Function
PAPER
Cite this: DOI: 10.1039/c5fo00675a
Received 6th June 2015, Accepted 20th July 2015 DOI: 10.1039/c5fo00675a www.rsc.org/foodfunction
In vitro fermentation of lupin seeds (Lupinus albus)
and broad beans (Vicia faba): dynamic modulation
of the intestinal microbiota and metabolomic output
Patricia Gullón,
† Beatriz Gullón,*‡ Freni Tavaria, Marta Vasconcelos and
Ana Maria Gomes
Broad beans (Vicia faba) and lupin seeds (Lupinus albus) are legumes rich in a wide range of compounds, which may represent a useful dietary approach for modulating the human gut microbiome. In this work, after in vitro digestion, legume samples were used as carbon sources in anaerobic batch cultures to evalu-ate their impact on the intestinal microbiota composition and on their metabolic products. The fermenta-tions were monitored by a decrease in pH, generation of short chain fatty acids (SCFA) and lactate and the changes in the dynamic bacterial populations byfluorescence in situ hybridization (FISH). The total SCFA at the end of fermentation was 81.52 mM for lupin seeds and 78.41 mM for broad beans accompanied by a decrease of the pH for both legumes. The microbial groups that increased significantly (P < 0.05) were Bifidobacterium spp., Lactobacillus–Enterococcus, Atopobium, Bacteroides–Pretovella, Clostridium coccoides–Eubacterium rectale, Faecalibacterium prausnitzii and Roseburia intestinalis. This impact on the intestinal microbiota suggests that lupin seeds and broad beans may be used in the development of novel functional foods, which can be included in dietary strategies for human health promotion.
Introduction
The compositional and functional link-up between intestinal microbiota and the host is mainly affected by the diet. So, the intestinal microbiota is capable of metabolizing the dietary components stimulating the proliferation and metabolic activity of bacterial populations.1
In this context, prebiotic carbohydrates are important com-ponents of healthy diets and represent a useful dietary approach supporting healthful hindgut microbiota.“A dietary prebiotic is a selectively fermented ingredient that promotes specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health”.2 Prebiotics are non-digestible by digestive enzymes in the human gut and enter the gut without modify-ing their structure. None are excreted in faeces, which may indicate that they are fermented by gut microbiota to produce
SCFA (acetate, propionate and butyrate),L-lactate, CO2and H2. The beneficial health effects of prebiotics are related to their influence on the gut microbiota composition, to the stimu-lation of growth, metabolism and activities of lactic acid bac-teria, bifidobacteria and other emergent strains such as Roseburia intestinales and Faecalibacterium prausnitzii3,4in the human intestine, exerting several functional properties, such as prevention of pathogen adhesion and colonization, modu-lation of bowel habits, regumodu-lation of lipid and glucose metab-olism, and the influence of the intestinal metabolome.5 Likewise, growth of these beneficial bacteria promotes indirectly the stimulation of the immune system through IgA and IL (IL1, IL6 andγ-IL) production.6
In recent years, a large number of studies in the area of pre-biotics have been focused on purified oligosaccharides from renewable sources to evaluate their impact on the gut micro-biota composition and their metabolic products and have been reported to potentially provide similar beneficial effects to those of inulin-type fructans.1,7–9However, few studies have been carried out using food sources rich in prebiotic com-ponents without the need to extract these compounds.
Very recently, it has been demonstrated that legumes could be used as a useful dietary tool to overcome gut dysbiosis.10 Pulses contain proteins, important vitamins and minerals (e.g., folate and iron, respectively) as well as antioxidants, very small amounts of unsaturated fats and complex carbohydrates †Present address: Chemical & Environmental Engineering Department,
Univer-sity of the Basque Country UPV/EHU, Plaza Europa 1, 20018 Donostia-San Sebastian, Spain.
‡Present address: Department of Chemical Engineering, Institute of Technology, University of Santiago de Compostela, 15782 Santiago de Compostela, Spain. CBQF– Centro de Biotecnologia e Química Fina – Laboratório Associado, Escola Superior de Biotecnologia, Universidade Católica Portuguesa/Porto, Rua Arquiteto Lobão Vital, Apartado 2511, 4202-401 Porto, Portugal. E-mail: beatriz.gullon@usc.es
Published on 28 July 2015. Downloaded by University of Winnipeg on 07/08/2015 15:29:49.
View Article Online
(e.g., fibers, resistant starch and oligosaccharides as α-galacto-sides or raffinose family oligosaccharides (RFOs)). Pulse ingre-dients could, therefore, constitute a very good source of growth factors and prebiotic components that are relevant to gastro-intestinal health and can be used for food supplementation offering the possibility of improving the formulation of func-tional foods.11Pulses such as lupin seeds (Lupinus albus) and broad beans (Vicia faba) are two good sources of prebiotic carbohydrates. Lupin seeds seem to contain the highest amount ofα-galactosides among the pulse species (7–15%)12 and ajugose is present in significant amounts (0.3–2%).13In broad beans verbascose is the main oligosaccharide in the seed.13 α-Galactosides are oligosaccharides, which are not digested in the upper part of the gastrointestinal tract, due to the absence of α-galactosidase among human endogenous enzymes, and are therefore available for bacterial fermentation in the intestine.14
To our knowledge, research on the prebiotic potential of lupin seeds and broad beans is very limited and none have investigated their effects on the metabolism and population dynamics of intestinal microbiota. Therefore, the main objec-tive of this work was to evaluate the impact of two traditional Portuguese pulses (lupin seeds and whole broad beans) on the intestinal microbiota composition and metabolomic output using an in vitro fermentation system.
Results and discussion
In the present study, we aimed at investigating the impact of lupin seeds and broad beans on the dynamics and meta-bolome of the colonic microbiota; these pulses are character-ized by a high content in dietary fiber that includes various complex polysaccharides and constitute a potential source of prebiotics scarcely explored.1Fermentations were followed by measuring the production of SCFA (acetate, propionate and butyrate) and lactate in the media, the pH shifts and the changes of the microbial populations by FISH. For comparative purposes, similar information was obtained in assays using either FOS (which have a recognized prebiotic effect, positive control),15or a medium without the carbon source (negative control).
Changes in bacterial populations in faecal batch cultures analyzed by FISH
The quantitative changes in the human fecal bacterial popu-lations observed in the in vitro cultures are summarized in Table 2. In the negative control cultures, all bacterial groups remained without significant variation throughout fermenta-tion; this was not the case for the two tested legumes and FOS where all groups revealed significant variations (P < 0.05) at the fermentation times assayed (see Table 2), with the excep-tion of the Bacteroides/Prevotella group and Clostridium histo-lyticum group clusters I, and II for which no significant variations were detected during the first 7 h of incubation between the addition of assayed substrates (legumes and FOS) and the control, but at 24 h of fermentation significant di ffer-ences were observed (P < 0.05). Moreover, no significant vari-ations were detected in total cell counts between lupin seeds, broad beans and FOS (P > 0.05).
In relation to the specific microbial groups, the results obtained with the selected whole legumes exhibited a certain degree of selectivity for Bifidobacterium spp., Clostridium coc-coides-Eubacterium rectale, Faecalibacterium prausnitzii and Roseburia intestinalis (see Table 2).
The increases in Bifidobacterium spp. were higher for media containing lupin seeds and broad beans (1.17–1.11 log-fold, respectively) and FOS (0.81 log-fold) than in the negative control. The shifts in the Bifidobacterium spp. population were similar for lupin seeds and broad beans (P > 0.05) and signifi-cant differences (P < 0.05) were found in comparison with FOS at 24 h of fermentation. This result confirms the suitability of these substrates as carbon sources for the growth of bifido-bacteria. Therefore, lupin seeds and broad beans caused stron-ger bifidogenic effects than FOS. The bifidogenic in vitro response of the fecal microbiota of purified GOS has been well reported.16–18However to our knowledge, there is no study that investigated the evaluation of the bifidogenic profile in legumes. Rycroft et al. (2001)19reported that commercial GOS increased the level of bifidobacteria (an increase of 0.61 log cells per mL) in human faecal batch culture fermentations. Sanz et al. (2005)20 showed that bifidobacterial populations increased with various galactose-containing disaccharides, so the increase of this group growing on 6β-galactobiose was 0.82 log cells per mL. Similar to our results, Cardelle-Cobas et al.
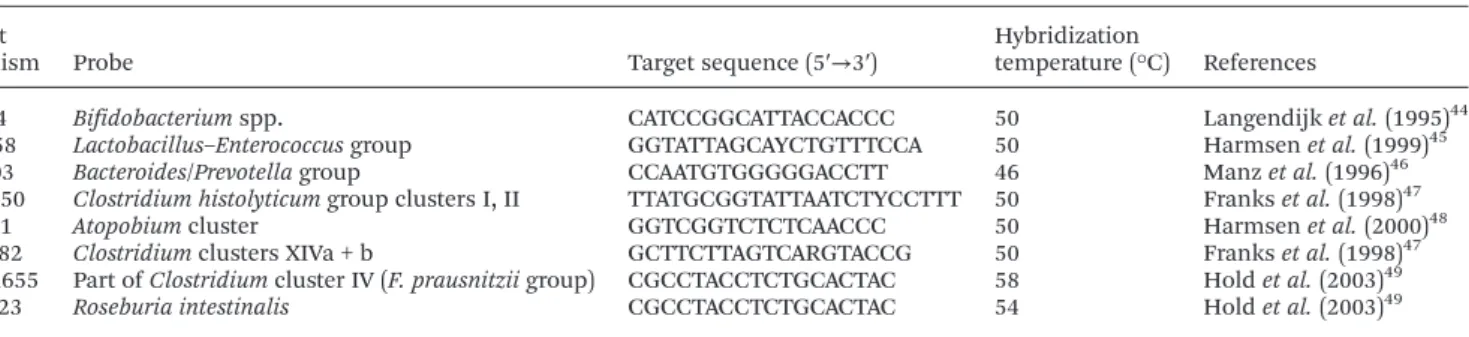
Table 1 Oligonucleotide probes for FISH used in this study Target
organism Probe Target sequence (5′→3′)
Hybridization
temperature (°C) References
Bif164 Bifidobacterium spp. CATCCGGCATTACCACCC 50 Langendijk et al. (1995)44 Lab158 Lactobacillus–Enterococcus group GGTATTAGCAYCTGTTTCCA 50 Harmsen et al. (1999)45
Bac303 Bacteroides/Prevotella group CCAATGTGGGGGACCTT 46 Manz et al. (1996)46 Chis150 Clostridium histolyticum group clusters I, II TTATGCGGTATTAATCTYCCTTT 50 Franks et al. (1998)47
Ato291 Atopobium cluster GGTCGGTCTCTCAACCC 50 Harmsen et al. (2000)48 Erec482 Clostridium clusters XIVa + b GCTTCTTAGTCARGTACCG 50 Franks et al. (1998)47
Fprau655 Part of Clostridium cluster IV (F. prausnitzii group) CGCCTACCTCTGCACTAC 58 Hold et al. (2003)49 Rint623 Roseburia intestinalis CGCCTACCTCTGCACTAC 54 Hold et al. (2003)49
(2009)18also observed high levels of bifidobacterial population when the faecal slurry culture grew in oligosaccharides derived from lactose and lactulose. Recently, Rodriguez-Colinas et al. (2013)16assessed the in vitro fermentation of several purified galactooligosaccharides (GOS) and reported minor changes in the content of bifidobacteria (an increase of 0.15 log cells per mL) in comparison with those obtained in this study.
The bacterial species that are responsible for the mainten-ance of the normobiosis in the human gut microbiota are not well clarified, but it is widely considered that bifidobacteria play an important role,21and the increases in their population might promote the well-being of the host. Favorable shifts in SCFA profiles were found in the present study in cultures using faecal inocula, attributed not only to the single bifido-genic effect previously described (shown by FISH analyses), but also to the change of other microbial groups that may metabolize acetate and produce propionate or butyrate via a metabolic cross-feeding mechanism22such as Roseburia spp.
The Lactobacillus–Enterococcus group and the Atopobium cluster showed a similar growth with all carbon sources tested, and no significant differences were found between the tested substrates (P > 0.05), having registered an increase in cell counts of 1.15 log-fold for legumes and 0.8 log-fold for FOS. This is an important observation to uphold the robustness of our results since FOS has previously been reported by other authors to enhance the growth of lactobacilli and Atopo-bium.9,19 However, to the best of our knowledge, this is the first evidence showing the stimulatory effect on intestinal lactobacilli and Atopobium populations after in vitro fermenta-tion with broad beans and lupin seeds. These results are in agreement with the data reported in related studies; Hernandez-Hernandez et al. (2011)23reported an increase in the population of these bacterial groups upon fermentation of the alternansucrase raffinose-derived oligosaccharides. Conver-sely, Rodriguez-Colinas et al. (2013)16did not observe stimu-lation of these bacterial groups on fermentation of different purified galactooligosaccharides.
The Bacteroides–Prevotella group, which comprises about 30% of the total colonic culturable microbiota, displayed a similar increase during the first 7 h of incubation with both legumes and FOS as shown in Table 2, whereas at 24 h of fer-mentation the growth was slightly more pronounced (P < 0.05) for broad beans and FOS (1.12 log-fold and 1.2 log-fold, respectively) than for lupin seeds (0.89 log-fold increase). Maccaferri et al. (2012)1demonstrated that PF (a pulse flour of lentils and chickpeas (50 : 50)) induced an overall increase of this bacterial group, known to be primary propionate and acetate producers.
A similar behaviour was observed for Clostridium histolyti-cum group clusters I, and II growing both on legumes and FOS where all growth substrates enabled a similar increase (P > 0.05) in cell numbers (0.63, 0.69 and 0.83 log-fold for lupin seeds, broad beans and FOS, respectively) during fer-mentation. Rodriguez-Colinas et al. (2013)16 observed slight increases of the Chis150 group (0.56 log cells per mL) growing on purified allolactose + 6-galactobiose; this group is specifi-cally related to propionate production as reported in this study in the next section.
Clostridium coccoides–Eubacterium rectale increased signifi-cantly over the 24 h fermentation representing the third most stimulated bacterial group by the supplementation with legumes. Significant differences (P < 0.05) were observed for the two legumes in comparison with the FOS cultures over fer-mentation (0.99, 1.06 and 0.81 log-fold for lupin seeds, broad beans and FOS, respectively), yet no significant differences between lupin seeds and broad beans were observed (P > 0.05). Interestingly, this result is not in agreement with that reported by Maccaferri et al. (2012)1who observed a decrease in the cell numbers of this bacterial group in a medium supplemented with PF. Such differences in behavior seem to indicate a legume-dependent effect, an observation that this study also corroborated with differences reported between lupin seeds and broad beans for some bacterial groups studied. On the other hand, Rodriguez-Colinas et al. (2013)16 reported that purified
Table 2 Bacterial populations in faecal batch cultures measured at 0, 7 and 24 h after the onset of fermentation (expressed as log cells per mL ± SD)
Lupin seeds Broad beans FOS Blank
Group 0 h 7 h 24 h 7 h 24 h 7 h 24 h 7 h 24 h DAPI 7.94 ± 0.08 9.01b± 0.03 9.61b± 0.02 8.90b± 0.13 9.61b± 0.06 8.99b± 0.15 9.59b± 0.05 8.37a± 0.11 8.49a± 0.17 Bif164 7.36 ± 0.03 8.21b± 0.05 8.53c± 0.05 8.06a,b± 0.06 8.47c± 0.08 7.99b± 0.04 8.17b± 0.10 7.37a± 0.07 7.59a± 0.09 Lab158 7.43 ± 0.07 8.14b± 0.08 8.53b± 0.10 8.00b± 0.12 8.47b± 0.15 8.09b± 0.05 8.23b± 0.05 7.34a± 0.08 7.49a± 0.06 Bac303 7.50 ± 0.06 8.10a± 0.04 8.39a± 0.07 8.07a± 0.12 8.62b± 0.04 8.34a± 0.05 8.70b± 0.08 8.15a± 0.18 8.29a± 0.10 Chis150 7.67 ± 0.05 8.35a± 0.06 8.30a± 0.10 8.21a± 0.09 8.36a± 0.05 8.29a± 0.11 8.50a± 0.16 8.11a± 0.14 8.29a± 0.04 Ato291 7.35 ± 0.07 8.29b± 0.06 8.70b± 0.13 8.19b± 0.15 8.46b± 0.12 8.18b± 0.06 8.41b± 0.03 7.53a± 0.08 7.85a± 0.15 Erec482 7.49 ± 0.07 8.40c± 0.03 8.48b,c± 0.07 8.34c± 0.08 8.55c± 0.12 8.13b± 0.05 8.30b± 0.02 7.76a± 0.11 7.92a± 0.10 Fprau655 7.38 ± 0.06 8.31b± 0.14 8.83c± 0.08 8.12b± 0.04 8.65b,c± 0.15 8.21b± 0.06 8.48b± 0.08 7.75a± 0.15 7.89a± 0.11 Rint623 7.35 ± 0.05 8.11b,c± 0.08 8.80c± 0.08 8.18c± 0.04 8.82c± 0.13 7.95b± 0.09 8.40b± 0.15 7.38a± 0.04 7.52a± 0.09
Total bacterial counts were obtained using DAPI and bacterial groups with FISH probes. Mean bacterial count ± standard deviation (n = 3). Different letters indicate significant differences (P < 0.05) for each bacterial group. Substrates were compared for two fermentation sampling times (7 and 24 h).
galactooligosaccharides caused slight changes in the population of C. coccoides–E. rectale, which is, in fact, the major butyrate-producing bacterial groups found in human faeces.
Faecalibacterium prausnitzii and Roseburia intestinalis were the two species most stimulated by the addition of either of the two legumes. Their numbers increased significantly over the 24 h fermentation period, independently of the substrate added, but these were significantly higher for both legumes than for FOS (P < 0.05), yet between lupin seeds and broad beans no significant differences were found (P > 0.05). F. praus-nitzii registered increases of 1.45, 1.27 and 1.10 log-fold for lupin seeds, broad beans and FOS, respectively and for R. intes-tinalis differences of 1.45, 1.47 and 1.13 log-fold with respect to the initial count were observed for lupin seeds, broad beans and FOS, respectively. The certain degree of selectivity exhibi-ted by lupin seeds and broad beans for these bacterial groups confirmed their better suitability as carbon sources for the metabolism of F. prausnitzii and R. intestinalis against FOS. However, our results are not in agreement with those reported by Maccaferri et al. (2012)1 who found a low increase in F. prausnitzii during the fermentation of a mixture of chickpeas and lentils (50 : 50).
The fact that these legumes promoted the growth dynamics of these two groups is very interesting because the populations of F. prausnitzii are reported to be decreased in Crohn’s disease and, in addition, recent evidence24 strongly suggests that F. prausnitzii produces a separate anti-inflammatory factor25 and is active in ulcerative colitis related to butyrate production.26Another feature of Crohn’s disease is the detec-tion of antibodies against commensal bacteria. Antibodies against flagellar antigens include those that target flagella of bacteria that are related to E. rectale and Roseburia spp.27 More-over, Roseburia spp. is associated with insoluble fibers and fer-ments these fibres as an energy source. Walker et al. (2008)28 and Duncan et al. (2007)29have previously shown a correlation between Roseburia spp. and butyrate production in obese patients on a carbohydrate-resistant diet. Based on this infor-mation, it is not yet clear that a decreased population of these
groups is beneficial for the host’s health. On the other hand, we must take into account that in vitro and in vivo experience indicates that a group affined to Roseburia and E. rectale per-forms a main function in mediating the butyrogenic effect of fermentable dietary carbohydrates.30
Short chain fatty acid (SCFA) production in faecal cultures The changes observed in the SCFA profile were in agreement with the microbial population dynamics data obtained through FISH described in the above section. Table 3 shows the concentrations of SCFA and lactate throughout the different fermentations, the ratio acetate/propionate (A/P) and the pH shifts resulting from the generation of acids. Both the increase in SCFA amount and the decrease in pH during the fermentation were considerably more pronounced in the pres-ence of carbon sources than in negative control cultures. Acetic acid was the most abundant SCFA followed by propionic and butyric acids that achieved similar concentrations by the end of the fermentation period (see Table 3). It should be noted that SCFA generation in cultures lacking a carbon source is due to degradation of proteins by putrefactive bac-teria present in the intestinal microbiota. Consequently, low SCFA formation in negative control cultures is obtained.
Lactic acid was detected only during the first 7 h of fermen-tation of carbon sources; a possible cause for its disappearance during the full fermentation process could be the fact that it can be converted into SCFA by different bacterial species31,32 that use the end products of other species as a substrate in a metabolic cross-talk.
Acetic acid was clearly the most prevalent SCFA with all the substrates tested, and reached the highest concentrations in media containing legumes (51 mM) after 48 h of fermentation. Acetic acid acts as an energy substrate for muscle tissue. The predominant formation of acetic acid is in agreement with the results reported by Rodriguez-Colinas et al. (2013)16for the faecal fermentation of purified galactooligosaccharides. Bifido-bacteria, lactobacilli and some microorganisms of the Atopo-bium cluster33,34 are lactic acid producers; acetic acid is
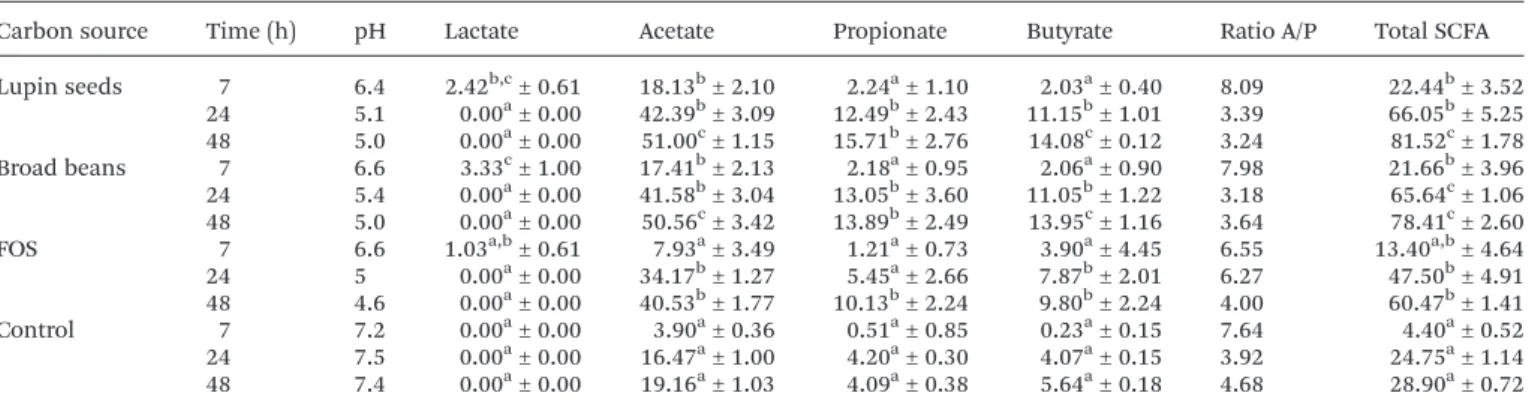
Table 3 Mean values of lactic acid and short chain fatty acid concentrations (mM) and pH of fermentation media prepared with lupin seeds, broad beans or FOS as carbon sources at 7, 24, and 48 h. The pH values and ratio acetate/propionate are also shown
Carbon source Time (h) pH Lactate Acetate Propionate Butyrate Ratio A/P Total SCFA Lupin seeds 7 6.4 2.42b,c± 0.61 18.13b± 2.10 2.24a± 1.10 2.03a± 0.40 8.09 22.44b± 3.52 24 5.1 0.00a± 0.00 42.39b± 3.09 12.49b± 2.43 11.15b± 1.01 3.39 66.05b± 5.25 48 5.0 0.00a± 0.00 51.00c± 1.15 15.71b± 2.76 14.08c± 0.12 3.24 81.52c± 1.78 Broad beans 7 6.6 3.33c± 1.00 17.41b± 2.13 2.18a± 0.95 2.06a± 0.90 7.98 21.66b± 3.96 24 5.4 0.00a± 0.00 41.58b± 3.04 13.05b± 3.60 11.05b± 1.22 3.18 65.64c± 1.06 48 5.0 0.00a± 0.00 50.56c± 3.42 13.89b± 2.49 13.95c± 1.16 3.64 78.41c± 2.60 FOS 7 6.6 1.03a,b± 0.61 7.93a± 3.49 1.21a± 0.73 3.90a± 4.45 6.55 13.40a,b± 4.64
24 5 0.00a± 0.00 34.17b± 1.27 5.45a± 2.66 7.87b± 2.01 6.27 47.50b± 4.91 48 4.6 0.00a± 0.00 40.53b± 1.77 10.13b± 2.24 9.80b± 2.24 4.00 60.47b± 1.41
Control 7 7.2 0.00a± 0.00 3.90a± 0.36 0.51a± 0.85 0.23a± 0.15 7.64 4.40a± 0.52 24 7.5 0.00a± 0.00 16.47a± 1.00 4.20a± 0.30 4.07a± 0.15 3.92 24.75a± 1.14
48 7.4 0.00a± 0.00 19.16a± 1.03 4.09a± 0.38 5.64a± 0.18 4.68 28.90a± 0.72 The starting concentration of the test substrates was 1% (w/v). Mean values ± standard deviation (n = 3). Different letters indicate significant differences (P < 0.05) for the same acid. Substrates were compared for three points of fermentation (7, 24 and 48 h).
formed by many anaerobic bacteria from the human tract,35 also being a major end product of carbohydrate fermentation for bifidobacteria. However, it is complicated to establish a relation-ship between the production of these acids with a specific bac-terial genus when the assays are carried out with faecal slurries. The exponential increase in acetic acid concentration during the first 7 h of fermentation was in agreement with the increment of total bacterial count as well as with the increases in lactobacilli, bifidobacteria and Atopobium (see Table 2).
Regarding propionic and butyric acid concentrations, the highest values were detected after 48 h of fermentation of legumes in comparison with FOS (see Table 3). Propionate is utilized primarily by the liver and converted to glucose, it may also modify hepatic metabolism and its role as a potential modulator of cholesterol synthesis has been proposed.36 Enhanced generation of acids in intestinal fermentation might be desirable because acidic environments can inhibit the growth of harmful microorganisms.15
Butyrate is the main source of energy for the colony epi-thelial cells that constitute the epiepi-thelial lining of the large intestine, and its increased production by gut bacteria has been linked to a reduced incidence of colon cancer21since it protects these cells against agents that lead to cellular differen-tiation and may even inhibit tumour growth.37An important observation is the good correlation observed between butyrate generation and growth of producer bacterial groups assayed in this study (see Tables 2 and 3); that was in agreement with the fact that the majority of butyrate-producing bacteria are thought to be included in the Clostridium clusters XIV a + b and IV and Roseburia/Eubacterium rectale and Faecalibacterium groups, belonging to the first and second clusters respectively, and are probably the most prolific butyrate producers in the human colon.38,39
The generation of acetate and propionate observed in this work, an indicator of the susceptibility to fermentability of lupin seeds and broad beans, is in agreement with the results reported by Maccaferri et al. (2012)1that demonstrated that a mixture of chickpeas and lentils (50 : 50) increased the concen-tration of acetate and propionate; this metabolic shift can be explained by the overall increase of Bacteroides/Pretovella species known to be primary propionate and acetate produ-cers, which represent a considerable share of the intestinal microbiota resulting from the supplementation with the pulse mixture. Similar findings were found in this work; so, the increase in the acetic and propionic acids coincided with the increase in the above cited bacterial groups.
Table 3 shows the ratio acetate/propionate (A/P) and we can observe that this proportion decreased over the incubation time. The ratio A/P decreased markedly during the period 7 h to 24 h in the case of both legumes whereas during the period 24 h to 48 h the decrease was less evident; this ratio declined smoothly for FOS during the period 7 h to 24 h and in the last 24 h the decrease was more marked (see Table 3). In the present study, the average ratio of acetic : propionic acids for lupin seeds was 3.39 and for broad beans was 3.18 at 24 h; these results compared favourably with those reported by other
authors.8,40 This result is interesting because the reduction in the acetic to propionic acid ratio has been proposed as an indi-cator of a potential hypolipidemic effect of prebiotics (inhi-bition of cholesterol and fatty acid biosynthesis in the liver, which finally results in a decrease in lipid levels in blood).41
Overall, SCFA production increased during the 48 h of fer-mentation in all the substrates studied and reported the highest production rates in the following order: lupin seeds > broad beans > FOS; at 24 h and 48 h of fermentation, both legumes registered similar fermentation patterns but showed statistical differences with FOS (P < 0.05). The moderately higher production of SCFA in the presence of legumes with respect to FOS could be attributed to the additive effects of the different compounds present in the legumes.
Experimental
Sample preparation
Lupin seeds (Lupinus albus) and broad beans (Vicia faba) were obtained from a local distributor in Porto (Portugal) and ground firstly by using a Bimby/Thermomix followed by using a coffee grinder to obtain a fine pulse powder.
In vitro simulation of human gastrointestinal transit of the legumes
An in vitro human gastrointestinal transit model that chemi-cally simulates the physiological conditions from the mouth to the small intestine42 was used to study the digestibility of lupin seeds and broad beans throughout the gastrointestinal tract. Digestibility was performed by enzymes and acid and basic solutions and absorption was simulated using a dialysis membrane of 1 kDa molecular weight cut-off to remove the low molecular mass digestion products. The sample obtained was stored immediately at −20 °C, before freeze-drying for 72 h and lyophilized. These samples were used for the in vitro anaerobic fermentation. HPLC (High Performance Liquid Chromatography) was performed to monitor the removal of small compounds from the digestion procedure, thus ensuring that they were not present in the fermentation step to better mimic the real situation.
Prebiotic effect assessment
Samples of lupin seeds and broad beans were assayed for their in vitro fermentability as per the method of Gullón et al. (2011).7 FOS (Sigma-Aldrich) was included as a positive control, and a medium without a carbon source was used as a negative control. Fermentation assays were carried out with 1% (w/v) of each carbon source. Samples were collected at 0, 7, 24 and 48 h of fermentation. Cells were recovered by centrifu-gation (10 000 rpm) and fixed in 4% paraformaldehyde for the analysis of bacterial populations by fluorescence in situ hybrid-ization (FISH), following the method previously described.7 The supernatants were used for the analysis of lactic acid and SCFA (by HPLC) and pH measurement. All additions and inoculations were carried out inside an anaerobic cabinet (5%
H2, 10% CO2and 85% N2). All experiments were performed in compliance with the relevant laws and institutional guidelines. Quantification of bacterial population in batch cultures by FISH
FISH was performed following the method reported else-where.7,38The probes used are reported in Table 1 and were commercially synthesized and labeled with the fluorescent dye Cy3 (Sigma-Aldrich). For the enumeration of total cells, samples were stained with the nucleic acid stain 4 ′,6-di-amidino-2-phenylindole (DAPI). The samples were examined using an epifluorescence microscope (Olympus BX41) equipped with Fluor 100 lenses. A minimum of 10 fields were counted per sample and analyzed.
Determination of fermentation products in batch cultures Supernatants from the anaerobic cultures were filtered through 0.20 μm cellulose acetate membranes, and analyzed for organic acids by HPLC. Analyses were performed using an Agilent 1200 series HPLC system with a RI detector (Agilent, Germany) and Aminex HPX-87H column (BioRad, Hercules, CA, USA). Other analysis conditions were as follows: mobile phase, 0.003 mol L−1H2SO4at 0.6 mL min−1and 50 °C.7 Statistical analysis
Univariate analysis of variance (ANOVA) and the post hoc Tukey test were used to determine the significance of the effect of lupin seeds and broad beans on bacterial group populations and SCFA production. All statistical analysis were performed using SPSS for Windows version 21.0 (IBM SPSS, Chicago, IL) and differences were considered to be statistically significant at P≤ 0.05.
Conclusions
The work presented here is the first study to investigate the influence of the fermentation of broad beans and lupin seeds on the complex faecal microbiota in vitro. The results indicated that compounds present in legumes have a potential prebiotic effect reflected in the increases in growth of several groups present in the intestinal microbiota and that are important for the normobiosis of the colon. Lupin seeds and broad beans were able to similarly stimulate the growth of certain numeri-cally predominant bacterial groups in the human intestinal microbiota, Bifidobacterium spp., Lactobacillus–Enterococcus group, Bacteroides–Pretovella group, F. prausnitzii and Roseburia intestinalis being the most favoured. As a consequence of the increase of the bacterial groups, the generation of SCFA occurred, the major SCFA being acetic, propionic and butyric acids. This effect is better than that observed for FOS which is recognized as the gold standard of a prebiotic ingredient and this fact can be attributed to the heterogeneous and rich com-position of legumes. Our findings support the incorporation of the tested legumes in the formulation and development of a variety of food products (for example: pasta from legume
flours) and could be a useful dietary tool to manipulate the gut microbiota-mediated well-being endpoint as reported by other authors.43
Abbreviations
A/P Ratio acetate/propionate ArXOS Arabinoxylooligosaccharides DAPI 4′-6-Diamidino-2-phenylindole FISH Fluorescent in situ hybridization FOS Fructooligosaccharides
GOS Galactooligosaccharides
HPLC High performance liquid chromatography
IgA Immunoglobulin A
IL Interleukin
RI Refraction index SCFA Short chain fatty acids TSB Trypticase soy broth YNB Yeast nitrogen base.
Acknowledgements
B. Gullón and P. Gullón are grateful to the Portuguese Foun-dation for Science and Technology (Fundação para a Ciência e Tecnologia, FCT, Portugal) for funding through the post-doctoral fellowship references SFRH/BPD/79941/2011 and SFRH/BPD/79942/2011, respectively. The authors are also grateful to FCT (Fundação para a Ciência e Tecnologia, Portu-gal) for financial support obtained via the project PROSKIN– Exploiting the use of Probiotics in skin (EXPL/BBB-BIO/1113/ 2013) and the project PEst-OE/EQB/LA0016/2011.
References
1 S. Maccaferri, A. Klinder, S. Cacciatore, R. Chitarrari, H. Honda, C. Luchinat, I. Bertini, P. Carnevali, G. R. Gibson, P. Brigidi and A. Costabile, Mol. Nutr. Food Res., 2012, 56, 1342–1352.
2 M. Roberfroid, G. R. Gibson, L. Hoyles, A. L. McCartney, R. Rastall, I. Rowland, D. Wolvers and A. Meheust, Br. J. Nutr., 2010, 104, S1–S63.
3 S. H. Duncan, G. L. Hold, A. Barcenilla, C. S. Stewart and H. J. Flint, Int. J. Syst. Evol. Microbiol., 2002, 52, 1615–1620. 4 K. P. Scott, J. C. Martin, S. H. Duncan and H. J. Flint, FEMS
Microbiol. Ecol., 2014, 87, 30–40.
5 M. Candela, S. Maccaferri, S. Turroni, P. Carnevali and P. Brigidi, Int. J. Food Microbiol., 2010, 140, 93–101.
6 C. Martínez-Villaluenga, J. Frias and C. Vidal-Valverde, Crit. Rev. Food Sci. Nutr., 2008, 48, 301–316.
7 B. Gullón, P. Gullón, Y. Sanz, J. L. Alonso and J. C. Parajó, LWT– Food Sci. Technol., 2011, 44, 1687–1696.
8 B. Gullón, P. Gullón, F. Tavaria, M. Pintado, A. M. Gomes and J. L. Alonso, J. Funct. Foods, 2014, 6, 438–449.
9 B. Gómez, B. Gullón, C. Remoroza, H. A. Schols, J. C. Parajó and J. L. Alonso, J. Agric. Food Chem., 2014, 62, 9769–9782.
10 C. R. Johnson, D. Thavarajah, G. F. Combs and P. Thavarajah, Food Res. Int., 2013, 51, 107–113.
11 F. Zare, C. P. Champagne, B. K. Simpson, V. Orsata and J. I. Boye, LWT– Food Sci. Technol., 2012, 45, 155–160. 12 C. Martínez-Villaluenga, J. Frias and C. Vidal-Valverde,
Food Chem., 2005, 91, 645–649.
13 N. R. Reddy, M. D. Pierson, S. K. Sathe and D. K. Salunkhe, Food Chem., 1984, 13, 25–69.
14 F. Guillon and M. M. Champ, Br. J. Nutr., 2002, 88, S293– S306.
15 M. Rossi, C. Corradini, A. Amaretti, M. Nicolini, A. Pompei and S. Zanoni, Appl. Environ. Microbiol., 2005, 71, 6150– 6158.
16 B. Rodriguez-Colinas, S. Kolida, M. Baran, A. O. Ballesteros, R. A. Rastall and F. J. Plou, Appl. Microbiol. Biotechnol., 2013, 97, 5743–5752.
17 A. Cardelle-Cobas, N. Corzo, A. Olano, C. Peláez, T. Requena and M. Ávila, Int. J. Food Microbiol., 2011, 149, 81–87.
18 A. Cardelle-Cobas, M. Fernandez, N. Salazar, C. Martinez-Villaluenga, M. Villamiel, P. Ruas-Madiedo and C. de los Reyes-Gavilan, J. Dairy Res., 2009, 76, 317–325.
19 C. E. Rycroft, M. R. Jones, G. R. Gibson and R. A. Rastall, J. Appl. Microbiol., 2001, 91, 878–887.
20 M. L. Sanz, G. R. Gibson and R. A. Rastall, J. Agric. Food Chem., 2005, 53, 5192–5199.
21 M. L. Connolly, J. A. Lovegrove and M. K. Tuohy, J. Funct. Foods, 2010, 2, 219–224.
22 A. Belenguer, S. H. Duncan, A. Graham Calder, G. Holtrop, P. Louis, G. E. Lobley and H. J. Flint, Appl. Environ. Micro-biol., 2006, 72, 3593–3599.
23 O. Hernandez-Hernandez, G. L. Côté, S. Kolida, R. A. Rastall and M. L. Sanz, J. Agric. Food Chem., 2011, 59, 10901–10906.
24 C. Manichanh, L. Rigottier-Gois, E. Bonnaud, K. Gloux, E. Pelletier, L. Frangeul, R. Nalin, C. Jarrin, P. Chardon, P. Marteau, J. Roca and J. Dore, Gut, 2006, 55, 205–211. 25 H. Sokol, B. Pigneur, L. Watterlot, O. Lakhdari,
L. G. Bermúdez-Humarán, J. J. Gratadoux, S. Blugeon, C. Bridonneau, J. P. Furet, G. Corthier, C. Grangette, N. Vasquez, P. Pochart, G. Trugnan, G. Thomas, H. M. Blottière, J. Doré, P. Marteau, P. Seksik and P. Langella, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16731– 16736.
26 J. Segain, D. R. De La Blétiere, A. Bourreille, V. Leray, N. Gervois, C. Rosales, L. Ferrier, C. Bonnet, H. Blottiere and J. Galmiche, Gut, 2000, 47, 397–403.
27 L. W. Duck, M. R. Walter, J. Novak, D. Kelly, M. Tomasi, Y. Cong and C. O. Elson, Inflamm. Bowel Dis., 2007, 13, 1191–1201.
28 A. W. Walker, S. H. Duncan, H. J. M. Harmsen, G. Holtrop, G. W. Welling and H. J. Flint, Environ. Microbiol., 2008, 10, 3275–3283.
29 S. H. Duncan, A. Belenguer, G. Holtrop, A. M. Johnstone, H. J. Flint and G. E. Lobley, Appl. Environ. Microbiol., 2007, 73, 1073–1078.
30 P. Louis, K. P. Scott, S. H. Duncan and H. J. Flint, J. Appl. Microbiol., 2007, 102, 1197–1208.
31 C. Bourriaud, R. J. Robins, L. Martin, F. Kozlowski, E. Tenailleau, C. Cherbut and C. Michel, J. Appl. Microbiol., 2005, 99, 201–212.
32 S. A. Hughes, P. R. Shewry, G. R. Gibson, B. V. McCleary and R. A. Rastall, FEMS Microbiol. Ecol., 2008, 64, 482–493. 33 F. E. Dewhirst, B. J. Paster, N. Tzellas, B. Coleman,
J. Downes, D. A. Spratt and W. G. Wade, Int. J. Syst. Evol. Microbiol., 2001, 51, 1797–1804.
34 J. Downes, M. A. Munson, D. A. Spratt, F. Kononen, E. Trakka, H. Jousimies-Somer and W. G. Wade, J. Med. Microbiol., 2001, 50, 947–951.
35 G. T. Macfarlane and G. R. Gibson, in Gastrointestinal Microbiology, ed. R. I. Mackie and B. A. White, Chapman and Hall, London, England, 1997, pp. 269–318.
36 L. D. Bourquin, E. C. Titgemeyer, K. A. Garleb, C. George and J. R. Fahey, J. Nutr., 1992, 122, 1508–1520.
37 G. P. Young and P. R. Gibson, Butyrate and the colorectal cancer cell, in Short Chain Fatty Acids, ed. H. J. Binder, J. Cummings and K. Soergel, 1994, pp. 148–160.
38 N. Salazar, P. Ruas-Madiedo, S. Kolida, M. Collins, R. Rastall, G. Gibson and C. G. de los Reyes-Gavilán, Int. J. Food Microbiol., 2009, 135, 260–267.
39 R. F. J. Benus, T. S. van der Werf, G. W. Welling, P. A. Judd, M. A. Taylor, H. J. M. Harmsen and K. Whelan, Br. J. Nutr., 2010, 104, 693–700.
40 S. F. Reis, B. Gullón, P. Gullón, S. Ferreira, C. J. Maia, J. L. Alonso, F. C. Domingues and N. Abu-Ghannam, Appl. Microbiol. Biotechnol., 2014, 98, 9365–9373.
41 N. M. Delzenne and N. Kok, Am. J. Clin. Nutr., 2001, 73, 456–458.
42 D. J. S. Mills, K. M. Tuohy, J. Booth, M. Buck, M. J. C. Crabbe, G. R. Gibson and J. M. Ames, J. Appl. Micro-biol., 2008, 105, 706–714.
43 F. Russo, C. Clemente, M. Linsalata, M. Chiloiro, A. Orlando, E. Marconi, G. Chimienti and G. Riezzo, Eur. J. Nutr., 2011, 50, 271–277.
44 P. S. Langendijk, F. Schut, G. J. Jansen, G. W. Raangs, G. R. Kamphuis, M. H. F. Wilkinson and G. W. Welling, Appl. Environ. Microbiol., 1995, 61, 3069–3075.
45 H. J. M. Harmsen, P. Elfferich, F. Schut and G. W. Welling, Microb. Ecol. Health Dis., 1999, 11, 3–12.
46 W. Manz, R. Amann, W. Ludwig, M. Vananneyt and K. H. Schleifer, Microbiology, 1996, 142, 1097–1106. 47 A. H. Franks, H. J. M. Harmsen, G. C. Raangs, G. J. Jansen,
F. Schut and G. W. Welling, Appl. Environ. Microbiol., 1998, 64, 3336–3345.
48 H. J. M. Harmsen, A. C. M. Wildeboer-Veloo, J. Grijpstra, J. Knol, J. E. Degener and G. W. Welling, Appl. Environ. Microbiol., 2000, 66, 4523–4527.
49 G. L. Hold, A. Schwiertz, R. I. Aminov, M. Blaut and H. J. Flint, Appl. Environ. Microbiol., 2003, 69, 4320–4324.