w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Toxicity
and
antitumor
efficacy
of
Croton
polyandrus
oil
against
Ehrlich
ascites
carcinoma
cells
Déborah
R.P.
Meireles
a,
Heloísa
M.B.
Fernandes
a,
Thaísa
L.
Rolim
a,
Tatianne
M.
Batista
a,
Vivianne
M.
Mangueira
a,
Tatyanna
K.G.
de
Sousa
a,
João
C.L.R.
Pita
a,
Aline
L.
Xavier
a,
Daiene
M.
Beltrão
a,
Josean
F.
Tavares
a,b,
Marcelo
S.
Silva
a,b,
Karina
K.P.
Medeiros
c,
Marianna
V.
Sobral
a,b,∗aProgramadePós-graduac¸ãoemProdutosNaturaiseSintéticosBioativos,CentrodeCiênciasdaSaúde,UniversidadeFederaldaParaíba,JoãoPessoa,PB,Brazil
bDepartamentodeCiênciasFarmacêuticas,UniversidadeFederaldaParaíba,JoãoPessoa,PB,Brazil
cDepartamentodeMorfologia,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received22March2016 Accepted30May2016 Availableonline1July2016
Keywords:
Antitumoractivity
Crotonpolyandrous
Ehrlichascitescarcinoma Essentialoil
Genotoxicity Toxicity
a
b
s
t
r
a
c
t
TheessentialoilfromCrotonpolyandrusSpreng.,Euphorbiaceae,leaveswastestedforthetoxicityand antitumoractivity.Theconcentrationproducing50%hemolysiswas141g/mlonmiceerythrocytes. Intheacutetoxicologicalstudy,theestimatedLD50was447.18mg/kg.Theessentialoildidnotinduce increaseinnumberofmicronucleatederythrocytes,suggestinglowgenotoxicity.Essentialoil(100or 150mg/kg)showedsignificantantitumor activityinEhrlichascitic carcinomamodel. Weobserved thatessentialoilinducescell-cyclearrestattheG0/G1phase,andincreasesthesub-G1peak,which representsamarkerofcelldeathbyapoptosis.Survivalalsoincreasedforthetreatedanimals.The toxi-cologicalanalysesrevealedreductioninbodyweight,increasedaspartateaminotransferaseandalanine aminotransferaseactivity,hematologicalchanges,andathymusindexreduction.Thesedatasuggest gas-trointestinalandlivertoxicity,anemia,leukopenia/lymphocytopenia,andimmunosuppressiveeffects. Histopathologicalanalysisrevealedtheweakhepatotoxicityofessentialoil.Insummary,essentialoilof C.polyandrusdisplaysinvivoantitumoractivityandmoderatetoxicity.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Canceristheoneoftheleadingcausesofdeathintheworld,and itaffectsmillionsofpeopleannually(Jainetal.,2011;Hanahan, 2014).Inthiscontext,thehigherplantsrepresentarichsourceof newsubstanceswhichmaybeusefulagainsttumors(Craggand
Newman,2013).
Manystudieshavebeenpublishedreportingthediverse thera-peuticpotentialofessentialoils,includingcancerpreventionand treatment.Themechanismsinvolvedincludeantioxidant, antimu-tagenic and antiproliferative effects, or by enhancing immune functionandsurveillance,inducingenzymesandenhancing detox-ification,andmodulatingmultidrugresistance(Bhallaetal.,2013). ManyEuphorbiaceaespeciesarerecognizedinvariouspartsof theworldasbeingbothtoxicandmedicinal.Crotonisalargegenus ofEuphorbiaceae,itcomprisesaround1300speciesoftrees,shrubs,
∗ Correspondingauthor.
E-mail:mariannavbs@ltf.ufpb.br(M.V.Sobral).
andherbsdistributedintropicalandsubtropicalregionsinboth hemispheres(Pereiraetal.,2002).Severalspeciesofthegenusare aromatic,indicatingthepresenceofvolatileconstituents(Oliveira
etal.,2001;Lopesetal.,2003).
EssentialoilsfromCrotonregelianus,andC.flavensleaves,aswell as isolated constituents␣-cadinol, -elemene and ␣-humulene
(Sylvestreetal.,2006;Bezerraetal.,2009)showedinvitro
anti-tumoractivity.Invivostudiesdescribeisoguanosineisolatedfrom C.tiglium,andascaridoleisolatedfromC.regelianuswhichhave shownantitumoractivityonsarcoma180murinemodel(Kinetal.,
1994;Bezerraetal.,2009).
CrotonpolyandrusSpreng.isfoundinBrazil,andistypicalofthe semi-aridregion,althoughitalsooccursintheAtlanticforestarea oftheBrazilianstatesAlagoas,Bahia,Ceará,Paraíba,Pernambuco, Piauí,RioGrandedoNorteandSergipe.Recentstudiesshowedthat extractsandessentialoilfromC.polyandrousleaveshavesignificant antifungalactivity,aswellasaweakcytotoxicityagainsttumorcell lines(Fernandesetal.,2012;2013).Someanticancerdrugswidely usedinclinicalpractice,suchascyclophosphamide,havepotent effectsinvivo,althoughtheyareineffectiveinvitro.Ingeneral,these
http://dx.doi.org/10.1016/j.bjp.2016.05.014
substancesarepro-drugsthatmustundergometabolicactivation toproducetheireffects(Shrivastavetal.,1980;Sunetal.,2006).
Then,theaimofthisstudywastoevaluatetheinvivoantitumor activityandtoxicityoftheessentialoilfromC.polyandrousleaves (EOC).
Materialsandmethods
Drugsandreagents
5-Fluorouracil(5-FU),TritonX-100,Tween80,and cyclophos-phamide were purchased from Sigma–Aldrich (St. Louis, MO, USA).Dimethylsulfoxide(DMSO)waspurchasedfrom Mallinck-rodt Chemicals® (Phillipsburg, NJ, USA). Sodium thiopental
(Thiopentax®)waspurchasedfrom Cristália(Itapira,SP, Brazil),
andheparin(Parinex®)fromHipolabor(Sabará,MG,Brazil).Kits
forbiochemicalandhematologicalanalysiswerepurchasedfrom LABTEST®(LagoaSanta,MG,Brazil).
Plantprocessing
CrotonpolyandrusSpreng.,Euphorbiaceae,leaveswerecollected inFebruary2011inSantaRita,ParaíbaState,Brazil.Voucher speci-mensnumberAgra&Gois1446wasdepositedatHerbariumLauro PiresXavieroftheFederalUniversityofParaíba,Brazil.
Extractionandanalysisofessentialoil
The fresh leaves of C. polyandrus (500g) were subjected to hydrodistillation for 4h using a Clevenger-type apparatus. The essentialoilobtainedwasdriedandanalyzedinGCanalysiswas performedonaShimadzuGC17-Agaschromatographusingfused silica capillary column DB-5 (30m×0.25mm id, 0.25M film thickness).Heliumwasusedascarriergasataflowrateof1ml/min. Splitratio1:100.Theoventemperaturewasprogrammedfrom60 to240◦to3◦C/min.Theinjectoranddetectortemperatureswere
220and230◦C,respectively(Fernandesetal.,2012).
Tumorcellline
TheinvivoantitumoractivityofEOC wastestedagainstthe Ehrlichcarcinomacell line, which wasgenerously provided by PharmacologyandToxicologyDivision,CPQBA,UNICAMP(Paulínia, SP,Brazil).Thecellsweremaintainedintheperitonealcavitiesof SwissmiceintheDr.ThomasGeorgeBioterium(ResearchInstitute inDrugsandMedicines/FederalUniversityofParaíba,Brazil).
Animals
MaleandfemaleSwiss albinomice(Musmusculus)obtained fromtheDr.ThomasGeorgeBioterium(ResearchInsituteinDrugs andMedicines/FederalUniversityofParaíba,Brazil)wereused.The animalsweighed28–32g,andwererandomlyhousedin polyethe-lene cages in a controlled environment (12h light/dark cycle, 24±1◦C,55%relativehumidity).Theywerefedonratchowpellets
andreceivedwateradlibitum.Animalswereusedingroupsofsix. Actionsonreducingpain,stressandanysufferingweretakenin accordancewithethicalguidelinesforanimalusage.Experimental protocolsandprocedureswereapprovedbythelocalanimalethics committee(CEUA-UFPB,no.0403/12)whichfollowsthe interna-tionalprinciplesinethicsforanimalexperimentation.
Pharmacologicalassays
Hemolysisassay
HemolyticEOCactivitywasevaluatedusingmiceerythrocytes (Kangetal.,2009).Briefly,freshbloodsampleswerecollected,and re-suspendedinPBStomakea0.5%(v/v)solution.Various concen-trationsofEOC(0–1000g/ml)dissolvedinDMSO(5%v/vinPBS), wereaddedtothesuspensionofredbloodcells.Theplateswiththe EOC-erythrocytemixtureswereincubatedonamixerfor60min andthencentrifuged.Thesupernatantwascarefullyremoved.After removal,200lofasolutionofTritonX-100(0.1%)wasaddedto eachwellcontainingtheEOC-erythrocytemixturesandthoroughly stirred.Thehemolysiscausedwasdeterminedby spectrophoto-metryat415nm.Theconcentrationthatproduces50%hemolysis (HC50)wasthen determined.Positive control(100%hemolysis), andnegativecontrol(0%hemolysis)incubatederythrocyteswith 0.1%TritonX-100inPBS,and5%DMSOinPBS,respectively,were used.
Acutepreclinicaltoxicitystudy
Theevaluationof acutepreclinicaltoxicityfor EOCwas per-formedbasedonthe“Guidefordrivingofnoclinicalstudiesof toxicologyandpharmacologicalsafetyrequiredtodevelopmentof drugs/Anvisa”,withsomemodifications(Anvisa,2013).Mice(six malesandsixfemales/group)weresubjectedtosingledosesof250, 375,or500mg/kgofEOC(intraperitoneally–i.p.)andthecontrol groupwasadministeredvehiclealone(5%(v/v)Tween80insaline). Thedoseslevelswerechosenbasedonpreviousscreening.For tox-icitydetection,signssuggestiveofcentralnervoussystem(CNS), orautonomicnervoussystem(ANS)activitywereevaluatedatthe intervals:0,15,30,and60min,after4h,anddailyfor14days.Body weightswereregisteredatthebeginningandendofthetreatment, andtheanimalswereobserveddailyforwaterandfeed consump-tion.Thenumberofdeadanimalsduringtheobservationperiod wascountedtodeterminethedoseresponsibleforthedeathof 50%oftheexperimentalanimals(LD50).
Genotoxicity
For the micronucleus assay, females mice (six/group) were treated(i.p.)with150or300mg/kgofEOC.Apositivecontrolgroup (cyclophosphamideat50mg/kgi.p.),andanegativecontrolgroup (Tween80 at5% in saline), were included.After48h, the ani-malswereanesthetizedwithsodiumthiopental(40mg/kg),and peripheralbloodsampleswerecollectedfromtheorbitalplexusfor makingslides.Foreachanimal,threebloodsmearswereprepared, andaminimumof2000erythrocyteswerecountedtodetermine thefrequencyofmicronucleatederythrocytes(OECD,1997).
Invivoantitumoractivity
Seven-day-old Ehrlich carcinoma cells, 0.5ml (2.0×106cells/ml) were implanted in the peritoneal cavity of thefemalemice(twelvefemalesmice/group)(ChenandWatkins,
1970;Dolaietal.,2012).Onedayafterinoculation,EOC(100or
cavity.Thevolumewasmeasuredinagraduatedcentrifugetube andexpressedinmilliliter.Analiquotwasremovedforviablecell countingbytestingwiththetrypanblueassay(Kiangetal.,2009;
Dolaietal.,2012).
Theremaininganimals(n=6/group)werekeptalivewithfood andwateradlibitumtocalculatetheanimal’ssurvivalrates.
Cellcycleanalyses
Forthecellcycleanalysis,mice(n=6)inoculatedwithEhrlich ascitescarcinomacellsweretreatedwithEOC(100or150mg/kg) fornine days,asdescribed above.Onedayaftertheendofthe treatment,ascitic fluidwascollectedfromtheperitonealcavity andonemillioncellswerecentrifugedat230×gfor7min.The supernatantwasremoved,thepelletwasresuspendedin0.3ml ofhypotonicpropidiumiodide(PI)solution(50g/ml),andthen incubatedfor4hat4◦Cinthedark.Theanalysiswasperformedby
cytometricflow(BDFACSCalibur®,USA),atotalof10,000events
wereacquired,anddatawasanalyzedusingWinMDI2.9software
(Maronietal.,2012).
Toxicityintransplantedmice
Fortheevaluationofpossibletoxiceffectsproducedby treat-mentwithEOC,theanimalswereweighedatthebeginningand theendofthetreatment(afterremoving/drainingoftheresidual ascitestumorvolume),whiledailyconsumptionofwaterandfood wereevaluated.Inaddition,theanimalorgans;liver,spleen, thy-mus,andkidneyswereexcised,weighed,andtheorganindexes werethencalculated.
Biochemical analyses were performed on serum samples obtainedaftercentrifugationoftotal blood,at160×gfor6min. Standardizeddiagnostickitswereusedtodeterminethelevelsof aspartateaminotransferase(AST),alanineaminotransferase(ALT), creatinine,andurea.
Thehematologicalanalysesusedheparinizedwholeblood.The hematologicalparametersforhemoglobin(Hb)level,redbloodcell (RBC)count,hematocrit(Hct), theredblood cellindices;mean corpuscularvolume(MCV),meancorpuscularhemoglobin(MCH), meancorpuscularhemoglobinconcentration(MCHC),andthetotal anddifferentialleukocytecountweredetermined.Thetestswere performedaccordingtothemanufacturer’sinstructions.
Liversandkidneyswerefixedin10%(v/v)formaldehydeand portionsoftheseorganswerecutintosmallpieces,theninto sec-tionsof5m,andstainedwithhematoxylin–eosin.Fordetectionof hepaticfibrosis,theliversectionswerestainedwithspecificstain
(GordonandSweet,1936).Histologicalanalysiswasperformedby
lightmicroscopytodeterminethepresenceandextentofliveror kidneylesions.
Statisticalanalysis
Alldataarepresentedasthemean±S.E.M.Theinvitroassays were performed in quadruplicate and repeated at least twice. TheHC50valueandtheir95%confidenceintervals(CI95%)were obtainedbynonlinearregression.Thedifferencesbetween exper-imentalgroupswerecomparedbyvarianceanalysis(ANOVA),and followedbyTukey’stest(p<0.05).
Resultsanddiscussion
Thepercentageofidentificationofvolatilecomponentsofoil was86.1%,withatotalof33identifiedcomponents.Monoterpenes (72.7%)andsesquiterpenes(24.2%)werethemaingroupsof chem-icalconstituents isolated,withthemajority: p-cymene (12.4%), bornylacetate(11%)andascaridole(6.4%).Thisisinaccordance
100
75
50
25
0
0 250 500 750
[OEC] (µg/ml)
% hemolysis
1000 1250
Fig.1.PercentageofhemolysisinredbloodcellsofSwissmiceupontreatmentwith EOC(g/ml).Eachdotrepresentstheaverage±SEMofthreeexperimentswiththree replicates,witha95%confidenceinterval.
withwhatwaspreviouslypublishedforessentialoilfromCroton polyandrousleaves(Fernandesetal.,2012).Inaddition,the chemi-calcompositionpresentedherewasconsistentwithliteraturedata forvolatileconstituentsofotherCrotonspecies(Sylvestreetal.,
2006;Bezerraetal.,2009;Correa-Royeroetal.,2009).
ThehemolyticactivityassaywitherythrocytesofSwissmice wasperformedtoevaluatenon-tumor celltoxicity. After treat-ment with EOC, the percentage of hemolysis increased in a concentration-dependentmanner.TheHC50valueobtainedwasin therangeof141.0(140.5–141.6)g/ml(Fig.1).
Anemia isthe mostcommonhematological cancer manifes-tation, and its incidence increases with the administration of chemotherapy/radiotherapy.Theredbloodcellsandhemoglobin maybedecreasedthroughdestructionand/ortheinabilityofthe bonemarrowtomakethesecells(Gasparetal.,2015).
ThedatashowedthatEOChadmoderatecytotoxicityagainst miceerythrocytes,inducing100% ofhemolysisfrom250g/ml. Thiscorroboratesfindingsintheliteraturewhichindicatethat cer-tainessentialoilsand/orcompoundsisolatedfromplantscanaffect cellmembranestructuresandproducehemolysis(Ngetal.,1986;
Grinbergetal.,1997;Zhangetal.,1997;Wuetal.,2012;Rodrigues
etal.,2013).Nevertheless,recentdatashowedthatEOCisnot cyto-toxictonon-tumorcellsoftheCHO(ovarian),andHaCaT(human keratinocyte)lines(Fernandesetal.,2012).
TheacutetreatmentwithEOCinduceddeathinmaleandfemale miceonlyat375and500mg/kg(Table1).TheLD50valueobtained wasapproximately447.18mg/kg.Itwasobservedthatinthefirst fewmomentsfollowingadministrationofEOC(0,15,30min)the animalsshowedsevereCNSstimulanteffectssuchashyperactivity, beingmorepronouncedatthehigherdoses.At4hafter adminis-tration,wecontradictorilyobservedCNSdepressanteffectssuch asdecreasedtouchresponse,lossofcornealandsoundreflexes, andptosis.Theoccurrenceofptosisisdescribedinsomeclassesof depressantdrugssuchasneurolepticsandanalgesicscentralaction. Alreadythereductionorlossofpainreflexsuggestsan antinoci-ceptive activity. There were also observed effects on the ANS, includingforcedbreathinganddiarrhea,whichsuggest parasym-patheticstimulation(Carlini,2003;Almeidaetal.,2001).However, theseeffects disappearedafter4hof treatment.Literaturedata reportedthat,ingeneral,ifthelethaldose(LD50)ofthetest sub-stanceisthreetimesmorethantheminimumeffectivedose,the substanceisconsideredagoodcandidateforfurtherstudies(Carol,
1995;Ameloetal.,2014).
Table1
Effectofsingledoses(i.p.)ofEOCinmice(n=6).
Dose(mg/kg) Sex M/T Symptoms
– M 0/6 None
F 0/6 None
250 M 0/6 Hyperactivity
F 0/6 None
375 M 2/6 Hyperactivity,lossofsoundreflexptosis,laboredbreathing,decreasedresponsetotouch,lossofcornealreflex,
F 1/6 Hyperactivity,ptosis,laboredbreathing,decreasedresponsetotouch,lossofcornealreflex, lossofsoundreflex
500 M 5/6 Hyperactivity,ptosis,ataxia,laboredbreathing,decreasedresponsetotouch,lossofcorneal
reflex,lossofsoundreflex,abductionofthehindpaws,diarrhea
F 3/6 Hyperactivity,ptosis,ataxia,laboredbreathing,decreasedresponsetotouch,lossofcorneal reflex,lossofsoundreflex,abductionofthehindpaws,diarrhea
M/T,numberofdeadmice/numberoftreatedmice.
Table2
Feedandwaterconsumptionandweightofanimals(n=6)subjectedtoacutetreatmentwithEOC(250or375mg/kg).
Group Sex Dose,mg/kg Waterconsumption,ml Feedintake,g Initialweight,g Finalweight,g
Control M – 46.92±1.45 40.72±0.63 31.22±0.57 37.68±1.68
F 39.38±0.71 34.42±0.97 30.45±0.84 33.85±0.70
EOC M 250 38.85±1.01
a 35.69
±1.21a 28.45
±0.92 30.03±1.05a
F 32.08±0.89a 34.16
±1.43 27.52±1.05 32.67±0.88
EOC M 375 33.08±2.16
a 27.43
±0.93a 30.65
±0.96 29.55±2.56a
F 26.15±1.28a 25.28
±1.99a 29.02
±0.92 32.84±0.63
Datapresentedasamean±SEMofsixanimalsanalyzedbyANOVAfollowedbyTukeytest.
ap<0.05comparedtocontrol.
Table3
Numberofmicronucleatederythrocytesinperipheralbloodofmicetreatedwith singledosesofEOCandcyclophosphamide(n=6).
Groups Dose,mg/kg Numberofmicronucleatedcells
Control – 2.80±0.37
Cyclophosphamide 50 14.50±2.60a
EOC 150 2.20±0.37
EOC 300 2.40±0.25
DataarepresentedasSEMofthemeanofsixanimalsanalyzedbyANOVAfollowed byTukeytest.
ap<0.05comparedtothecontrolgroupwithANOVA,andfollowedbyTukey.
Almost all anticancer drugs cause gastrointestinal disorders
(Boussiosetal.,2012).Inthiscontext,metabolicparameters,such
asweight,andfeedintakeassessmentsmustbeevaluated dur-ingpreclinical studiesto investigategeneraltoxicity. Then, the decreaseonwaterandfeedconsumption,anddecreaseonbody weightinducedbyEOCdemonstratealltogethertoxicity.
Thepreclinical toxicologicalevaluation alloweddetermining thesafepharmacologicaldosestoproceedwithinvivo pharma-cologicalstudies.
To evaluate in vivo genotoxic effects of EOC we performed micronucleustesting(Table3).AnimalstreatmentwithEOCdidnot induceincreasesinthenumberofmicronucleatederythrocytesin peripheralbloodascomparedtothecontrolgroup.Then,theresults didnotshowgenotoxiceffectsforEOC,inthisexperimentalmodel.
Plantsproduceawidevarietyofsubstances,whichmayhave ther-apeuticimportance;however,manyofthemmayhavemutagenic effects.Inaddition,manyanticancerdrugscancausesideeffects thatincludeinductionofgenotoxicityinnon-tumorcells(Vieira
etal.,2010).
Forinvivoantitumoractivityassay,weusedEhrlichascites car-cinoma cells.This celllineis referred toasan undifferentiated carcinoma,andisoriginallyhyperdiploid,hashightransplantable capability, no-regression, rapid proliferation, shorter life span, 100%malignancyandalsodoesnothavetumorspecific transplan-tationantigen(TSTA)(Ozaslanetal.,2011).Therefore,anexcellent modelforstudyingexperimentalneoplasia(Salgadoetal.,2002;
Nascimentoetal.,2006;Verc¸osaJúnioretal.,2006;Araújoetal.,
2009).Theanalyzedparameters(tumorvolume,and cell viabil-ity)significantlydecreasedcomparedtothetumorcontrolgroup, featuringatumorgrowthinhibitoryactivityinbothdosestested ofEOC(100or150mg/kg).Therewasnosignificantdifferencein theparametersbetweenthegroupstreatedwiththeEOCand5-FU
(Table4).
Some of the constituents present in EOC are described in the literature as having significant antitumor activity, specifi-cally ascaridole (Bezerra et al., 2009), limonene (Gould, 1997),
␣-humulene(Silvaetal.,2008), terpinen-4-ol(Wuetal.,2012), caryophyllene(Zheng etal.,1992),1,8-cineole,␣-pineneand -pinene(Wangetal.,2012).Nevertheless,arecent reviewofthe anticancer activityof essential oils reported that thetheory of
Table4
Effectsof5-FUandEOConcellviabilityandtumorvolumeinmice(n=6)transplantedwithEhrlichascitescarcinomacellssubjectedtodifferenttreatments(9days).
Groups Dose,mg/kg Cellviability,×106cells/ml Tumorvolume,ml
Tumorcontrol – 219.8±27.77 9.34±1.40
5-FU 25 2.99±0.96a 0.09±0.02a
EOC 100 4.85±1.28a 0.10
±0.02a
EOC 150 3.95±0.49a 0.04
±0.02a
Datapresentedasmean±SEMofsixanimalsanalyzedbyANOVAfollowedbyTukeytest.
Table5
Feedandwaterconsumptionandweightofanimals(n=6)subjectedtodifferenttreatments(9days).
Groups Dose,mg/kg Waterconsumption,ml Feedconsumption,g Initialweight,g Finalweight,g
Healthyanimals – 35.67±1.12 30.85±1.57 28.62±0.37 32.27±0.86
Tumorcontrol – 34.69±2.16 29.33±1.45 27.74±1.20 31.02±1.10
5-FU 25 33.44±1.57 32.84±1.21 27.56±0.62 25.78±0.35a,b
EOC 100 32.19±2.70 24.41±0.88a,b 27.90±1.31 26.88±0.94a,b
EOC 150 24.38±1.70a,b 15.49
±1.17a,b 29.30
±0.86 24.34±0.93a,b Datapresentedasmean±SEMofsixanimalsanalyzedbyANOVAfollowedbyTukeytest.
ap<0.05comparedtotumorcontrol. bp<0.05comparedtohealthyanimals.
Table6
Effectsof5-FUandEOConthemiceorganindices(n=6)subjectedtodifferenttreatments(9days).
Groups Dose,mg/kg Heartindex,mg/g Liverindex,mg/g Kidneysindex,mg/g Thymusindex,mg/g Spleenindex,mg/g
Healthyanimals – 4.22±0.25 50.83±2.07 10.85±0.47 3.73±0.54 5.54±0.46
Tumorcontrol – 3.86±0.12 69.42±4.04 13.17±0.59 2.61±0.15 6.19±0.28
5-FU 25 4.84±0.48 57.39±1.92 12.29±0.30 2.85±0.08 6.32±0.51
EOC 100 4.38±0.15 64.35±4.09 13.15±0.64 2.88±0.60 7.12±0.98
EOC 150 4.73±0.31 62.35±5.46 14.55±0.32 1.84±0.13a 5.49
±0.83
Datapresentedasmean±SEMofsixanimalsanalyzedbyANOVAfollowedbyTukeytest.
ap<0.05comparedtohealthyanimals.
Table7
Effectsof5-FUandEOConbiochemicalparametersofperipheralbloodofmice(n=6)subjectedtodifferenttreatments(9days).
Groups Dose,mg/kg AST,U/L ALT,U/L Urea,mg/dL Creatinine,mg/dL
Healthyanimals – 283.2±24.94 53.6±6.55 39.0±1.87 0.42±0.08
Tumorcontrol – 287.8±20.19 71.8±7.31 66.0±11.64 0.46±0.04
5-FU 25 242.0±12.17 67.8±7.11 43.0±7.14 0.32±0.02
EOC 100 348.0±32.35 54.0±8.50 52.8±16.86 0.63±0.15
EOC 150 405.2±24.43a,b 240.8±27.88a,b 30.6±4.41 0.60±0.02
Datapresentedasmean±SEMofsixanimalsanalyzedbyANOVAfollowedbyTukeytest.
ap<0.05comparedtotumorcontrol. bp<0.05comparedtohealthyanimals.
synergisticaction appears tobea significantaspect, emphasiz-ingtheimportancetostudythewholeessentialoilratherthanits componentsseparately(Bhallaetal.,2013).
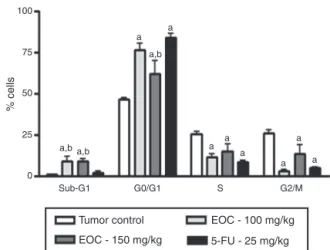
Oneofthemainwaystostudythemechanismofactionof anti-cancerdrugsistoexamineifthedrugexertsitseffectsbyinducing cellcyclearrest.EOCinducedsignificantchangeinthe distribu-tionofEhrlichcarcinomacellsindifferentcellcyclephases.There wereincreasesinthepercentageofcellsinG0/G1,and simulta-neousreductionofcellsintheSphase,andintheG2/Mphase.In addition,weobservedasignificantincreaseinthecontentof sub-diploidDNA(fragmentedDNA)inthecellsofanimalstreatedwith EOC(Fig.2),whichisconsideredasamarkerofcelldeathby apo-ptosis(Darzynkiewiczetal.,1992).Inductionofapoptosisisone themostimportantmarkerofcytotoxicantitumoragents.Ithas beenshownthatsomenaturalcompoundsincludingplantsinduce apoptoticpathwaysthatareblockedincancercells (Safarzadeh
etal.,2014).
Consideringthevarioustoxicsideeffectsofanticanceragents onnormalcells,weproceededtoinvestigatepossibleEOC toxic-ity.EOCinducedadecreaseinwaterandfeedconsumptionwhen comparedtothehealthyandtumorcontrolgroups(Table5).We foundasignificantdecreaseinthefinalweightsforalloftheanimals treated,includingthosetreatedwith5-FU.Theresultscorroborate thedataobservedonacutetoxicitystudy,confirmingthepossible gastrointestinalEOCtoxicity.Similarly,5-FUalsoinduceda reduc-tioninbodyweightthatwasexpectedsincethisisaneffectwell describedintheliteratureforthischemotherapy(El-Sayyadetal.,
2009).
In regarding to the organ indexes, there was a significant decrease in the thymus index for the group treated with EOC (150mg/kg)compared tothehealthygroup(Table6).Thedata
100
75
50
% cells
25
a,b a,b
a,b
a a
a a a
a a
a
0
Sub-G1 G0/G1
Tumor control
EOC - 150 mg/kg
EOC - 100 mg/kg
5-FU - 25 mg/kg
S G2/M
Fig.2.PercentageofEhrlichascitescarcinomacellsindifferentphasesofthecell cycleaftertreatmentwith5%Tween80solution(control),EOC(100mg/kg),EOC (150mg/kg)and5-FU(25mg/kg),ap<0.05comparedtocontrolgroup,bp<0.05
comparedtogrouptreatedwith5-FUwithANOVAandthenfollowedbytheTukey test.
for the thymusindex indicatethat EOC promotedan apparent immunosuppression,whichcorroborateswiththehematological datashowingadecreaseinlymphocytesaftertreatmentwiththe highestEOCdose.Thiseffectisoneofthemostcommonsideeffects of chemotherapeutic agents currently used in clinical practice
(RasmussenandArvin,1982).
Fig.3.Histopathologyofliverofexperimentalgroups:(A)portalspacewithvasculobiliartriadandhepaticcordslobular–control;(B)parenchymalnecrosisfoci–EOC (100mg/kg);(C)Kupffercellhyperplasia–EOC(150mg/kg);(D)moderateincreasesinthenumberoflymphocytesinportalareas–5-FU(25mg/kg);(E)hepatocellular polyploidyphenomena–5-FU(25mg/kg).
grouptreatedwithEOC(150mg/kg),inrelationtothetumor con-trolandhealthygroups,wasobserved(Table7).Thedatasuggest thatEOCinducedlivertoxicity,asevidencedbyincreasedAST,but moreimportantlybytheincreaseinALT.Significantly,weobserved thatthechangeswerenotwithinnormalvariationlimitsformice enzymaticactivity(referencevalues:AST–formaleandfemale mice,70–400IU/l,ALT –formales,25–200IU/landfor females, 25–100IU/l)(Gad,2007).
Inthehematologicalevaluations,EOC (150mg/kg)induceda significantdecreaseintheredbloodcellcount,hemoglobinand hematocrit(Table8).Inaddition,significantincreasewasobserved
forMCVandMCH(Table8).Thissuggestsclinicalfeaturesof
ane-mia(Nissensonetal.,2003).Thiscomplicationiscommonformany
patientsinchemotherapy(Gasparetal.,2015)andtheseresults corroborate the data observed on hemolytic assay, confirming the toxicity of the oil to erythrocytes. Based on hematimetric indices,wesuggestthattheanemiacausedbytreatmentwithEOC (150mg/kg)fitsthemacrocytic andnormochromicanemia pro-file.
The leukopenia and lymphocytopenia observed for EOC (150mg/kg)isoneofthemajorsideeffectsofcancertreatment, drugaggressiontoward cells of theimmune system(Liu et al., 2013).Yet,itwaspossibletodemonstrateamarkedleukopenia, withincreaseoflymphocytesandreductionofneutrophilsinthe treatmentwith5-FU(Table8),knownsideeffectsofthisanticancer drug(Linsetal.,2009).
Nohistopathologicalchangeswereobservedinthekidneysof animalstreatedwithEOC(datanotshown).Inthemajorityof ani-malstreatedwithbothdosesofEOCweobservedliverchanges suchasKuppfercellhyperplasia,moderateincreasesinthe num-beroflymphocytesinportalareas,andparenchymalnecrosisfoci (randomlyseeninzonesI,IIandIII)(Fig.3BandC).Inthe ani-malsof5-FUgroup,beyondthesechanges,wefoundperi-portal inflammation,peri-septalnecrosisfeaturingdiscrete(piecemeal) areasofhepaticcytolysis,inflammationwithintheportalspaces, parenchymalactivity,withfocalhepatocytenecrosissurrounded bylymphohistiocyticaggregatesinmanyplaces,and hepatocellu-larpolyploidyphenomena(Fig.3DandE).Inthetreatmentgroup (5-FU),thehistologicalchangeswereconsistentwithmoderately activetoxichepatitis.
ThedatacorroboratethebiochemicalresultsobtainedforAST andALTforthehighestdose(150mg/kg)ofEOC.However,allof thechangescommontobothtreatedgroupsarereportedinthe literatureasevidenceofweakhepatotoxicity.Withdrawalofthe drug,oradosageadjustmentusuallyleadstoarapidimprovement andreversalofthedamage(Tortietal.,2001;Montenegroetal.,
2008).
Table8
Effectsof5-FUandEOConhematologicalparametersofperipheralbloodofmice(n=6)subjectedtodifferenttreatments(9days).
Parameters Healthyanimals Tumorcontrol 5-FU EOC
25mg/kg 100mg/kg 150mg/kg
Redbloodcells,106/mm3 9.36
±0.14 8.20±0.50 8.48±0.16 8.79±0.23 6.21±0.74a,b
Hemoglobin,g/dl 14.84±0.24 12.50±0.80 12.88±0.08 14.14±0.17 10.46±0.18b
Hematocrit,% 43.84±0.55 40.76±2.38 37.32±1.01 40.40±0.69 34.22±4.07b
MCV,fm3 46.60±0.97 49.60±0.25 43.80±0.58a,b 46.00±0.84a 54.80±0.20a,b
MCH,pg 15.84±0.47 15.26±0.19 15.22±0.23 16.14±0.26 16.88±0.24a
MCHC,g/dl 33.74±0.38 30.68±0.28 34.52±0.83 34.04±0.52 32.62±1.79
Totalleukocytes,103/mm3 8.18
±0.43 13.66±1.0 4.12±0.59a 10.86
±2.87 4.86±1.0a
Lymphocytes,% 60.60±4.24 36.20±6.53b 78.40
±2.21a 41.40
±8.48 24.23±2.88a,b
Neutrophils,% 34.60±4.21 54.20±9.22 17.40±2.5a 64.0
±6.63b 63.40
±5.5b
Monocytes,% 4.40±0.74 4.20±1.2 3.40±0.75 4.0±1.13 5.60±1.12
Eosinophils,% 0.40±0.24 0.29±0.20 0.40±0.24 0.22±0.11 0.60±0.40
Dataarepresentedasmean±SEMofsixanimalsanalyzedbyANOVAfollowedbyTukeytest.
ap<0.05comparedtotumorcontrol. bp<0.05comparedtohealthyanimals.
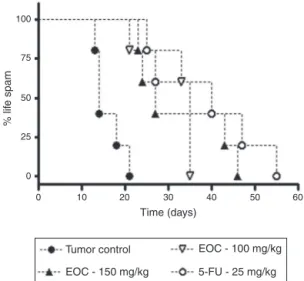
100
75
50
25
0
0 10 20
Tumor control
EOC - 150 mg/kg
EOC - 100 mg/kg
5-FU - 25 mg/kg
30
Time (days)
% lif
e spam
40 50 60
Fig.4.SurvivaltimesoffemalemiceinoculatedwithEhrlichcarcinomacellsand treatedwithEOCand5-FU.Datapresentedasmean±SEMofsixanimalsanalyzed byKaplan–Meiertest.
observedtoxicitytotreatmentwith100mg/kgwassignificantly lower,wehaveshowntheadvantagesofEOCatadoseof100mg/kg.
Conclusions
EOChaspotentinvivoantitumoractivity,andinducesmoderate gastrointestinal,hematologicalandlivertoxicity,underthe condi-tionsevaluated.Nevertheless,itdoesnotrepresentalimitingfactor forthecontinuityofpre-clinicalpharmacologicalstudies,whereas antineoplasticdrugstypicallyexhibithightoxicity.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contribution
DRPM,HMBF,TLR,TMB,VMM,TKGS,JCLRP,ALX,DMB,MVS par-ticipatedinstudyconceptanddesign,acquisitionofdata,analysis andinterpretationofdata,andcriticalrevisionofthemanuscriptfor importantintellectualcontent.JFTandMSScarriedoutthe extrac-tionofessentialoiland participatedindraftingthemanuscript. KKPMperformedthehistopathologicalanalysis.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
ThisworkwassupportedbytheBrazilianagenciesCAPESand CNPq. “Pontual Traduc¸ões” (Londrina/Paraná, Brazil) performed Englisheditingofthemanuscript.
References
Almeida,R.N.,Navarro,D.S.,Barbosa-Filho,J.M.,2001.Plantswithcentralanalgesic activity.Phytomedicine8,310–322.
Amelo,W.,Nagpal,P.,Makonnen,E.,2014.Antiplasmodialactivityofsolvent frac-tionsofmethanolicrootextractofDodonaeaangustifoliainPlasmodiumberghei infectedmice.BMCComplement.Altern.Med.14,462–468.
Anvisa,2013.Determinaapublicac¸ãodoGuiaparaaconduc¸ãodeestudosnão clíni-cosdetoxicologiaeseguranc¸afarmacológicanecessáriosaodesenvolvimento demedicamentos.MinistériodaSaúde.AgênciaNacionaldeVigilânciaSanitária. DiárioOficialdaUnião,PoderExecutivo,Brasília,DF,31January2013. Araújo,M.J.A.M.,Dutra,R.P.,Costa,G.C.,Reis,A.S.,Assunc¸ão,A.K.M.,Libério,S.A.,
Maciel,M.C.G.,Silva,L.A.,Guerra,R.N.M.,Ribeiro,M.N.S.,Nascimento,F.R.F., 2009.EffectofpropolisofScaptotrigonaaff.posticaonthedevelopmentofthe tumorofEhrlichinmice.Rev.Bras.Farmacogn.20,580–587.
Bezerra,D.P.,MarinhoFilho,J.D.,Alves,A.P.N.N.,Pessoa,C.,DeMoraes,M.O.,Pessoa, O.D.L.,Torres,M.C.M.,Silveira,E.R.,Viana,F.A.,Costa-Lotufo,L.V.,2009. Anti-tumoractivityoftheessentialoilfromtheleavesofCrotonregelianusandits componentascaridole.Chem.Biodivers.6,1224–1231.
Bhalla,Y.,Gupta,V.K.,Jaitak,V.,2013.Anticanceractivityofessentialoils:areview. J.Sci.FoodAgric.93,3643–3653.
Boussios, S., Pentheroudakis, G., Katsanos, K., Pavlidis, N., 2012. Systemic treatment-inducedgastrointestinaltoxicity:incidence, clinicalpresentation andmanagement.Ann.Gastroenterol.25,106–118.
Carol,A.,1995.Acute,subchronicandchronictoxicology.In:CRC(Ed.),Handbook ofToxicology.CRCPressInc.,U.S.,pp.51–104.
Carlini,E.A.,2003.Plantsandthecentralnervoussystem.Pharmacol.Biochem. Behav.75,501–512.
Chen,L.,Watkins,J.F.,1970.EvidenceagainstthepresenceofH2histocompatibility antigensinEhrlichascitestumourcells.Nature225,734–735.
Correa-Royero,J.,Tangarife,V.,Durán,C.,Stashenko,E.,Mesa-Arango,A.,2009. Invitroantifungalactivityandcytotoxiceffectofessentialoilsandextractsof medicinalandaromaticplantsagainstCandidakruseiandAspergillusfumigatus. Rev.Bras.Farmacogn.20,734–741.
Darzynkiewicz,Z.,Bruno,S.,DelBino,B.G.,Gorczyca,W.,Hotz,M.A.,Lassota,P., Traganos,F.,1992.Featuresofapoptoticcellsmeasuredbyflowcytometry. Cytometry13,795–808.
Dolai,N.,Karmakar,I.,Kumar,R.B.S.,Kar,B.,Bala,A.,Haldar,P.K.,2012.Evaluation ofantitumoractivityandinvivoantioxidantstatusofAnthocephaluscadamba onEhrlichascitescarcinomatreatedmice.J.Ethnopharmacol.142,865–870. El-Sayyad,H.I.,Ismail,M.F.,Shalaby,F.M.,Abou-El-Magd,R.F.,Rajiv,L.G.,Augusta,
F.,Madhwa,H.G.R.,Allal,O.,2009.Histopathologicaleffectsofcisplatin, doxoru-bicinand5-flurouracil(5-FU)ontheliverofmalealbinorats.Int.J.Biol.Sci.5, 466–473.
Fernandes,H.M.B.,Oliveira-Filho,A.A.,Sousa,J.P.,Oliveira,T.L.,Lima,E.O., Meire-les,D.R.,Brito,M.T.,Zelioli,I.A.M.,Queiroz,N.C.A.,Foglio,M.A.,Ruiz,A.L.T.G., Carvalho,J.E.,Silva,M.S.,Castello-Branco,M.V.S.,Tavares,J.F.,2012.Antitumor, antimicrobialeffectandchemicalcompositionoftheessentialoilofCroton polyandrusSpreng.Lat.Am.J.Pharm.31,1430–1434.
Fernandes,H.M.B.,Leão,A.D.,Oliveira-Filho,A.A.,Sousa,J.P.,Oliveira,T.L.,Lima, E.O.,Silva,M.S.,Tavares,J.F.,2013.Antimicrobialactivityandphytochemical screeningofextractsfromleavesofCrotonpolyandrusSpreng.Int.J.Pharmacogn. Phytochem.Res.5,223–226.
Gad,S.C.,2007.AnimalModelsinToxicology.Taylor&Francis,ISBN0824754077. Gaspar,B.L.,Sharma,P.,Das,R.,2015.Anemiainmalignancies:pathogeneticand
diagnosticconsiderations.Hematology1,18–25.
Gordon,H.,Sweet,H.H.,1936.Asimplemethodforthesilverimpregnationof reti-culin.Am.J.Pathol.12,545–551.
Gould,M.N.,1997.Cancerchemopreventionandtherapybymonoterpenes.Environ. HealthPerspect.105,977–979.
Grinberg,L.N.,Newmark,H.,Kitrossky,N.,Rahamim,E.,Chevion,M.,Rachmilewitz, E.A.,1997.Protectiveeffectsofteapolyphenolsagainstoxidativedamagetored bloodcells.Biochem.Pharmacol.54,973–978.
Hanahan,D.,2014.Rethinkingthewaroncancer.Lancet383,558–563.
Jain,D.,Pathak,N.,Khan,S.,Raghuram,G.V.,Bhargava,A.,Samarth,R.,Mishra,P.K., 2011.EvaluationofcytotoxicityandanticarcinogenicpotentialofMenthaleaf extracts.Intern.J.Toxicol.30,225–236.
Kang,C.,Munawir,A.,Cha,M.,2009.Cytotoxicityandhemolyticactivityof jelly-fishNemopilemanomurai(Scyphozoa:Rhizostomeae)venom.Comp.Biochem. Physiol.150,85–90.
Kiang,J.G., Smith, J.T.,Agravante, N.G.,2009. Geldanamycin analog17-DMAG inhibitsiNOSandcaspasesingamma-irradiatedhumanTcells.Radiat.Res.172, 321–330.
Kin,J.H.,Lee,S.J.,Han,Y.B.,1994.IsolationofisoguanosinefromCrotontigliumand itsantitumoractivity.Arch.Pharmacol.Res.17,115–118.
Lins,K.O.,Bezerra,D.P.,Alves,A.P.N.N.,Alencar,N.M.,Lima,M.W.,Torres,V.M.,Farias, W.R.,Pessoa,C.,deMoraes,M.O.,Costa-Lotufo,L.V.,2009.Antitumorproperties ofasulfatedpolysaccharidefromtheredseaweedChampiafeldmannii (Diaz-Pifferer).J.Appl.Toxicol.29,20–26.
Liu,W.,Zhang,C.C.,Li,K.,2013.Prognosticvalueofchemotherapy-induced leukope-niainsmall-celllungcancer.CancerBiol.Med.10,92–98.
Lopes,D.,Bizzo,H.R.,Sobrinho,A.F.S.,Pereira,M.V.G.,2003.Essentialoilfromleaves ofCrotonsacaquinhaBenth.J.Essent.OilRes.15,48–49.
Maroni,L.C.,Silveira,A.C.O.,Leite,E.A.,Melo,M.M.,Ribeiro,A.F.C.,Cassali,G.D., Souza,C.M.,Fagundes,E.M.S.,Caldas,I.R.,Araújo,M.S.S.,etal.,2012.Antitumor effectivenessandtoxicityofcisplatin-loadedlong-circulatingandpH-sensitive liposomesagainstEhrlichascitictumor.Exp.Biol.Med.237,973–984. Montenegro,R.C.,Farias,R.A.F.,Pereira,M.R.P.,Alves,A.P.N.N.,Bezerra,F.S.,Neto,
M.A.,Pessoa,C.,Moraes,M.O.,Costa-Lotufo,L.V.,2008.Antitumoractivityof pisosterolinmicebearingwithS180tumor.Biol.Pharm.Bull.31,454–457. Nascimento,F.R.F.,Cruz,G.V.B.,Pereira,P.V.S.,2006.AsciticandsolidEhrlichtumor
inhibitionbyChenopodiumambrosioidesL.treatment.LifeSci.78,2650–2653.
Ng,T.B.,Li,W.W.,Yeung,H.W.,1986.Asterylglycosidefractionwithhemolytic activityfromtubersofMomordicacochinchinensis.J.Ethnopharmacol.18,5–6. Nissenson,A.R.,Goodnough,L.T.,Dubois,R.W.,2003.Anemia.Notjustaninnocent
bystander?Arch.Intern.Med.163,1400–1404.
OECD(TheOrganisationforEconomicCo-operationandDevelopment),1997. Mam-malianErythrocyteMicronucleusTest:GuidelinesforTestingofChemicals.n◦
474.
Oliveira,A.C.,Leal-Cardoso,J.H.,Santos,C.F.,Morais,S.M.,Coelho,A.N.S.,2001. AntinociceptiveeffectsoftheessentialoilofCrotonzehntneriinmice.Braz.J. Med.Biol.Res.34,1471–1474.
Ozaslan,M.,Karagoz,I.D.,Kilic,I.H.,Guldur,M.E.,2011.Ehrlichascitescarcinoma. Afr.J.Biotechnol.10,2375–2378.
Pereira,A.S.,Carbonell,A.S.,AquinoNeto,F.R.,Amaral,A.C.F.,Barnes,R.A.,2002. High-temperaturegaschromatography–massspectrometrywithglasscapillary columnsforthescreeningofnaturalproducts.J.Chromatogr.A947,255–265. Rasmussen,L.,Arvin,A.,1982.Chemotherapy-inducedimmunosuppression.
Envi-ron.HealthPerspect.43,21–25.
Rodrigues,K.A.F.,Amorim,L.V.,Oliveira,J.M.G.,2013.EugeniaunifloraL.essential oilasapotentialanti-Leishmaniaagent:effectsonLeishmaniaamazonensis andpossiblemechanismsofaqction.Evid.BasedComplement.Altern.Med., http://dx.doi.org/10.1155/2013/279726.
Safarzadeh,E.,Shotorbani,S.S.,Baradaran,B.,2014.Herbalmedicineasinducersof apoptosisincancertreatment.Adv.Pharm.Bull.4,421–427.
SalgadoOloris,S.C.,Dagli,M.L.Z.,Guerra,J.L.,2002.Effectofh-caroteneonthe devel-opmentofthesolidEhrlichtumorinmice.LifeSci.71,717–724.
Shrivastav,S.,Stone,K.R.,Paulson,D.F.,Bonar,R.A.,1980.Activationof cyclophos-phamideforinvitrotestingofcellsensitivity.CancerRes.40,4443–4445. Silva,S.L.,Chaar,J.S.,Figueiredo,P.M.S.,Tomomasa,Y.,2008.Cytotoxicevaluationof
essentialoilfromCaseariasylvestrisSwonhumancancercellsanderythrocytes. ActaAmaz.38,107–112.
Sun,Q.,Li,R.T.,Guo,W.,2006.Novelclassofcyclophosphamideprodrug: cyclophos-phamidespiropiperaziniums(CPSP).Bioorg.Med.Chem.Lett.16,3727–3730. Sylvestre,M.,Pichette,A.,Longtin,A.,Nagau,F.,Legault,J.,2006.Essentialoilanalysis
andanticanceractivityofleafessentialoilofCrotonflavensL.fromGuadeloupe. J.Ethnopharmacol.103,99–102.
Torti,V.R.,Cobb,A.J.,Everitt,J.L.,Marshall, M.W.,Boorman, G.A.,Butterworth, B.E., 2001. Nephrotoxicity and hepatotoxicity induced by inhaled bro-modichloromethaneinwild-typeandp53-heterozygousmice.Toxicol.Sci.64, 269–280.
Verc¸osaJúnior,D.,Souza-Fagundes,E.M.,Cassali,G.D.,Ribeiro,E.L.,Zani,C.L.,Melo, M.M.,2006.EfeitodomiriadenolídeoisoladodeAlomiamyriadenia(Asteraceae) sobreotumordeErlichascíticonocamundongo.Arq.Bras.Med.Vet.Zootec.58, 788–798.
Vieira,P.,Santos,S.,Chen-Chen,L.,2010.Assessmentofmutagenicityand cytotox-icityofSolanumpaniculatumL.extractsusinginvivomicronucleustestinmice. Braz.J.Biol.70,601–606.
Wang,W.,Li,N.,Luo,M.,Yuangang,Z.,Efferth,T.,2012.Antibacterialactivityand anticanceractivityofRosmarinusofficinalisL.essentialoilcomparedtothatof itsmaincomponents.Molecules17,2704–2713.
Wu,C.S.,Chen,Y.J.,Chen,J.J.W.,Shieh,J.J.,Huang,C.H.,Lin,P.S.,Chang,G.C., Tsai-Chang,J.H.,Lin,C.C.,2012.Terpinen-4-olinducesapoptosisinhumannonsmall celllungcancerinvitroandinvivo.Evid.BasedComplement.Altern.Med., http://dx.doi.org/10.1155/2012/818261.
Zhang,A.,Zhu,Q.Y.,Luk,Y.S.,Ho,K.Y.,Fung,K.P.,Chen,Z.Y.,1997.Inhibitoryeffects ofjasminegreenteaepicatechinisomersonfreeradical-inducedlysisofred bloodcells.LifeSci.61,383–394.