w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Schinus
terebinthifolius
administration
prevented
behavioral
and
biochemical
alterations
in
a
rotenone
model
of
Parkinson’s
disease
Adriana
Sereniki
a,
Cybelle
F.B.
Linard-Medeiros
a,
Shirliane
N.
Silva
b,
Juciene
B.R.
Silva
a,
Tadeu
J.S.
Peixoto
Sobrinho
b,
Juliano
R.
da
Silva
a,
Lariza
D.S.
Alves
a,
Soraya
S.
Smaili
c,
Almir
G.
Wanderley
a,b,∗,
Simone
S.L.
Lafayette
a,baDepartamentodeCiênciasFarmacêuticas,UniversidadeFederaldePernambuco,Recife,PE,Brazil
bDepartamentodeFisiologiaeFarmacologia,UniversidadeFederaldePernambuco,Recife,PE,Brazil
cDepartamentodeFarmacologia,UniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8July2015 Accepted30November2015 Availableonline29January2016
Keywords:
Antioxidanteffects Lipidicperoxidation Parkinson’sdisease Rotenone
Schinusterebinthifolius
a
b
s
t
r
a
c
t
Parkinson’sdiseaseisaneurodegenerativedisordercharacterizedbymotorimpairment,cognitive declineandpsychiatricsymptoms.SchinusterebinthifoliusRaddi,Anacardiaceae,hadbeenstudiedfor itsanti-inflammatoryandantioxidantproperties,andinthisstudy,thestembarkwasevaluatedforthe neuroprotectiveeffectsonbehavioralandbiochemicalalterationsinducedbyadministrationsofrotenone inrats.Behavioralevaluationswereperformedusingopen-fieldandrotarod.Theinvitroandinvivo
antioxidantactivitiesweredeterminedbytheDPPHradicalscavengingactivityandlipidperoxidation methodrespectively.Theadministrationofrotenone(3mg/kg,s.c.)producedhypolocomotion,increaseof immobilityandmuscleincoordination,whilethetreatmentwithS.terebinthifoliusstembarkextract(150, 300and600mg/kgp.o.)forsevendayspreventedrotenone-induceddysfunctionalbehavior.Biochemical analysisofthesubstantianigra,striatumandcortexrevealedthatrotenoneadministrationsignificantly increasedlipidperoxidation,whichwasinhibitedbytreatmentwithalldosesofS.terebinthifolius.The resultssuggestedneuroprotectiveeffectofS.terebinthifoliuspossiblymediatedthroughitsantioxidant activity,indicatingapotentialtherapeuticbenefitofthisspeciesinthetreatmentofParkinson’sdisease. ©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
The increase of elderly populationhas led to an increasing incidenceofneurodegenerativediseasesworldwide,leading,thus, totheinterestin studiesforpreventionandtreatmentofthese pathologies.Naturalconstituentsderivedfromplantsare impor-tanttoinvestigate,sincestudieshaveshownthatthesecompounds exhibitedarangeofbiologicalactivities,withtherapeuticpotential, forinstanceantioxidantandanti-inflammatoryeffects.
Parkinson’sdisease(PD)isoneifthemajorneurodegenerative disorder in the entire world and is characterized by progres-sivedegenerationof dopamine-containingneurons thatproject from substantia nigra pars compacta to the striatum. Besides, motorimpairment(bradykinesia,rigidity,tremoratrestand dis-turbancesinbalance),cognitivedeclineandpsychiatricsymptoms (likedepression)arethecardinalsymptomsofthepathology(Dutta
∗ Correspondingauthor.
E-mail:almir.wanderley@ufpe.br(A.G.Wanderley).
and Mohanakumar, 2015; Fernandez, 2015).Main mechanisms have beenproposed toexplain the eventsculminating in neu-ronal death in PD, which are associated with oxidative stress, mitochondrialdysfunction,neuroinflammationand environmen-talexposures,likepesticides,contributingfortheappearanceof behavioral and biochemical alterations (Dauer andPrzedborski, 2003; Renaud et al., 2015). Several animal models have been usedtomimetizeandelucidatethepathogenesisofPD,inspecial rotenone,MPTPand6-hydroxydopamine(DauerandPrzedborski, 2003;Blesaetal.,2012).
Rotenone(Derrissp.,Fabaceae)hasbeenapotenthydrophobic pesticideanditsenvironmentalexposuremimicstheclinicaland pathologicalfeaturesofPD,suchasbehavioral,biochemicaland pathologicalchanges,providingareproducibleanimalmodelfor PD(Betarbetetal.,2000).Itprovokedneurodegenerationvia mul-tiplemechanisms,mainlybyinhibitionofcomplexI,increaseof oxidativestressandinductionofapoptosis(Zaitoneetal.,2012; vonWrangeletal.,2015).
SchinusterebinthifoliusRaddi,Anacardiaceae,isanativeplantof SouthAmerica(Corrêa,1974).InBrazilitispopularlycalledAroeira
http://dx.doi.org/10.1016/j.bjp.2015.11.005
orCabuí.Infolkmedicine,ithasbeenusedforthetreatmentof ulcers,respiratoryproblems,woundsandarthritis(Morton,1978) andasanantiseptic,antiinflammatoryandhaemostatic(Medeiros etal.,2007).Manyofitspropertiesorhealingeffectsattributed byfolkmedicineareassociatedtothepresenceofpolyphenolsin theplant,whichgivetheplantitsantioxidantproperties(Medeiros etal.,2007;Abdouetal.,2015).Theaimofthisstudywasto investi-gatethepossibleneuroprotectiveeffectsofS.terebinthifoliusinthe behavioractivityandontheoxidativestressinducedbyrotenone asexperimentalmodelofParkinson’sdiseaseinrats.
Materialsandmethods
Plantmaterial
StembarkofSchinusterebinthifoliusRaddi,Anacardiaceae,were collectedintheremains oftheAtlanticrainforestlocatedinthe municipality of Cabo de Santo Agostinho, Pernambuco, Brazil betweenFebruaryandApril2013(8◦20′33′′Sand34◦56′59′′W).A voucherspecimenwasauthenticatedintheDepartmentofBotany oftheFederalUniversityofPernambucobythecuratorM. Bar-bosaandwasdepositedatGeraldoMarizHerbariumunderrecord n◦.8758.Extractionwasperformedbymacerationandairdried, and500gofpulverizedS.terebinthifoliusbarkwasaddedto1.0lof ethanol70%atroomtemperature,for7days,andwasoccasionally shaken.Inthelaboratory,thecrudeethanolicextractwas evapo-ratedtodrynessunderreducedpressureforthetotalelimination ofalcohol,followedbylyophilization,yieldingapproximately35g ofdryresidue.ThelyophilizedextractofS.terebinthifoliuswaskept atroomtemperatureuntiluseandsuspendedindistilledwater.
Highperformanceliquidchromatography(HPLC)analysis
Themainphytochemicalmarkers(gallicacid,ellagicacid, cate-chinandepicatechin)inS.terebinthifoliussampleswereanalyzed bywaybywayofliquid chromatography-diodearraydetection (LC-DAD)analysisusingaShimadzusystem(LC-20AT)equipped witha photo diodearray detector (SPD-M20A).The chromato-graphicseparationwasperformedusingaGeminiRP-18column 5mparticlesizeand250mm×4.60mmi.d.(Phenomenex), pro-tectedbyaguardcolumnofthesamematerial.Agradientelution wasperformedbyvaryingtheproportionofSolventA(0.5%acetic acidindistilled water, v/v) and solventB(methanol)at a flow rateof0.8ml/minfollowinggradientprogram:20–40%B(10min), 40–60%B(10min),60%B(10min),60–40%B(10min),and40–20% B(10min). Thedried extracts and standardswere dissolvedin methanol:water(20/80,v/v)andfilteredthroughamembraneof 0.45m(Millipore®,USA)priortoinjectionof20l.Thepeaks ofeachmarkingondrysubstancewereidentifiedbycomparing retentiontimesandUVspectraofDAD.
Animals
Adultmale Wistarrats(Rattus norvegicusvar.albinus),age3 monthsandweighingbetween250and300g,wereobtainedfrom theDepartmentof PhysiologyandPharmacology attheFederal Universityof Pernambuco,Brazil.The animalsweremaintained instandardenvironmentalconditions(22±2◦C,12:12hdark/light cycle).Commercialfood(Presence®,Purina,Brazil)andwaterwere
availableadlibitum.AllprotocolswereapprovedbytheAnimal Experimentation Ethics Committee of theFederal University of Pernambuco,underlicensen◦.23076.050131/2013-82in accor-dancewiththeNationalInstituteofHealthGuidefortheCareand UseofLaboratoryAnimals.
Drugs
Butylated hydroxytoluene (BHT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), malondialdehyde (MDA), thiobarbituric acid(TBA),epinephrine,1,1,3,3-tetrametoxipropane(TMP),Tween 80, DMSO and rotenone,were purchased fromSigma–Aldrich®
(St.Louis,MO,USA).Rotenonewasdissolvedinsunfloweroilone daybeforebeginning treatment.On subsequentdays, solutions remainedintherefrigerator(4◦C).Sunfloweroilwasobtainedin localmarket.
Experimentalgroups
The animals were randomly divided into five experimental groups (n=10). Rats from Group 1 received water by gavage (1ml/kg)and1hlaterasubcutaneous(s.c.)administrationofthe vehicle(90%,w/vsunfloweroil,+10%w/vDMSO)forsevendays. Group2alsoreceivedwaterbygavageandrotenone(3mg/kg,s.c.) diluted invehicle after1hduring sevendays. Groups 3,4 and 5receivedbygavage150,300or600mg/kgofS.terebinthifolius, respectively,suspendedinwater,and1hlaterweregiventhem rotenone(3mg/kg,s.c.)alsoduringsevendays.24hafterthelast dayoftreatment,theratswereevaluatedwithbehavioraltestsand biochemicalassays(Linard-Medeirosetal.,2015).
Openfieldtest
TheopenfieldtestwascarriedouttoBroadhurst(1957)and
Bernardi andPalermo-Neto(1979).Toquantifyexploratoryand generallocomotor activity,each ratwas placed intothecenter ofanopen-fieldarena(acircularwoodenboxwithadiameterof 100cmand40cmhigh,withfloordividedinto19regions).Rats wereassessedindividuallyfor5min,whilefourparameterswere analyzed:(i)latencytostartthemovement(timetoleavetheinner circle,ins),(ii)locomotionfrequency(numberofsquarecrossed withfourpaws),(iii)rearingfrequency(numberoftimesthe ani-malstoodontheirhindpaws)and(iv)immobilitytime(lackof movementduringtesting,ins).Theapparatuswascleanedwitha 5%ethanolsolutionbeforebehavioraltestingtoeliminatepossible biasduetoodorsleftbypreviousrat.
Rotarodactivitytest
Inordertoquantifymotordeficiency,therotarodtestwasused atafixedspeed,accordingtoMonvilleetal.(2006).Toperformthis test,theanimalwasplacedwithallfourpawsonabarwitha diam-eterof7cmandset25cmabovethefloor.Thebarrotatedataspeed of25rpm.Beforebeingsubmittedtothedifferenttreatments,the ratsweretrainedintwosessionsof180seachforhabituation. Ani-malswereplacedontherotatingbarandthetimespentonthe rotatingbarwasrecorded.Acut-offtimeof180swasmaintained throughouttheexperiment.Theaverageresultswererecordedas timeoffall.
Biochemicalanalysis
DPPHradicalscavengingactivityinvitro
Antioxidant radicalscavenging activity was evaluated using DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals (Brand-Williams etal.,1995).1mlofDPPHsolution(30mM,in95%ethanol)was incubatedwith2.5mlofvaryingconcentrationsofS.terebinthifolius
extract(2.5–250g/ml).Thereactionmixturewasshakenwelland incubatedfor 30minat roomtemperature,and theabsorbance of the resulting solution was read at 515nm against a blank. Theradicalscavengingactivitywasmeasuredasanabsorbance decreaseofDPPHandwascalculatedusingthefollowingequation: (Absorbanceofcontrol−Absorbanceoftest)×100/Absorbanceof control.Thesyntheticantioxidantbutylatedhydroxytoluene(BHT) wasincludedinexperimentsasapositivecontrol.TheEC50value
foreachsample(treereplicates),wascalculatedfromacalibration curve obtained for the percentage of radical scavenging activ-ity versus concentration of the sample required to reduce the absorbanceofthenegativecontrol(DPPHsolution)by50%.The BHTcurveantioxidantwasy=15.695ln(x)+16.477(R2=0.9544).
TheS.terebinthifolius extract curvewasy=18.322ln(x)−24.936 (R2=0.9026).
Measurementoflipidperoxidationinvivo
Thequantitativemeasurementoflipidperoxidationinthe subs-tantianigra,striatumandcortexwasperformedaccordingtothe methoddescribedbyBuegeandAust(1978).Theamountof malon-dialdehyde(MDA)oflipidperoxidationwasmeasuredbyreaction withthiobarbituricacid.Briefly,aliquots(500l)ofsupernatant were placed in test tubes and added to 1ml of TBA reagent: 0.38%(w/w)TBA,250ml of1Nhydrochloricacid,15%(w/w)of trichloroaceticacidand20mlof2%(w/v)ethanolicBHT.The solu-tion wasshaken and heated to 100◦C for 15min, followed by coolinginanicebath.Then,1.5mlofn-butanolwasadded.The mixturewasshakenandcentrifugedto3000×gfor20min.After centrifugation,theupperlayerwascollectedandassessedwitha spectrophotometer(CARY3EUV–VisibleSpectrophotometer Var-ian,Inc. Brazil)at532nm.ResultsofTBARSofsubstantia nigra, striatumandcortexwereexpressedasnmolMDA/mgproteinusing astandardcurvegeneratedwithdifferentconcentrationsof 1,1,3,3-tetramethoxypropanesolution.
Statisticalanalysis
Datawereexpressedasmean±standarderrorofmean(SEM). Thedifferencebetweengroupswasanalyzedbyone-way analy-sisofvariance(ANOVA)followedbyposthocNewman–Keulstest. ThecomparisonswerecarriedoutbyGraphPadPrism® 5.0 and
significantatp≤0.05.
Results
Highperformanceliquidchromatography(HPLC)analysis
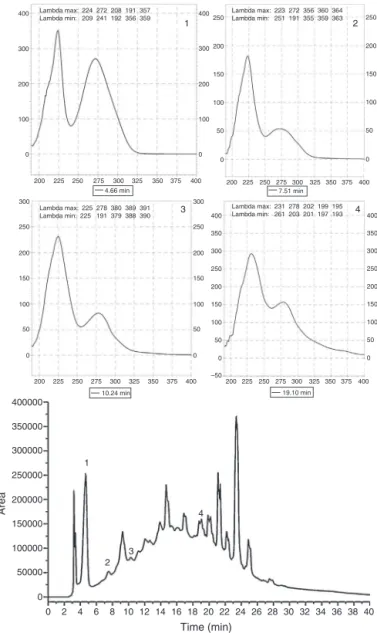
Fromthecomparisonofretentiontimeswithextractstandards previouslyinjectedintotheHPLC,itwaspossibletoconfirmthat,S. terebinthifoliussampledemonstratedthepresenceofphenolicacids (gallicacid,4.66min;ellagicacid, 7.51min)and alsoflavonoids (catechin,10.24min;epicatechin19.05min)(Fig.1).
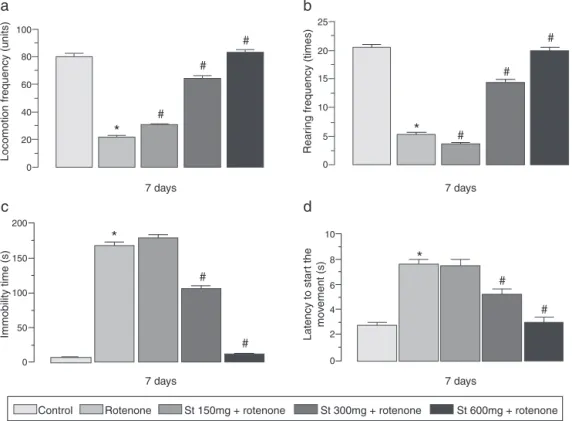
EffectsofS.terebinthifoliusonbehavioraltests
Rotenoneadministration(3mg/kg,s.c.)during7 days signif-icantlyreduced thefrequencies of locomotion and rearing and increasedthetimeofimmobilityandlatencytostartthe move-ment in theopen field test. Pre-treatment with thestem bark
400000 350000 300000 250000 200000 150000 100000 50000 0
0 2 4 6 8 10 12 14 16 18 20 22 24 26
Time (min)
28 30 32 34 36 38 40
Area
1
2 3
4
4.66 min Lambda max: 224 272 208 191 357 Lambda min: 209 241 192 356 359
10.24 min Lambda max: 225 278 380 389 391 Lambda min: 225 191 379 388 390
19.10 min Lambda max: 231 278 202 199 195 Lambda min: 261 203 201 197 193
7.51 min Lambda max: 223 272 356 360 364 Lambda min: 251 191 355 359 363 400
300
200
100
0
200 225 250 275 300 325 350 375 400 200 225 250 275 300 325 350 375 400
200 225 250 275 300 325 350 375 400 200 225 250 275 300 325 350 375 400 300
250
200
150
100
50
0
300
400
350
300
250
200
150
100
50
0
400
350
300
250
200
150
100
50
0
–50 250
200
150
100
50
0 400
250
200
150
100
50
0
250
200
150
100
50
0 300
200
100
0
1
3 4
2
Fig.1. ChromatogramofSchinusterebinthifoliusstembarkdetectedat278nm. Peaks:gallicacid(Rt=4.66),catechin(7.51),epicatechin(10.24)andellagicacid (Rt=19.05)obtainedbyHPLCmethod.
(150,300and600mg/kg)significantlyimprovedlocomotor activ-ityandrearinganddecreasedthetimeofimmobilityandlatency tostartthemovementwhencomparedtorotenoneanimals. How-ever, thelower dose of theextract (150mg/kg) was unableto preventtherotenone-inducedlocomotordeficitsintheopenfield test(Fig.2a–d).
Onrotarodtest,rotenoneadministration(3mg/kg,s.c.)induced asignificantdecreaseinthetimeofpermanencyoftheanimals comparedwithcontrolratsduringtheobservationinthe appa-ratus.Dailypre-treatmentof300and600mg/kgdoseswasable toincrease thetime ofpermanency incomparison torotenone group.However,thelowerdoseoftheextractwasunableto pre-venttherotentone-inducedlocomotordeficitsintheopenfieldtest (Fig.3).
Reductionof2,2-diphenyl-1-picrylhydrazylradicalinvitro
*
*
*
*
#
# #
#
#
#
# #
# #
100 80 60 40 20 0
25 20 15 10 5 0
Locomotion frequency (units)
Latency to star
t the
mo
vement (s)
Rear
ing frequency (times)
7 days
Immobility time (s)
7 days
7 days 7 days
a
c
d
b
200
150
100 50
0
10 8 6 4 2 0
Control Rotenone St 150mg + rotenone St 300mg + rotenone St 600mg + rotenone
Fig.2.EffectofSchinusterebinthifolius(St,150,300and600mg/kg,p.o.)onopenfieldtestinratsbeforerotenone(3mg/kg,s.c.)administration.(a)Totalambulatoryactivity, (b)rearingfrequency,(c)immobilitytimeand(d)latencytostartdemovement.Thevaluesareexpressedasmean±S.E.M.(n=10/group).One-wayANOVAfollowedby Newman–Keulstest(*p≤0.05vs.controlgroupand#p≤0.05vs.rotenonegroup).
*
*
#
#
Control Rotenone St 150mg + rotenone
St 300mg + rotenone St 600mg + rotenone
100 80
Motor perf
or
mance (s)
40 60
20
0
7 days
Fig.3.EffectofSchinusterebinthifolius(St,150,300and600mg/kg,p.o.)onrotarod testinratsbeforerotenone(3mg/kg,s.c.)administration.Thevaluesareexpressed asmean±S.E.M.(n=10/group).One-wayANOVAfollowedbyNewman–Keulstest (*p≤0.05vs.controlgroupand#p≤0.05vs.rotenonegroup).
synthetic antioxidant BHT showed EC50 25.768±0.147g/ml. This activity was about 2.1 times smaller when compared to
S.terebinthifolius.
Measurementoflipidperoxidationinvivo(TBARS)
Rotenoneadministration(3mg/kg)during7daysprovokedan increaseonoxidativestressasindicatedbythesignificantincrease ofMDAconcentrations in substantianigra, striatumand cortex whencomparedtocontrolgroup.However,thepre-treatmentwith alldosesofS.terebinthifoliuswasabletoinhibitthelipid peroxida-tionincomparisontorotenoneanimals(Fig.4a–c).
Discussion
This report showed the neuroprotective effect of S. tere-binthifolius in Parkinson’s diseaseusing the model of rotenone subcutaneousadministrationduring7days.AlldosesofS. tere-binthifolius(150,300and600mg/kg)significantlypreventedmotor deficitsintheopenfieldandrotarodtests,andmainly,wasableto inhibittheoxidativestressinducedbysystemicrotenone adminis-trationobservedininvivoTBARSmethod.Itwasalsodemonstrated theinvitroantioxidanteffectofS.terebinthifolius,aswellitsmainly compoundsinHPLCmethod.Theantioxidantactivityoftheextract wasalsomeasuredintheDPPHradicalassay,whichprimarily eval-uatesprotonradical-scavengingability.
Rotenone is a potent inhibitor of complex I (NADH: ubiquinoneoxidoreductase)of themitochondrial electron trans-portchain. Inrats,this toxincausesa syndromethat replicates both neuropathological findings and behavioral symptoms of Parkinson’sdisease.Applicationoflowdosesofrotenoneinvitro
andinvivohavebeenshowntoaffectmanyofthemechanisms involvedinthepathogenesisofParkinson’sdisease,suchasaltered calcium signaling, induction of oxidative stress and apoptosis, lossoftyrosinehydroxlase,proteasomaldysfunction,nigraliron accumulationandtheformationoffibrillarcytoplasmicinclusions thatcontainubiquitinand␣-synuclein(vonWrangeletal.,2015).
*
*
*
#
#
#
#
#
#
#
#
#
Control Rotenone St 150mg + rotenone
St 300mg + rotenone St 600mg + rotenone
1.0 0.8 0.6 0.4 0.2 0.0 1.0 0.8
0.6
0.4 0.2
0.0
1.0 0.8 0.6 0.4 0.2 0.0
nMol MD
A/mg protein
nMol MD
A/mg protein
nMol MD
A/mg protein
a
b
c
Fig.4.EffectofSchinusterebinthifolius(St,150,300and600mg/kg,p.o.)onlipid peroxidationlevelsinratsbeforerotenone(3mg/kg,s.c.)administration.(a) Subs-tantianigra,(b)striatumand(c)cortexofbrainrats.Thevaluesareexpressed asmean±S.E.M.(n=10/group).One-wayANOVAfollowedbyNewman–Keulstest (*p≤0.05vs.controlgroupand#p≤0.05vs.rotenonegroup).
largelyaresultoftheperoxidationofpolyunsaturatedfattyacids with more than two double bonds (Sanders and Greenamyre, 2013).
Inourstudy,administrationofrotenonepromotedincreased levelsofMDAinsubstantianigra,striatumand cortex, suggest-ing an increased lipid peroxidation and, thus, oxidative stress. In this way, there was an increase in brain MDA levels (an indicatorof lipid peroxidationdue tofree radicals) which was inhibited by theadministration of stem bark of all doses of S. terebinthifolius,suggestingitsantioxidantaction.Indeed,recently
Costaet al.(2015) shown thatextract fromdifferenttissues of
S. terebinthifolius present antioxidant activity by DPPH radical assay. Abdou et al. (2015) investigated the curative and pro-tectiveeffects of S. terbenthifolius ethanolic extract (350mg/kg,
p.o.)againstCCl4-inducedacutehepatotoxicityinratsandshown
that the administration significantly reinstated the antioxidant capacity.Oxidativestressduetoanincreaseinfreeradical gen-erationoranimpairedendogenousantioxidantmechanismisan
importantfactorthathadbeenimplicatedinvarious neurodegen-erativediseases (Sanders and Greenamyre,2013;Solanki etal., 2015).
Thebrainishighlysusceptibletofreeradicaldamagebecause ofitshighutilizationofoxygenandthepresenceofarelatively lowconcentrationofantioxidantenzymesandfreeradical scaven-gers.Thereisagrowinginterestinestablishingtherapeuticand dietarystrategiestocombatoxidativestressinduceddamageto thecentralnervoussystem(Gaoetal.,2012;Virmanietal.,2013). Studiesin humansandanimals havesuggested thatflavonoids, a large group of polyphenolic compounds found in fruits and vegetablesarecapableofcounteractingneuronalinjury,thereby delayingtheprogressionofneurodegenerativediseases, motivat-ingresearcheseffortstoidentifythemechanismsofneuronaldeath aswelltodiscovernewcompoundstocontrolthem(Duttaand Mohanakumar,2015;Chenetal.,2015;Solankietal.,2015;Renaud etal.,2015).
ThemajorcompoundsofstembarkofS.terebinthifoliusRaddi areflavonoids(cathechinandepicatechin)andphenolicsacids (gal-licacidandellagicacid).Catechinsarepowerfulantioxidantsand freeradicalscavengersinvitroassaysandinvivomodels(Srividhya etal.,2009;Solankietal.,2015).Ithasbeenfoundthatcatechins havemuchhigherantioxidantactivityincomparisontovitamins Cand Einvitro.Thechemicalstructureshavebeencontributed tothiseffecttochelatemetalions,preventthegenerationoffree radicals,allowthedisplacementofelectronsandprovidehigh reac-tivitytoeliminatethefreeradicals(Mandeletal.,2006;Khanand Mukhtar,2007;Senthil,2008).Furthermore,thecatechinsand epi-catechinswerealsoabletoindirectlymodulateexpressionofsome protectiveenzymesantioxidants(HidgonandFrei,2003;Weinreb etal.,2004).Thechronictreatmentwithcatechinspreventedthe declineintheactivityofSODandGSHenzymesintheserumof oldmice,preventingalsosignificantlyincreasedTBARSinthe hip-pocampusoftheseanimals(AbdElMohsenetal.,2002;Mandel etal.,2006).Otherwise,gallicacidandellagicacidarehydrolyzed tanninsofnaturalproductsfoundinabundanceingrapes,tea,fruit and wine (Singhet al., 2004). Theseacids wereable toreduce theproductionofROSandsuperoxide,suchashydrogen perox-ide,hydroxyl radicals and hypochlorous acid;alsodue to their antimutagenicandanticarcinogenicactivities(Kim,2007).Inthe cognitiveimpairmentinducedbyanotheranimalmodelof Parkin-son’sdiseaseinanimals,6-OHDA,thetreatmentwithgallicacid significantlyreduced thelevel of TBARSin theratbrains(Kim, 2007).
Inconclusion,theresultsofthisstudyconfirmthatthe subcuta-neousadministrationofrotenoneinratsinducesneurobehavioral andbiochemicalchangesmimickingthoseobservedinParkinson’s disease.TreatmentofratswithS.terebinthifoliusproduced signif-icantneuroprotectionprobablymediatedthroughitsantioxidant activity.Takentogether,ourdatasuggestedneuroprotectiveeffects ofS.terebinthifoliusRaddi,probablymediatedthroughits antiox-idantactivity.Thus, thepresent workbroughtoutnewinsights forthetreatmentofneurodegenerativediseasesandhighlighted thepharmacologicalpropertiesforParkinson’sdiseaseandother neurodegenerativepathologies.
Author’scontributions
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorswouldliketothankCAPESforfinancialsupport.The authorsalsowouldliketodedicatethispapertoSimoneSetteLopes Lafayette.
References
AbdElMohsen,M.M.,Kuhnle,G.,Rechner,A.R.,Schroeter,H.,Rose,S.,Jenner,P., Rice-Evans,C.A.,2002.Uptakeandmetabolismofepitatechinanditsaccessto thebrainafteroralingestion.FreeRadic.Biol.Med.33,1693–1702.
Abdou,R.H.,Saleh,S.Y.,Khalil,W.F.,2015.Toxicologicalandbiochemical stud-iesonSchinus terebinthifoliusconcerningitscurative andhepatoprotective effectsagainstcarbontetrachloride-inducedliverinjury.Pharmacogn.Mag.11, S93–S101.
Bernardi,M.M.,Palermo-Neto,J.,1979.Effectsofabruptandgradualwithdrawal fromlong-termhaloperidoltreatmentonopenfieldbehaviorofrats. Psy-chopharmacology65,247–250.
Betarbet,R.,Sherer,T.B.,Mackenzie,G.,Garcia-Osuma,M.,Panov,A.V.,Greenamyre, J.T.,2000.Chronicsystemicpesticideexposurereproducesfeaturesof Parkin-son’sdisease.Nat.Neurosci.3,1301–1306.
Blesa,J.,Phani,S.,Jackson-Lewis,V.,Przedborski,S.,2012.Classicandnewanimal modelsofParkinson’sdisease.J.Biomed.Biotechnol.2012(ID),845618. Brand-Williams,W.,Cuvelier,M.E.,Berset,C.,1995.Useoffreeradicalmethodto
evaluateantioxidantactivity.Lebensm-Wiss.U.Technol.28,25–30.
Broadhurst,P.L.,1957.Determinationofemotionalityintherat.I.Situationalfactors. Br.J.Psychol.48,1–12.
Buege,J.A.,Aust,S.D.,1978.Microssomallipidperoxidation.MethodsEnzymol.52, 302–310.
Chen,M.,Wang,T.,Yue,F.,Li,X.,Wang,P.,Li,Y.,Chan,P.,Yu,S.,2015.Tea polyphe-nolsalleviatemotorimpairments,dopaminergicneuronalinjury,andcerebral
␣-synucleinaggregationinMPTP-intoxicatedParkinsonianmonkeys.
Neuro-science286,383–392.
Corrêa,M.P.,1974.DicionáriodeplantasúteisdoBrasiledasplantasexóticas culti-vadas,vol.3.ImprensaNacional,RiodeJaneiro,Brazil,pp.125–126. Costa,C.O.D’S.,Ribeiro,P.R.,Loureiro,M.B.,Simões,R.C.,Castro,R.D.,Fernandez,
L.G.,2015.Phytochemicalscreening,antioxidantandantibacterialactivitiesof extractspreparedfromdifferenttissuesofSchinusterebinthifoliusRaddithat occursinthecoastofBahia,Brazil.Phcog.Mag.11,607–614.
Dauer,W.,Przedborski,S.,2003.Parkinson’sdisease:mechanismsandmodels. Neu-ron39,889–909.
Dutta,D.,Mohanakumar,K.P.,2015.TeaandParkinson’sdisease:constituentsoftea synergizewithantiparkinsoniandrugstoprovidebettertherapeuticbenefits. Neurochem.Int.89,181–190.
Fernandez,H.H.,2015.UpdateonParkinsondisease.Clev.Clin.J.Med.82,563–568. Gao,X1,Cassidy,A.,Schwarzschild,M.A.,Rimm,E.B.,Ascherio,A.,2012. Habit-ualintakeofdietaryflavonoidsandriskofParkinsondisease.Neurology78, 1138–1145.
Hidgon,J.V.,Frei,B.,2003.Teacatechinsandpolyphenols:healtheffects,metabolism andantioxidantfunctions.Crit.Rev.FoodSci.Nutr.43,89–143.
Khan,N.,Mukhtar,H.,2007.Teapolyphenolsforhealthpromotion.LifeSci.81, 519–533.
Kim,Y.J.,2007.Antimelanogenicandantioxidantpropertiesofgallicacid.Biol. Pharm.Bull.30,1052–1055.
Linard-Medeiros,C.F.B.,Sales,V.A.W.,Ramos,A.C.,Sereniki,A.,Trevisan,M.T.S., Wan-derley,A.G.,Lafayette,S.S.,2015.NeuroprotectiveeffectofextractofAnacardium occidentaleLinnonarotenonemodelofParkinson’sdisease.Int.J.Pharm.Sci. Res.6,123–129.
Mandel,S.,Amit,T.,Reznichenko,L.,Weinreb,O.,Youdim,M.B.,2006.Greentea cate-chinsasbrain-permeable,naturalironchelators-antioxidantsforthetreatment ofneurodegenerativedisorders.Mol.Nutr.FoodRes.50,229–234.
Medeiros,K.C.P.,Monteiro,J.C.,Diniz,M.F.F.M.,Medeiros,I.A.,Silva,B.A.,Piuvezam, M.R.,2007.EffectoftheactivityoftheBrazilianpolyherbalformulation: Euca-lyptusglobulusLabill,PeltodonradicansPohlandSchinusterebinthifoliusRaddiin inflammatorymodels.Rev.Bras.Farmacogn.7,23–28.
Monville,C.,Torres,E.M.,Dunnett,S.B.,2006.Comparisonofincrementaland accel-eratingprotocolsoftherotarodtestfortheassessmentofmotordeficitsinthe 6-OHDAmodel.J.Neurosci.Methods158,219–223.
Morton,J.F.,1978.Brazilianpepper:itsimpactonpeople,animalsandthe environ-ment.Econ.Bot.32,353–359.
Renaud, J., Nabavi, S.F., Daglia, M., Nabavi, S.M., Martinoli, M.G., 2015. Epigallocatechin-3-gallate,apromisingmoleculeforParkinson’sdisease? Reju-venationRes.18,257–269.
Sanders, L.H.,Greenamyre,J.T.,2013.Oxidativedamage tomacromoleculesin humanParkinsondiseaseandtherotenonemodel.FreeRadic.Biol.Med.62, 111–120.
Senthil,K.,2008.RepletionofantioxidantstatusbyEGCGandretardationof oxida-tivedamageinducedmacromolecularanomaliesinagedrats.Exp.Gerontol.43, 143–183.
Sherer,T.B.,Kim,J.H.,Betarbet,R.,Greenamyre,J.T.,2003.Subcutaneousrotenone exposure causes highly selective dopaminergic degeneration and alpha-synucleinaggregation.Exp.Neurol.179,9–16.
Singh,R.P.,Sharad,S.,Kapur,S.,2004.Freeradicalsandoxidativestressin neurode-generativediseases:relevanceofdietaryantioxidants.J.IndianAcad.Clin.Med. 5,218–225.
Solanki,I.,Parihar,P.,Mansuri,M.L.,Parihar,M.S.,2015.Flavonoid-basedtherapies intheearlymanagementofneurodegenerativediseases.Adv.Nutr.6,64–72. Srividhya,R.,Zarkovic,K.,Stroser,M.,Waeg,G.,Zarkovic,N.,Kalaiselvi,P.,2009.
Mito-chondrialalterationsinagingratbrain:effectiveroleof(−)-epigallocatechin gallate.Int.J.Dev.Neurosci.27,223–231.
Virmani, A., Pinto, L., Binienda, Z., Ali, S., 2013. Food, nutrigenomics, and neurodegeneration–neuroprotection bywhat you eat!Mol. Neurobiol. 48, 353–362.
vonWrangel,C.,Schwabe,K.,John,N.,Krauss,J.K.,Alam,M.,2015.The rotenone-inducedratmodelofParkinson’sdisease:behavioralandelectrophysiological findings.Behav.BrainRes.279,52–61.
Weinreb,O.,Mandel,S.,Amit,T.,Youdim,M.B.,2004.Neurologicalmechanismsof greenteacatechinpolyphenolsinAlzheimer’sandParkinson’sdiseases.J.Nutr. Biochem.15,506–516.
Zaitone,S.A.,Abo-Elmatty,D.M.,Shaalan,A.A.,2012.Acetyl-l-carnitineand␣-lipoic