromC.fluminensis.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Synthetic insecticides were widely used after World War II (McGraw and O’Neill, 2013) and have played important roles inthecontrol ofagriculturalpestsandtropical diseasevectors. Thesecompounds,however,arehighlytoxicandcontaminatethe environmentcausingserioushumanhealthproblems(Longnecker etal.,2001;Weissetal.,2004).
In addition, the frequent use of pesticides has enhanced insectresistanceandresultedinincreaseddosagesand/or subse-quentreplacementbyotherchemicalswithevenhighertoxicity (Hemingway and Ranson, 2000). Therefore, new strategies for insect control are under development, especially with natural
∗ Correspondingauthor.
E-mail:mdfeder@id.uff.br(D.Feder).
productsofplantoriginthatarelessharmfultotheenvironment (Stoateetal.,2009).
Co-evolution mechanisms between insects and plants have resultedintheselectionofplantsecondarymetabolites, includ-ing pyrethrins, alkaloids, rotenoids and terpenoids, with killer, repellentorgrowthanddevelopmentalinhibitoryactivitiesagainst insects(Isman,2006;AlexenizerandDorn,2007;Miresmailliand Isman,2014).
ThevastBrazilianfloraisofprimaryimportanceinthesearchfor alternativenaturalinsecticides(Mendonc¸aetal.,2005;Giorgietal., 2013).ClusiafluminensisPlanch.&Triana,Clusiaceae,isanative plantfromBrazil,foundinregionsofhighluminousintensityand waterrestriction,suchasthe“restinga”environment(sandycoastal plains)androckyoutcrops(Bittrich,2010).Moreoverinnature,C. fluminensisleavesarerarelyattackedbyinsects.
Previousreports showedthat theleavesofspecies fromthe genus Clusia are rich in terpenes, especially triterpenes and sesquiterpenes (Barrios et al., 1990; De Andrade et al., 1998;
http://dx.doi.org/10.1016/j.bjp.2016.08.004
Compagnone et al., 2008; Marín et al., 2008; Guimarãeset al., 2013).Nagemetal.(1993)identifiedthepentacyclictriterpenes, amyrin, friedelin, ␣-friedelinol and -friedelinol as wellas the tetracyclic triterpene, lupenone, in the leavesof C. fluminensis. Recently,lanosterolandclusianonefromthefruitsandmale flow-ersofC.fluminensis,respectively,wereisolatedandstructurally characterized(Silvaetal.,2012;Oliveiraetal.,2014).Subsequently, clusianonewasshowninlaboratoryassaystoinducehighmortality andtoinhibitdevelopmentofAedesaegyptilarvae(Anholetietal., 2015).
Clusianoneisabenzophenoneofwhichtherearemany deriva-tives(BeerhuesandLiu,2009;Lietal.,2014)butonlyafewstudies of theirinsecticide properties have beenmade (Middleton and Chadds,1970; Ranganatha et al., 2013). For example, the ben-zophenones,cariphenone Aandcariphenone B,fromHypericum carinatumGriseb.,Hypericaceae,haveanti-mosquitoactivity(da Silvaet al.,2013),while benzophenonespresent incommercial UVfiltersmimicecdysoneactivityinChironomusriparius(Meigen, 1804) (Diptera: Chironomidae) and affect development (Ozáez etal.,2014).
Lanosterolisknowngenerallytobetheprecursorofergosterol infungiandcholesterolinanimals(Phillipsetal.,2006).Although cycloartenolisthemainprecursorofsteroidsinvegetable, stud-ieshave shownthat plants arecapableof producing lanosterol directlyfrom2,3-oxidosqualenecyclase(Suzukietal.,2006). Stud-iesinvolvingterpenesshowthatthesemetabolitesarecapableof causingchangesintheendocrinesystemofinsects,actingas ago-nistsorhormoneantagonists,killingthemorpreventingthemfrom reachingadulthood(Bowersetal.,1976).Inapreviousstudywith
C.fluminensisclusianoneshowedasignificantreducedsurvivalof
Aedesaegyptilarvae.However,noactivitywasdetectedwith lanos-terolonthesurvivalordevelopmentofA.aegyptilarvae(Anholeti etal.,2015).
Dysdercusperuvianus(Guérin-Menéville)(Hemiptera: Pyrrho-coridae)andOncopeltusfasciatus(Dallas)(Hemiptera:Lygaeidae) areoftenusedasmodelsfordrugtestingin basicresearchand appliedentomology(Fernandesetal.,2013;Tietbohletal.,2014). Thecottonstainerbug,D.peruvianus,damagescottonseedsand spoilsthe cottonfibers. It is also a vector for phytopathogenic microorganismsandmayresultingreatlossesincottonproduction (Gallo,1988).InBrazil,Dysdercusspp.aretargetsforinsecticidesin cottonplantations,however,therearefewspecificproductsforthe controlofthesepests(Gallo,1988).Themilkweedbug,O.fasciatus, isalsoahemipteraninsect,butunlikeDysdercusspp.,isnotusually regardedasapestspeciesbuthasbeenusedextensivelyasamodel inbiologicalresearch(LiuandKaufman,2009).
ThepresentpaperinvestigatestheactivityofC.fluminensis hex-anicextracts,andtheirmajorisolatedcomponents,lanosteroland clusianone,againsttwopestinsectspecies,D.peruvianusandO. fas-ciatus.TheresultsshowthatcompoundsfromC.fluminensishave selectiveactivitieswithlanosterolkillingO.fasciatuswithgreater efficiencythanD.peruvianuswhileclusianonekilledD.peruvianus
butnotO.fasciatus.
Materialandmethods
Insectcolonies
ColoniesofOncopeltusfasciatusandDysdercusperuvianuswere establishedintheLaboratoryofInsectBiologyoftheUniversidade FederalFluminense,andkeptatconstanttemperature(26±1◦C),
photoperiod (16L:8D) and relative humidity (60%±5) (Milano etal.,1999).Theinsectswerehousedin transparentglasspots, covered in netting and water provided ad libitum. O. fasciatus, werereared under similar conditions to D. peruvianus but fed,
respectively,onsunflowerseeds(HelianthusannuusL.,Asteraceae) and cotton seeds (Gossypium hirsutum L., Malvaceae) (Feir and Beck,1963; Feir,1974).The seedswere placed inside the pots duringthe matingand layingperiod and uptothe firstinstar. Fromthesecondinstaron,theinsectsweretransferredtoclean potseachweek,inordertoavoidseedcontaminationwithinsect fecesandfacilitatecleaning,theseedswereplacedontopofthe nettingclosingthepots.Alltheinsecticidalactivityexperiments wereconductedataconstanttemperatureof26±1◦C.
Plantmaterialandextractpreparation
Flowersfrommaleindividualandfruitsfromfemaleindividual ofClusiafluminensisPlanch.&Triana,Clusiaceae,werecollectedin thesummerandautumn,respectively,inNiterói(RiodeJaneiro State,Brazil).Theidentificationofplantsandthepreparationof extractswereasdescribedpreviously(Silvaetal.,2012;Anholeti etal.,2015).
Isolationofsubstancesandchemicalanalysisofcrudeextracts
The polyisoprenylated benzophenone, clusianone, and the triterpene,lanosterol,wereisolatedandanalyzedfromtheflowers andfruits,respectively,ofC.fluminensisusingvarious chromato-graphicandmassspectrometrictechniquesasdescribedbySilva etal.(2012),Oliveiraetal.(2014)andasmodifiedinAnholetietal. (2015).
Insectbioassays
Randomlyselected,4thinstarnymphsofO.fasciatusandD. peru-vianus,weretreatedwithsamplesolutions,whichwereapplied topicallytothedorsalcuticleofeach insect.Thecrudeextracts fromC.fluminensisweredissolvedinethanol ataconcentration of1mg/ml,and1lofeachsamplewasapplied.Theisolated sub-stances(clusianoneandlanosterol)wereappliedsimilarly,except thattheyweredissolvedinacetonetoaconcentrationof0.7mg/ml (0.7g/insect).
Thecontrolgroupswereuntreated(C)ortreatedsolelywiththe solvents(SC)usedtodissolvethesamples.Biologicalevaluationof theresultsofthedifferenttreatmentswasperformeddailyfrom thebeginningofthe4thinstaruptotheadultstage.Observations
weremadeofsurvival(mortality),theintermoltand metamorpho-sisperiods,andthepresenceofprematureadultcharacteristicsand bodydeformities.Theexperimentswereterminatedatthedeath oremergencetoadultsofallinsectsfromthecontrolgroups.
Allexperimentswererepeatedatleasttwicewithbatchesoften fullyengorgedinsectswithreplicatesofsixforeachofthethree groups(experimental,CandSC).Theresultsarederivedfromthe mediaofthepercentageofeachreplicatefromthedayafter top-icalapplicationon4th instarnymphs(1stday)tothelastdayof
observation.
Dataandstatisticalanalysis
AllgraphswerecreatedwithGraphPad Prism6.05 software (GraphPadSoftware,SanDiego,CA,USA),showingthesurvivaland thedevelopmentalcourseofthejuvenilestageandadults.
0
2 4 6
FR x SC P=.2433, SC x C P=.6326 FR x SC P=5865–09, SC x C P=.05538 FR x SC P<.0001, SC x C P=.0034 8 10
Days
Sur
viv
al, %
12 14 16 20 40 60 80 100 0 2 4 6
FR x SC P=.0198, SC x C P=.9591 8 10
Days
Sur
viv
al, %
12 14 16 18 20 22 24 20
40 60 80 100
D
E
F
A
B
C
0 2 4 6
FR x SC P=4237–04, SC x C P=.7495
FR SC C
8 10 Days
5
th instar
, %
12 14 16 18 20 22 24 20 40 60 80 100 0 2 4 6
FR x SC P=.0002, SC x C P=.3917 8 10
Days
Adults
, %
12 14 16 18 20 22 24 20 40 60 80 100 0
2 4 6 8 10 Days
5
th instar
, %
12 14 16 20 40 60 80 100 0
2 4 6 8 10 Days
Adults
, %
12 14 16 20
40 60 80 100
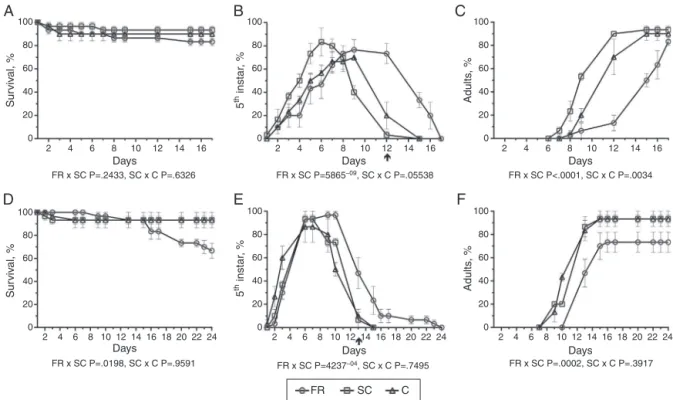
Fig.1.Effectsofextractsoffruit(FR)fromClusiafluminensisonsurvival(A,D)anddevelopmentofnymphs(B,E),adults(C,F)ofDysdercusperuvianus,(A,B,C)andOncopeltus fasciatus(D,E,F),atdifferentdaysafterexperimentaltreatment.(FR,)comparedwiththesolventcontrol(SC,),thatwascomparedwiththeuntreatedcontrol(C,). Fourthinstarhemipterannymphweretopicallytreatedwith1gofextractin1lofsolvent.Statisticalanalyses(underthegraphs)withtheBarnard’stestwereused(B, E)onarepresentativedayindicatedbyanarrowandtheGehan-Breslow-Wilcoxontestwasused(A,C,D,F)tocomparetheentirecurvebetweenthegroups(FR×SCand SC×C).EachpointrepresentsthemediumofatleastsixreplicateswithteninsectsandbarsshowSE.Significantdifferences(pvalue<0.05)areinbold.
graphs.Thep-valuesgeneratedbyExactpackagewereconfirmed byFortranprogramXUN2X2version2.0(Berger,1996)andBarnard packageversion1.6forRprogram(Erguler,2015).Inall experi-ments,onlyp-values<0.05wereconsideredstatisticallysignificant andnocorrectionsformultiplecomparisonsweremade(Rothman, 1990).
Results
ThebioassayswiththehexanicextractofC.fluminensisfruit(FR) showedthatinthetreatedgroupstherewasnoeffectonsurvival ratesofD.peruvianus(Fig.1A).Incontrast,inexperimentswithO. fasciatus,thesurvivalcurveshowedasignificant(p<0.02,inbold)
0
2 4 6 8
Days
FL x SC P=.0218, SC x C P=.6326
Sur
viv
al, %
10 12 14 20
40 60 80 100
A
B
C
D
E
F
0
2 4 6 8
Days
FL x SC P=.1055, SC x C P=.9591
Sur
viv
al, %
10 12 14 16 20 40 60 80 100 0
2 4 6 8
Days
FL x SC P=8683–09, SC x C P=.7495
FL SC C
5
th instar
, %
10 12 14 16 20 40 60 80 100 0
2 4 6 8
Days
FL x SC P<.0001, SC x C P=.3917
Adults
, %
10 12 14 16 20 40 60 80 100 0
2 4 6 8
Days
FL x SC P=7969–06, SC x C P=2036–04
5
th instar
, %
10 12 14 20 40 60 80 100 0
2 4 6 8
Days
FL x SC P<.0001, SC x C P=.0002
Adults
, %
10 12 14 20
40 60 80 100
Abundance
1.7e+07
1.6e+07
1.5e+07
1.4e+07
1.3e+07
1.2e+07
1.1e+07
1e+07
9 000 000
8 000 000
7 000 000
6 000 000
5 000 000
4 000 000
3 000 000
2 000 000
1 000 000
0 4.00
4748 6427
8354
8807 10 428
12 938 13 064 13 179 13 336
14 521 14 729
16 167 17 529
19 497 8629 10 289
19 159
Lanosterol
TIC: EM01_14_03_0780.D/data.ms
H HO
18 778 19 924
20 424 2222 163
Time 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00 24.00 26.00 28.00 30.00
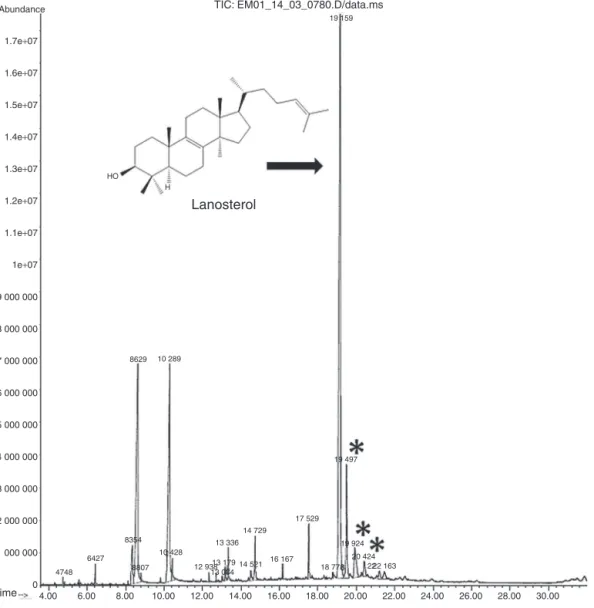
Fig.3.ChromatogramobtainedforthehexanicextractoffruitsofClusiafluminensisbyGC–MSshowingthetriterpenelanosterolasthemajorcomponent.*Lanosterol isomers.
mortalityrateinFRgroupcomparedwiththesolventcontrol(SC) (Fig.1D),sothatbythelastdayoftesting(24th),33.3%ofthetreated
insectsweredeadcomparedto6.7%ofthecontrolgroups(Fig.1D). Inaddition,thedevelopmentofnymphsandadultsinFRtreated
O.fasciatusandD.peruvianusshowedsignificantdelays(p≤0.0002) incomparisonwiththecontrols(Fig.1B,CandE,F).Forexample, withD.peruvianusatday12,90%oftheinsectsfromtheSCgroup hadmetamorphosed(Fig.1C),while73.3%oftheinsectsoftheFR groupwerestilljuveniles(Fig.1B).Likewise,atday13withO. fas-ciatus86.7%oftheinsectsfromtheSCgroupwereadults(Fig.1F) while46.7%oftheFRgroupwerestillnymphs(Fig.1E).
IncontrastwiththeFR,thebioassayswiththehexanicextract ofC.fluminensisflowers(FL),resultedinsignificantmortalityin theD.peruvianus(p=0.0218)experimentalgroupcomparedwith theSC(day14.3%versus6.7%,respectively,Fig.2A)aswellasno significanteffectontheO.fasciatussurvivalincomparisonwiththe SC(p=0.1055,Fig.2D).
Similarto theeffects of FR ondevelopment to adults, both insectspeciesshowed significantdelays in molting(p≤0.0001) betweentheFL-treatedandtheSCgroups(Fig.2CandF).For exam-ple,onday10withD.peruvianus,76.7%oftheinsectsintheSC grouphad moltedtoadults,while noneofthem had metamor-phosedintheFLgroup(Fig.2Band C).Likewise,ontheday13 withO. fasciatus,86.7% oftheinsectshad becomeadultsinthe
SCgroup,whileonly16.7%oftheFLgrouphadreachedthisstage (Fig.2EandF).
Thehexanicextractsofthefruitsandflowersweresubmitted toGC–MS.Thechromatogramobtainedforthehexanicextractof fruitsofC.fluminensisshowedapeakat19.16min,corresponding toasubstancethatrepresents40.6%ofthesamplecomposition. Themassspectrumofthissubstanceissimilartothatprovidedby theequipment’sdatabaseforthetriterpene,lanosterol.Themass fragmentationpatternobservedis alsoconsistentwiththedata providedbyShinetal.(2000)forthissubstance.Thetriterpene isaccompaniedbythreeisomers(retentiontimes:19.50,19.92, 20.42min)withthesamemassfragmentationpatternoflanosterol, and that togethercorrespondto10.73% of sample composition (Fig.3).Othersubstances presentinappreciableamountinthis extract,withretentiontimesof8.63minand 10.29min(Fig.3), showedmassfragmentationpatternsconsistentwithfattyacids, andwereidentified,respectively,aspalmiticandoleicacids.
Moreover,theGC–MSdataobtainedforthehexanicextractof flowersofC.fluminensissuggeststhepresenceoflanosterol(9.7%) andclusianone(54.8%),as shownina previouspaper(Anholeti etal.,2015).
100
A
B
C
D
E
F
80
60
40
20
0
2 4 6 8 10 Days
Sur
viv
al, %
12 14 16
100
80
60
40
20
0
2 4 6 8 10 Days
5
th instar
, %
12 14 16
100
80
60
40
20
0 2 4
LN x SC P=.0003, SC x C P=.0243 LN x SC P=0.02133, SC x C P=.6876
LN x SC P=.0140, SC x C P=.1498
100
80
60
40
20
0
2 4 6 8 10
Days
Sur
viv
al, %
12 14
LN x SC P=.0069, SC x C P=.6713
100
80
60
40
20
0
2 4 6 8 10
Days
LN SC C
5
th instar
, %
12 14
LN x SC P=5308–05, SC x C P=.2364
100
80
60
40
20
0
2 4 6 8 10
Days
Adults
, %
12 14
LN x SC P=.0199, SC x C P=.4193 6 8 10
Days
Adults
, %
12 14 16
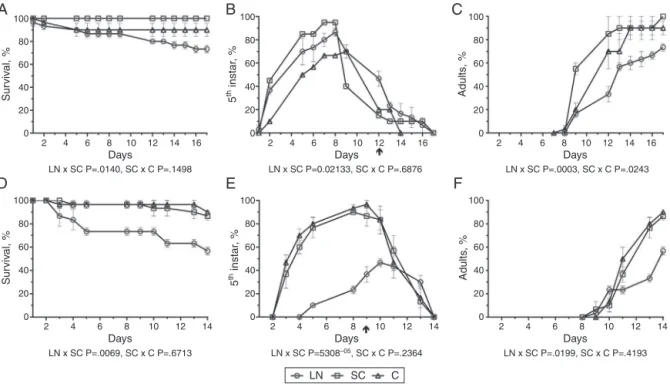
Fig.4. Effectsofextractsoflanosterol(LN)isolatedfromClusiafluminensisonsurvival(A,D)anddevelopmentofnymphs(B,E),adults(C,F)ofDysdercusperuvianus,(A,B, C)andOncopeltusfasciatus(D,E,F),atdifferentdaysafterexperimentaltreatment.(LN,)comparedwiththesolventcontrol(SC,),thatwascomparedwiththeuntreated control(C,).TheassayswereexecutedandanalyzedasinFig.1legendand“Materialsandmethods”section.
2014).Subsequently,bioassayswiththesetwopurifiedsubstances wereperformedagainstD.peruvianusandO.fasciatus.
Inthebioassayswithlanosterol-treatedD. peruvianusandO. fasciatus, thesurvival rates were significantly reduced in com-parison with the SC groups (p=0.014, Fig. 4A and p =0.0069,
Fig.4D,respectively).Regardingthedevelopmentofthenymphs toadults, significantdelays occurredwith lanosterol treatment although variations were observed between the D. peruvianus
andO. fasciatusgroups.WithD.peruvianus,there wasa signifi-cantdifferenceintheadultdevelopmentbetweenthelanosterol andSCcurves(p=0.0003,Fig.4C).Thus,byday12,33.3%ofthe nymphs from thelanosterol grouphad moltedcompared with 85%intheSCgroup(Fig.4Band C).Similarly,withO.fasciatus, atday9oflanosteroltreatment,only36.7%ofthenymphsofO. fasciatushadmoltedto5thinstarwhereasinthecontrolgroups (p=5.308−05)at least93.9% ofinsects had already reachedthis
samenymphalstage(Fig.4E).Thedifferencesbetweenthecurves ofdevelopmenttoadultwerealsostatisticallysignificantforthe lanosterol-treatedinsects comparedtotheSCgroup(p=0.0199,
Fig.4F).
Inexperimentswithlanosterol-treatedO.fasciatusandD. peru-vianus, 3.3%(SE±3.3) malformed insects withdeformed wings andruffledcuticlewereobserved(Fig.5).Theseabnormalforms appeared after4–6days testing with4th to5th instar nymphs
thatshouldhavemoltedto5thinstars. Thesemalformed speci-mensdiedafterorduringmolting,oftenconfinedwithintheold cuticle.
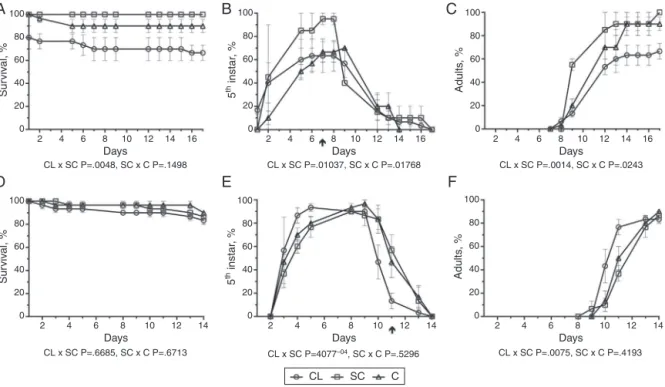
Thebioassayswithclusianoneshowedasignificantreductionin survivalofD.peruvianus(p<0.0048,Fig.6A)butnotofO.fasciatus
(p>0.6685,Fig.6D)incomparisonwiththeSCgroups.Onthelast daywithD.peruvianus(17thday),only66.7%ofinsectssurvived comparedwith100%oftheSCgroup(Fig.6A).Regarding develop-ment,significantdelays(p=0.01037at7days,Fig.6BandC)were recordedwithD.peruvianusbut,surprisingly,clusianone acceler-atedsignificantly(p=4.077−04at11days)thedevelopmentofO. fasciatus(Fig.6EandF).
Fig.5. Normal5thinstar(A)andadult(B)ofOncopeltusfasciatusfromcontrol group(PC).DeformedspecimensofO.fasciatusfrom4thinstarabnormalmoltafter treatmentwithlanosterol(CtoF).Bar=1cm.
0
2 4 6
CL x SC P=.0048, SC x C P=.1498 8 10
Days
Sur
viv
al, %
12 14 16 20
40 60 80 100
A
B
C
D
E
F
0
2 4 6
CL x SC P=.6685, SC x C P=.6713 8 10 Days
Sur
viv
al, %
12 14 20
40 60 80 100
0
2 4 6
CL x SC P=4077–04, SC x C P=.5296 8 10 Days
CL SC C
5
th instar
, %
12 14 20
40 60 80 100
0
2 4 6
CL x SC P=.0075, SC x C P=.4193 8 10 Days
Adults
, %
12 14 20
40 60 80 100 0
2 4 6
CL x SC P=.01037, SC x C P=.01768 8 10
Days
5
th instar
, %
12 14 16 20
40 60 80 100
0
2 4 6
CL x SC P=.0014, SC x C P=.0243 8 10
Days
Adults
, %
12 14 16 20
40 60 80 100
Fig.6.Effectsofextractsofclusianone(CL)isolatedfromClusiafluminensisonsurvival(A,D)anddevelopmentofnymphs(B,E),adults(C,F)ofDysdercusperuvianus,(A,B, C)andOncopeltusfasciatus(D,E,F),atdifferentdaysafterexperimentaltreatment(CL,)comparedwiththesolventcontrol(SC,),thatwascomparedwiththeuntreated control(C,).TheassayswereexecutedandanalyzedasinFig.1legendand“Materialsandmethods”section.
Discussionandconclusion
Inthepresentstudy,thetriterpene,lanosterol,wasfurther iden-tified and purified from the hexanic extractsof the fruits (FR) andflowers(FL)ofC.fluminensis,followingtheoriginalprotocols describedbyOliveiraetal.(2014).Incrudeextractsoftheflowers, lanosterolrepresentsonly10%ofthecontents(Anholetietal.,2015) whileinextractsofthefruits,thiscompoundmakesup40.46%of theextract.Thehighlanosterolcontentin theFRextract prob-ablyaccountsforsomesimilaritiesinsurvivalanddevelopment obtainedwiththetwoinsectspeciestestedwiththeFRextractand purifiedlanosterol(compareFigs.1and4).O.fasciatuswasmore sensitivetotheFRextractandlanosterol,intermsofmortality,than
D.peruvianus,andnoactivitywasdetectedagainstAe.aegyptiwith eithertreatment(Anholetietal.,2015).Moltingaberrationinlast stageOncopeltusnymphs,treatedwithIGR’spesticideshavebeen described(Redfernetal.,1982).However,theappearanceofsome precociousadultswithdeformationsafterthe4thinstar
hemipter-answeretreated(Fig.5)couldindicatetheanti-juvenilehormone activityoflanosterol(Bowersetal.,1976).
Theeffectofplantmetaboliteswithantijuvenilehormone activ-ityhasbeendescribedagainstmanyinsects,especiallyafterthe identificationoftheprecocenespurifiedfromplantsofthegenus
Ageratum(Bowersetal.,1976;Prattetal.,1980).Thesesubstances havemarkedeffectsonhemipteransandinducetheappearanceof earlyadults(adultoids)withcharacteristicssimilartothosefound inthepresumptiveadultoidbugsresultingfromthetreatmentwith lanosterolinthepresentstudy(Masneretal.,1979;Unnithanetal., 1977;Jurbergetal.,1984).
The lanosterol effect is similar to theprecocenes, have less effectsin holometabolousinsects(Kellyand Fuchs,1978;Staal, 1986;Erezyilmazetal.,2006),whichcouldexplainwhyA.Aegypyi
wasnotsusceptibletolanosterol(Anholetietal.,2015)evenupto concentrationsof100g(unpublishedresult).
Apparently,theprecocenesinactivate thecorpora allata and consequently interrupt the production of juvenile hormone (Bowers and Aldrich,1980).However, anti-JH activitycan also
occurwithsubstancesthatcompetewithJHfortherecognition receptorsinthecellsoftargettissues(Staal,1986).Inaddition, somesubstances,suchaslanosterol,canactasprecursorsinthe biosynthesisofJH,producingahormonewithalteredspecificity (Staal,1986).Lanosterolcanbeaprecursorofcholesterolandthe biosynthesisofjuvenilehormoneissimilartothatofcholesterol (Miaoetal.,2002).
Following application, the uptake of lanosterol into the hemolymphmaynotonlymodifythesynthesisofJHbutalsotheJH receptorsonthetargetcellsbysubstitutingforcholesterolinthe cellmembraneandsignificantlyalteringthestructuraland func-tionalintegrityofthecell(Miaoetal.,2002)tobindorrespond appropriatelytoJH.Cholesterolsubstitutionbylanosterolmayalso interfere/modifytheentryofsmallmoleculesandionsintoandout ofepithelialcellsoftheexoskeletonandcompromisethechitin syn-thasesecretorypathwayproducingthecuticle(Merzendorferand Zimoch,2003)andresultininsectdeformations.
Inthepresentstudy,andincontrasttotheireffectsonAedes aegypti (Diptera: Culicidae) (Anholeti et al., 2015), FLand clu-sianonehadnoorlimitedeffectsonkillingthehemipteransand delaying their development. Moreover, clusianone was further identifiedandpurifiedfromthehexanicextractsofflowers(FL) ofC.fluminensis.Clusianoneat55%isthemajorcomponentofC. fluminensisflowersextract(Anholetietal.,2015)withlanosterol alsopresentinFLbutmakinguplessthan10%ofthecomposition. In O. fasciatus, clusianoneactually accelerated development, aswellthesolventcontrolsinD.peruvianus.Wehaveobserved inourlaboratorythatsomecompoundsthatstressthenymphs canacceleratetheirdevelopmenttotheadultsstage(unpublished observation),asifattemptingtoescapeanunfavorable environ-ment.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authorscontributions
MCA,MRF,MACK,SRPpreparedextracts,isolatedsubstances andanalyzedchemicaldatafromtheplantmaterial.MGScollected theplantmaterialandidentifiedtheplant.RCD,MSG,DF,CBM,NAR conceived,designedresearchandanalyzeddataofinsectbioassays. MCA,RCD,DF,NARandCBMwrotethemanuscript.RCD,BPS,JPFP, MCAconductedinsectbioassays.Allauthorsreadandapprovedthe manuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
The authors thank the Universidade Federal Fluminense (PROPPI-UFF), CNPqand FAPERJfor financial support. Theyare gratefultoRodrigoMexas(Fiocruz)forhelpingwiththe illustra-tions,toDrCéliaCarlini(UFRGS)forthedonationofcottonseeds andtoFelipeLeite(UFF)forhistechnicalassistance.Thecommand ofForteBarãodoImbuhy(21stGroupofFieldArtillery),for
permis-siontocollectC.fluminensis.
References
Alexenizer,M.,Dorn,A.,2007.Screeningofmedicinalandornamentalplantsfor insecticidalandgrowthregulatingactivity.J.Pest.Sci.80,205–215.
Anholeti,M.C.,Duprat,R.C.,Figueiredo,M.R.,Kaplan,M.A.C.,Santos,M.G.,Gonzalez, M.S.,Ratcliffe,N.A.,Feder,D.,Paiva,S.R.,Mello,C.B.,2015.Biocontrolevaluation ofextractsandamajorcomponent,clusianone,fromClusiafluminensisPlanch. &TrianaagainstAedesaegypt.Mem.I.OswaldoCruz110,629–635.
Barrios,M.,Calvo,M.,Arguedas,E.,Castro,O.,1990.ConstituentsofClusiauvitana. Fitoterapia61,479.
Beerhues,L.,Liu,B.,2009.Biosynthesisofbiphenylsandbenzophenones–evolution ofbenzoicacid-specifictypeIIIpolyketidesynthasesinplants.Phytochemistry 70,1719–1727.
Berger, R.L., 1996. A Fortran program – XUN2X2 version 2.0, http://www.public.asu.edu/∼rlberge1/software/xun2x2v2.f.Onlineinteractive versionavailableat:http://www4.stat.ncsu.edu/∼boos/exact/.
Bittrich,V.,2010.ClusiaceaeinListadeespéciesdafloradoBrasil.JardimBotânicodo RiodeJaneiro,Brasilhttp://floradobrasil.jbrj.gov.br/2010/FB000089[accessed August2010].
Bowers,W.S.,Aldrich,J.R.,1980.Invivoinactivationofdenervatedcorporaallataby precoceneIIinthebug,Oncopeltusfasciatus.Experientia36,362–364. Bowers,W.S.,Ohta,T.,Cleere,J.S.,Marsella,P.A.,1976.Discoveryofinsect
anti-juvenilehormonesinplants.Science193,542–547.
Calhoun, P., 2015. Exact: Unconditional Exact Test. R package version 1.6. https://CRAN.R-project.org/package=Exact.
Compagnone,R.S.,Suarez,A.C.,Leitao,S.G.,DelleMonache,F.,2008.Flavonoids, benzophenonesandaneweuphanederivativefromClusiacolumnarisEngl.Rev. Bras.Farmacogn.18,6–10.
daSilva,F.C.,deBarros,F.M.C.,Prophiro,J.S.,daSilva,O.S.,Pereira,T.N.,Bordignon, S.A.L.,Eifler-Lima,V.L.,vonPoser,G.L.,2013.Larvicidalactivityoflipophilic extractofHypericumcarinatum(Clusiaceae)againstAedesaegypti(Diptera: Culi-cidae)andbenzophenonesdetermination.Parasitol.Res.112,2367–2371. DeAndrade,M.R.,Almeida,E.X.,Conserva,L.M.,1998.Alkylchromoneandother
compoundsfromClusianemorosa.Phytochemistry47,1431–1433.
Erezyilmaz,D.F.,Riddiford,L.M.,Truman,W.,2006.Thepupalspecifierbroaddirects progressivemorphogenesisinadirect-developinginsect.Proc.Natl.Acad.Sci. U.S.A.103,6925–6930.
Erguler,K.,2015.Barnard:Barnard’sUnconditionalTest.Rpackageversion1.6. https://CRAN.R-project.org/package=Barnard.
Feir,D.,1974.Oncopeltusfasciatus:aresearchanimal.Annu.Rev.Entomol.19,81–96. Feir,D.,Beck,S.D.,1963.Feedingbehaviorofthelargemilkweedbug,Oncopeltus
fasciatus.Annu.Entomol.Soc.Am.56,224–229.
Fernandes,C.P.,Xavier,A.,Pacheco,J.P.F.,Santos,M.G.,Mexas,R.,Ratcliffe,N.A., Gon-zalez,M.S.,Mello,C.B.,Rocha,L.,Feder,D.,2013.Laboratoryevaluationofthe
Manilkarasubsericea(Mart.)Dubardextractsandtriterpenesondevelopmentof
DysdercusperuvianusandOncopeltusfasciatus.Pest.Manag.Sci.69,292–301.
Gallo,D.,1988.ManualdeEntomologiaAgrícola,2nded.Ceres,SãoPaulo,Brazil,pp. 649.
Giorgi, A., De Marinis, P., Granelli, G., Chiesa, L.M., Panseri, S., 2013. Sec-ondary metabolite profile, antioxidant capacity, and mosquito repel-lent activity of Bixa orellana from Brazilian Amazon region. J. Chem., http://dx.doi.org/10.1155/2013/409826.
Guimarães,A.L.A.,Bizarri,C.H.B.,Barbosa,L.S.,Nakamura,M.J.,Ramos,M.F.S.,Vieira, A.C.M.,2013.CharacterisationoftheeffectsofleafgallsofClusiamyianitida
(Cecidomyiidae)onClusialanceolataCambess.(Clusiaceae):anatomicalaspects andchemicalanalysisofessentialoil.Flora208,165–173.
Hemingway,J.,Ranson,H.,2000.Insecticideresistanceininsectvectorsofhuman disease.Annu.Rev.Entomol.45,371–391.
Isman,N.B.,2006.Botanicalinsecticides,deterrents,andrepellentsinmodern agri-cultureandanincreasinglyregulatedworld.Annu.Rev.Entomol.51,45–66. Jurberg,J.,Costa,J.M.,Gonc¸alves,T.C.,Garcia,E.S.,Azambuja,P.,1984.Morphogenetic
effectsofprecoceneIIonnymphsofRhodniusprolixus(Stal,1859) (Hemiptera-Triatominae).Mem.I.OswaldoCruz79,397–407.
Kelly,T.J.,Fuchs,M.S.,1978.Precoceneisnotaspecificantigonadotropicagentin adultfemaleAedesaegypti.Physiol.Entomol.3,297–301.
Li,W.Q.,Zhang,Z.J.,Nan,X.,Liu,Y.Q.,Hu,G.F.,Yu,H.T.,Zhao,X.B.,Wua,D.,Yana, L.T.,2014.Design,synthesisandbioactivityevaluationofnovelbenzophenone hydrazonederivatives.Pest.Manag.Sci.70,667–673.
Liu,P.,Kaufman,T.C.,2009.Morphologyandhusbandryofthelargemilkweedbug,
Oncopeltusfasciatus.ColdSpringHarb.Protoc.8,127.
Longnecker,M.P.,Klebanoff,M.A.,Zhou,H.,Brock,J.W.,2001.Associationbetween maternalserumconcentrationoftheDDTmetaboliteDDEandpretermand small-for-gestational-agebabiesatbirth.Lancet358,110–114.
Marín,R.M.,Montes,P.O.R.,Bello,A.A.,Nival,V.L.A.,2008.Caracterizaciónpor cro-matografíadegases/espectrometríademasasdelextractoapolardelashojas
deClusiaminorL.Lat.Am.J.Pharm.27,747–751.
Masner,P.,Bowers,W.S.,Kälin,M.,Mühle,T.,1979.EffectofprecoceneIIonthe endocrineregulationofdevelopmentandreproductioninthebug,Oncopeltus
fasciatus.Gen.Comp.Endocrinol.37,156–166.
McGraw,E.A.,O’Neill,S.L.,2013.Beyondinsecticides:newthinkingonanancient problem.Nat.Rev.Microbiol.11,181–193.
Mendonc¸a,F.A.C.,Silva,K.F.S.,Santos,K.K.,Ribeiro,K.A.L.,Santana,A.E.G.,2005. ActivitiesofsomeBrazilianplantsagainstlarvaeofthemosquitoAedesaegypti. Fitoterapia76,629–636.
Merzendorfer,H.,Zimoch,L.,2003.Chitinmetabolismininsects:structure, func-tionandregulationofchitinsynthasesandchitinases.J.Exp.Biol.206,4393– 4412.
Miao,L.,Nielsen,M.,Thewalt,J.,Ipsen,J.H.,Bloom,M.,Zuckermann,M.J.,Mouritsen, O.G.,2002.Fromlanosteroltocholesterol:structuralevolutionanddifferential effectsonlipidbilayers.Biophys.J.82,1429–1444.
Middleton, W.J., Chadds, F., 1970. Benzophenone hydrazones containing peruoroalkyl, peruoroalkoxy and peruoroalkylthio substituents. United StatespatentUS3732307,1970Set24,1973May8.
Milano,P.,Consoli,F.L.,Zerio,N.G.,Parra,J.R.P.,1999.Thermalrequirementsofthe cottonstainerDysdercusperuvianusGuerin-Meneville(Heteroptera: Pyrrhocori-dae).Ann.Soc.Entomol.Bras.28,233–238.
Miresmailli,S.,Isman,M.B.,2014.Botanicalinsecticidesinspiredbyplant–herbivore chemicalinteractions.TrendsPlantSci.19,29–35.
Nagem,T.J.,Mesquita,A.A.L.,Silva,R.,1993.ConstituentsofClusiafluminensis. Fitoterapia64,380.
Oliveira,E.C.,Anholeti,M.C.,Domingos,T.F.,Faioli,C.N.,Sanchez,E.F.,Paiva,S.R., Fuly,A.L.,2014.InhibitoryeffectoftheplantClusiafluminensisagainstbiological activitiesofBothropsjararacaSnakeVenom.Nat.Prod.Commun.9,21–25. Ozáez,I.,Martínez-Guitarte,J.L.,Morcillo,G.,2014.TheUVfilterbenzophenone3
(BP-3)activateshormonalgenesmimickingtheactionofecdysoneandalters embryodevelopmentintheinsectChironomusriparius(Diptera).Environ.Pollut. 192,19–26.
Pratt,G.E.,Jennings,R.C.,Hamnett,A.F.,Brooks,G.T.,1980.Lethalmetabolismof precocene-Itoareactiveepoxidebylocustcorporaallata.Nature284,320–323. Phillips,D.R.,Rasbery,J.M.,Bartel,B.,Matsuda,S.P.,2006.Biosyntheticdiversityin
planttriterpenecyclization.Curr.Opin.PlantBiol.9,305–314.
Ranganatha,V.L.,Begum,A.B.,Prashanth,T.,Gurupadaswamy,H.D.,Madhu,S.K., Shivakumar,S.,Khanum,S.A.,2013.Synthesisandlarvicidalpropertiesof ben-zophenonecompriseindoleanaloguesagainstCulex quinquefasciatus. Drug Invent.Today5,275–280.
Redfern,R.E.,Kelly,T.J.,Borkovees,A.B.,Hayes,D.K.,1982.Ecdysteroidtitersand moultingaberrationinlaststageOncopeltusnymphs,treatedwithIGR’s pesti-cides.Biochem.Physiol.118,351–356.
R Core Team, 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/.
Shin,Y.,Tamai,Y.,Terazawa,M.,2000.ChemicalconstituentsofInonotusobliquusI.:a newtriterpene,3ˇ-hydroxy-8,24-dien-lanosta-21,23-lactonefromsclerotium. Eur.J.For.Res.1,43–50.
Silva,M.C.A.,Heringer,A.P.,Figueiredo,M.R.,Paiva,S.R.,2012.Separationof clu-sianonefromClusiafluminensisPlanch.andTriana(Clusiaceae)byhighspeed counter-current chromatography (HSCCC). J. Liq. Chrom.Rel. Technol.35, 2313–2321.
Staal,G.B.,1986.Antijuvenilehormoneagents.Ann.Rev.Entomol.31,391–429. Stoate,C.,Báldi,A.,Beja,P.,Boatman,N.D.,Herzon,I.,vanDoorn,A.,deSnoo,G.R.,
Rakosy,L.,Ramwell,C.,2009.Ecologicalimpactsofearly21stcenturyagricultural changeinEurope–areview.J.Environ.Manage.91,22–46.
Suissa,S.,Shuster,J.J.,1985.Exactunconditionalsamplesizesforthe2x2binomial trial.J.R.Stat.Soc.Ser.A148,317–327.
Suzuki,M.,Xiang,T.,Ohyama,K.,Seki,H.,Saito,K.,Muranaka,T.,Hayashi,H.,Katsube, Y.,Kushiro,T.,Shibuya,M.,Ebizuka,Y.,2006.Lanosterolsynthasein dicotyle-donousplants.PlantCellPhysiol.47,565–571.
Tietbohl,L.A.C.,Barbosa,T.,Fernandes,C.P.,Santos,M.G.,Machado,F.P.,Santos, K.T.,Mello,C.B.,Araújo,H.B.,Gonzalez,M.S.,Feder,D.,Rocha,L.,2014. Labo-ratoryevaluationoftheeffectsofessentialoilofMyrciariafloribundaleaveson thedevelopmentofDysdercusperuvianusandOncopeltusfasciatus.Rev.Bras. Farmacogn.24,316–321.
Unnithan,G.C.,Nair,K.K.,Bowers,W.S.,1977.Precocene-induceddegenerationof thecorpusallatumofadultfemalesofthebugOncopeltusfasciatus.J.Insect. Physiol.23,1081–1094.