Tânia Catarina Ferreira
Dissertation presented to obtain the Ph.D degree in Biology
Instituto de Tecnologia Química e Biológica | Universidade Nova de Lisboa
THE ROLE OF PKCs IN
MORPHOGENESIS
Ferreira, T.
The role of PKCs in Morphogenesis
PhD thesis
Instituto Gulbenkian de Ciência, Universidade Nova de Lisboa, 2016
In English, with abstract in Portuguese
To my family and friends.
Declaration/Declaração
I declare that this dissertation is a result of my own research, carried out
in the laboratory of Dr. Rui Gonçalo Martinho at Instituto Gulbenkian de
Ciência (IGC) in Oeiras, Portugal; with the cosupervision of Dr. António
Jacinto at Chronic Diseases Research Center (CEDOC), Lisbon, Portugal.
Chapter 3 has been partially published in Development 139 (3), 503-513,
entitled "Drosophila aPKC is required for mitotic spindle orientation during
symmetric division of epithelial cells", Leonardo Gaston Guilgur, Pedro
Prudêncio, Tânia Ferreira, Ana Rita Pimenta-Marques and Rui Gonçalo
Martinho. Chapter 4 has been partially published in Developmental Biology
394 (2), 277-291, entitled "Drosophila protein kinase N (Pkn) is a negative
regulator of actin–myosin activity during oogenesis", Tânia Ferreira, Pedro
Prudêncio and Rui Gonçalo Martinho.
Declaro que esta dissertação é o resultado do meu próprio trabalho
desenvolvido no laboratório do Dr. Rui Gonçalo Martinho, no Instituto
Gulbenkian de Ciência (IGC) em Oeiras, Portugal; com co-supervisão do Dr.
António Jacinto, Centro de Investigação em Doenças Crónicas (CEDOC),
Lisboa, Portugal. O capítulo 3 foi parcialmente publicado na revista
Development 139 (3), 503-513, como "Drosophila aPKC is required for mitotic
spindle orientation during symmetric division of epithelial cells", Leonardo
Gaston Guilgur, Pedro Prudêncio, Tânia Ferreira, Ana Rita Pimenta-Marques
e Rui Gonçalo Martinho. O capítulo 4 foi parcialmente publicado na revista
Developmental Biology 394 (2), 277-291, como "Drosophila protein kinase N
(Pkn) is a negative regulator of actin–myosin activity during oogenesis", Tânia
Financial Support/Apoio Financeiro
This dissertation had the financial support from the FCT doctoral fellowship
SFRH/BD/37587/2007 and Fundação Calouste Gulbenkian.
Esta dissertação teve o apoio financeiro da FCT, bolsa de doutoramento
Acknowledgments
Thank to all that have crossed my path, it has been a long journey, and
we are finally here!!!
First of all I thank my supervisor Rui, for giving me the opportunity of
doing my PhD in his lab, for accepting my scientific naiveness and
stubbornness. But most, for teaching me, and for letting me learn and explore
my ideas. For showing me that no matter where I go anything is possible.
I have been very lucky with the people that worked with me at the Early
Fly Development lab: Pedro, Ana Rita, Gaston, Barbara, Paulo, Xana, Rui,
Denisa e André. We are colleagues, but so much more than that! I am
indebted to André Rosa, my sidekick in the craziness of screening… It was
truly fun to do it with you! Ana Rita, Pedro and Gaston… you have been
priceless in my live. Thank you for sharing these years with me. Ana, thank
your for teaching me all about flies and pretty much mentoring my early days
at the lab. But thank you for much more than that: for your honesty, for your
endless strength and brilliant sense of humor. Gaston, thank you for your
friendship and for being always full of surprises. You are an amazing mixture
of different worlds, you are an amazing friend! Pedrito… my Prud, obrigada
por tudo não é suficiente! Thank you for never leaving me alone, for always
being there, helping me carry my burdens, for being my accomplice and giving
me strength so often without me realizing it. Friendships like this are treasured
moments in a lifetime!
I also have to thank my Kikas, first for sharing Pedro with me =), but also
for the non-complicated friendship that we have built. For the never-endless
support and comprehension; and for ensuring our daily dose of pastéis de
nata! =)
In this journey, I have also been very lucky with the next step! Thank you
Miguel for accepting me in your lab, for trusting me and pushing me to always
Teresa, Catarina, Clara, Vanessa, Inês, Margarida, Bia, Marika, Kirsten, Akila,
Pamela, Sebastian, Gianluca, Gonçalo, Cornelia, Ksenia, Zé Manel and
Eddi…
Jujuca, a very pink and special thanks to you! Thank you for your true
friendship, and for never giving up on me! We are indeed the same type of
crazy...but also different sides of the same coin! I hope we will always be…
A special thanks to Sónia Rosa, our wing fairy but so much more than
that! Thank you for taking care of us (and I do not only mean work!) and for
putting up with our insanity with a smile on your face.
The Zheng He wing is an amazing place to work. It is almost a living
organism! Many thanks to all that are part, specially: Paulo Duarte, Catarina,
Inês Bento, Zita, Gaelle, Pipo, Beatriz and Nita!
The IGC has been a truly wonderful place to be. The scientific
environment and discussions are priceless and I am truly grateful for all that
had the patience to engage in some of them with me!
Aos meus pais, por serem quem são! Por me amarem e me apoiarem
incondicionalmente. Por serem os meus maiores cr ticos e maiores apoiantes.
Tudo o que conquisto devo-o a vocês, e é em parte vosso!
Finally to you, Duarte! For believing in me, for never quitting on me,
even when you felt you should. This journey has not only been the journey of
my PhD, it has been also our journey and our test… and we made it! And we
made it brilliantly, blessed by our little L. that now makes us so much more
Table Of Contents
Table Of Contents ... VII
List Of Figures ... XI
List Of Tables ... XIII
List Of Abbreviations ... XIII
Abstract ... XIX
Sum
á
rio ... XXI
Chapter 1 - General Introduction ... 1
1.1. Tissue Morphogenesis ... 3
1.2. The Cytoskeleton Is A Dynamic Network Of Filaments ... 4
1.2.1. The Cytoskeleton And Its Components ... 4
1.2.1.1. The Microtubule Cytoskeleton ... 4
1.2.1.2. The Intermediate Filaments Cytoskeleton ... 5
1.2.1.3. The Actin Cytoskeleton ... 6
1.2.1.3.A. The Structural Dynamics Of The Actin Cytoskeleton
... 6
1.2.1.3.B. The Actin Cytoskeleton And Its Regulators ... 6
1.2.2. The Cytoskeleton And The Regulation Of Contractility ... 10
1.3. RhoGTPases And The Regulation Of The Actin-Myosin
Cytoskeleton ... 13
1.3.1. Rho-GTP Effector Targets ... 17
1.3.1.1. ROK Regulation Of Actin-Myosin Activity ... 17
1.3.1.2. The PKC Family Of Kinases ... 19
1.4. Model Systems To Study Epithelium Morphogenesis ... 24
1.4.1. Wing Disc Epithelium Is A Powerful Tool To Study
Symmetric Cell Division ... 24
1.4.1.1. Wing Disc Morphogenesis ... 25
1.4.1.2. Mitotic Spindle Position In Epithelial Cells ... 25
1.4.1.3. Molecular Landmarks Of Spindle Positioning ... 27
1.4.2. Drosophila Oogenesis Is A Powerful Tool To Study
Actin-Myosin Regulation ... 31
1.4.2.2. Stages and Dynamics of Female Follicle Epithelium
Development ... 34
1.4.2.3. Actin-myosin Cytoskeleton Regulation and Dynamics in
Drosophila oogenesis ... 37
1.5. References ... 38
Chapter 2 - 2R Maternal Screen ... 53
2.1. Introduction ... 57
2.1.1. Early Stages Of Drosophila Embryogenesis ... 57
2.2. Materials And Methods ... 62
2.2.1. Fly Husbandry ... 62
2.2.2. 2R Maternal Screen ... 62
2.2.3. Generation Of Maternal Mutant Embryos ... 63
2.2.4. Mapping Of Complementation Groups 5 And 8 ... 64
2.2.5. Identification Of Point Mutations In Complementation Groups
5 And 8 ... 65
2.2.6. Western Blot Analysis ... 65
2.2.7. Immunohistochemistry ... 66
2.2.8. Generation Of The P[FRT42B], P{Pkn[06736]}/CyO
Recombinant Stock ... 66
2.2.9. Image Treatment ... 68
2.3. Results ... 68
2.3.1. Nine Complementation Groups Were Identified Through A
2R Maternal Mutant Screen ... 68
2.3.2. Mapping Of Complementation Group 5 ... 70
2.3.3. Complementation Group 5 Alleles Are Allelic To The aPKC
Gene ... 71
2.3.4. Mapping Of Complementation Group 8 ... 73
2.4. Discussion ... 75
2.5. References ... 78
Chapter 3 - Novel Roles Of Drosophila aPKC In Epithelial
Morphogenesis ... 84
Section A - Drosophila aPKC Is Required For Mitotic Spindle
Orientation During Symmetric Division Of Epithelial Cells ... 87
3.A.1. Guilgur et al. (2012). ... 88
Section B - Drosophila aPKC PB1 Domain Is Essential For Follicle
Epithelial Integrity ... 107
3.B.1. Introduction ... 109
3.B.1.1. aPKC Is Essential For Epithelial Morphogenesis ... 109
3.B.1.2. The Organisation Of Epithelial Polarity And Its
Regulators ... 109
3.B.2. Materials And Methods ... 114
3.B.2.1. Fly husbandry ... 114
3.B.2.2. Generation Of Maternal Mutant Embryos ... 114
3.B.2.3. Generation Of Mutant Clonal Tissue ... 114
3.B.2.3.1. Generation Of Clonal Germline And Follicle Cells
... 114
3.B.2.4. Immunohistochemistry ... 115
3.B.2.4.1. Embryogenesis ... 115
3.B.2.4.2. Oogenesis ... 115
3.B.2.5. Image Treatment ... 116
3.B.3. Results ... 116
3.B.3.1. apkc[pb1] Has A Point Mutation Within The PB1 Domain
Of aPKC ... 116
3.B.3.2. apkc[pb1] Maternal Mutant Embryos Show Germband
Extension Defects ... 117
3.B.3.3. Females Mutant For apkc[pb1] Fail To Form An
Epithelium During Oogenesis ... 118
3.B.4. Discussion ... 121
3.B.4.1. Drosophila aPKC Is Required For Mitotic Spindle
Orientation During Symmetric Division Of Epithelial Cells ... 122
3.B.4.2. aPKC PB1 Domain Is Essential For All Tested aPKC
Functions ... 124
3.B.5. References ... 127
Chapter 4 - Pkn Is A Negative Regulator Of Actin-Myosin Activity ... 131
Section A - Drosophila Protein Kinase N (Pkn) Is A Negative
Regulator Of Actin-Myosin Activity During Female Germline
Development ... 135
4.A.1. Ferreira et al. (2014). ... 136
4.A.2. Supplementary Materials And Methods ... 156
4.A.2.1. Quantification Of Germline Cell Number ... 156
4.A.2.2. Quantification Of Cytokinesis ... 157
4.A.2.3. Quantification Multi-Nucleated Germline Cells ... 157
4.A.2.4. In Vivo Analysis Of Nurse Cell To Oocyte Cytoplasmic
Transfer ... 157
4.A.3. Supporting Figures ... 159
Section B - Drosophila Protein Kinase N (Pkn) Is Required For
Epithelial Morphogenesis ... 161
4.B.1. Introduction ... 163
4.B.1.1. Protein Kinase N Subfamily Members ... 163
4.B.1.2. Pkn Regulation And Signalling ... 165
4.B.1.3. Follicle Epithelium Morphogenesis ... 167
4.B.2. Materials And Methods ... 170
4.B.2.1. Fly Husbandry ... 170
4.B.2.2. Generation Of Mutant Clones ... 171
4.B.2.3. Immunohistochemistry ... 171
4.B.2.4. Quantification Of Follicle Cell Migration ... 172
4.B.2.5. Quantification Of Follicle Epithelia Integrity ... 172
4.B.2.6. Quantification Of Follicle Apical Cell Area ... 173
4.B.2.7. Quantification Of Follicle Cell Basal Actin Levels ... 174
4.B.2.8. Quantification Of Follicle Basal Actin Fibres Orientation
... 175
4.B.2.9. Quantification Of Follicle Cell Basal Myosin Levels .... 176
4.B.2.10. Statistical Analysis ... 177
4.B.3. Results ... 177
4.B.3.1. Pkn Is Not Required For The Initial Formation Of The
Follicle Epithelium ... 177
4.B.3.2. Pkn Is Not Required For Epithelial Polarity And
Adherens Junctions ... 178
4.B.3.3. Pkn Is Required For Follicle Cell Morphogenesis ... 179
4.B.3.4. Pkn Is Required For Follicle Cell Migration ... 181
4.B.3.6. Pkn Regulates F-actin Polymerisation At The Basal
Domain Of Follicle Cells ... 185
4.B.3.7. Pkn Regulates Myosin Activation At The Basal Domain
Of Follicle Cells ... 188
4.B.4. Discussion ... 194
4.B.4.1. Pkn Is Required For Morphogenesis But It Is Not For
Cytokinesis And Cell-Cell Adhesion. ... 194
4.B.4.2. Pkn Is A Negative Regulator Of Actin-Myosin Activity 195
4.B.4.3. Future Work ... 198
4.B.5. References ... 200
Chapter 5 - General Discussion ... 209
5.1. References ... 217
List Of Figures
Figure 1.1: Cellular components of the eukaryotic cytoskeleton. ... 5Figure 1.2: Actin filaments are formed by two parallel strands of head–tail polymers of actin monomers. ... 7
Figure 1.3: Within the cell actin filaments can be arranged to form multiple structures. ... 8
Figure 1.4: Actin binding proteins influence actin structure. ... 9
Figure 1.5: Actin-myosin network organization and cell adhesion, and force generation dynamics. ... 11
Figure 1.6: Non-muscle myosin. ... 12
Figure 1.7: Formation of protrusive actin structures by Rho-family GTPases. 15 Figure 1.8: Cellular processes regulated by Rho GTPases and the actin cytoskeleton. ... 16
Figure 1.9: Rho GTPases and epithelial morphogenesis. ... 16
Figure 1.10: Involvement of GTPases in the assembly and contractility of actin-myosin fibres. ... 18
Figure 1.11: The PKC protein family. ... 21
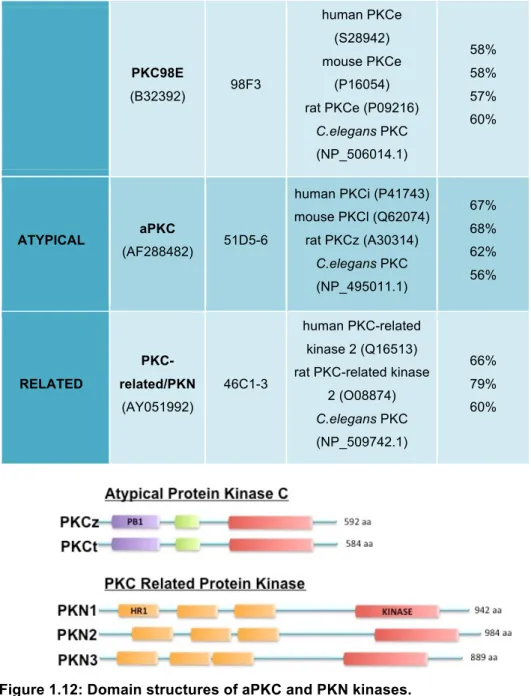
Figure 1.12: Domain structures of aPKC and PKN kinases. ... 23
Figure 1.13: Drosophila wing discs give rise to the adult structures. ... 25
Figure 1.14: Drosophila wing discs. ... 26
Figure 1.15: Diverse Polarity Cues Converge on Gαi–LGN–NuMA and the Dynein-Dynactin Complex to Control Mitotic Spindle Orientation. ... 28
Figure 1.16: Distribution of apical-basal polarity markers during mitosis. ... 30
Figure 1.18: Drosophila female germline development - Dumping. ... 35
Figure 1.19: Drosophila oogenesis - follicular cell populations. ... 35
Figure 1.20: Drosophila oogenesis - Border cell migration. ... 36
Figure 2.1: Drosophila melanogaster nuclear cycles during early embryogenesis. ... 58
Figure 2.2: Formation of the cellularization furrows. ... 59
Figure 2.3: Four stages of gastrulation. ... 61
Figure 2.4: 2R Maternal Screen Outline. ... 69
Figure 2.5: Outline of complementation tests. ... 69
Figure 2.6: CG5 mapps to the 47E3- 47F5 2R interval. ... 71
Figure 2.7: CG 5 alleles are allelic to the aPKC gene. ... 71
Figure 2.8: apkc[ts] and apkc[pb1] protein levels are normal. ... 72
Figure 2.9: aPKC localization is normal in apkc[ts] but not apkc[pb1] mutant embryos. ... 73
Figure 2.10: During early GBE there is aPKC delocalization. ... 74
Figure 2.11: CG8 was mapped to the genetic interval within the Hig and l(2)03659 genes, in the 2R arm, corresponding to the 45A6 - 45A9 cytological interval. ... 76
Figure 3.1: Various types of cell polarity in which the PAR-aPKC system is involved. ... 110
Figure 3.2 apkc mutants gastrulate but display progressive epithelial breakdown. ... 111
Figure 3.3: aPKC canonical signalling in the follicle epithelium. ... 113
Figure 3.4: aPKC signaling regulates the actin cytoskeleton. ... 113
Figure 3.5: The FLP-FRT-mediated mitotic recombination system. ... 116
Figure 3.6: In apkc[pb1] the PB1 domain, and most likely the aPKC-Par6 interaction, is disrupted. ... 118
Figure 3.7: apkc[pb1] maternal mutant embryos show germband extension defects. ... 119
Figure 3.8: apkc[pb1] protein fails to apically localize. ... 120
Figure 3.9: apkc[pb1] mutant cells fail mesenchymal-to-epithelial transition and acquire a multi-layer organization. ... 120
Figure 3.10: apkc[pb1] mutant cells fail mesenchymal-to-epithelial transition. ... 121
Figure 4.1: Drawing illustrating germline cell divisions and appearance of the ring canals during the four consecutive mitotic divisions of the germline cell in the egg chamber. ... 157
Figure 4.2: Drosophila Female Germline Development. ... 159
Figure 4.3:flw[6] is a dominant enhancer of the pkn oogenesis defects. ... 160
Figure 4.4: Domain structure of PKN family kinases. ... 164
Figure 4.5: A model for the coordinated regulation of dorsal closure by distinct Rho family GTPases. ... 168
Figure 4.7: Schematic representation of the three classes of filament
orientation scored. ... 175
Figure 4.8: PKN is not required for epithelium formation. ... 178
Figure 4.9: pkn mutant follicle cells are polarized and adherens junctions are normal. ... 180
Figure 4.10: PKNis required for epithelial cell morphogenesis. ... 181
Figure 4.11: pkn mutant follicle migration is delayed. ... 184
Figure 4.12: pkn mutant follicles have reduced apical cell area. ... 186
Figure 4.13: pkn mutant follicles have higher levels of basal F-actin when compared to control cells ... 187
Figure 4.14: Basal actin fibres orientation is affected in pkn mutant follicle cells. ... 188
Figure 4.15: Myosin is apically localized in pkn mutant follicles. ... 190
Figure 4.16: Myosin localization is normal in both the apical and basal domains of pkn mutant follicle cells. ... 190
Figure 4.17: Activated myosin, p-myosin, localization is normal in both the apical and basal domains of pkn mutant follicle cells ... 191
Figure 4.18: Hyperactivated myosin, 2p-myosin, is enriched in the basal domain of pkn mutant follicle cells ... 192
Figure 4.19: PKN negatively regulates actin-myosin contractility. ... 193
List Of Tables
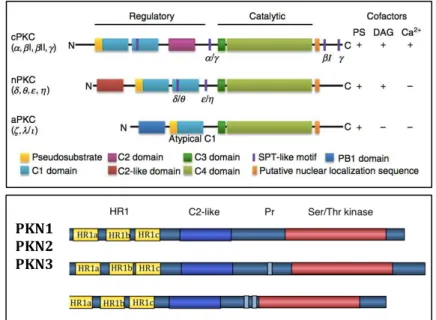
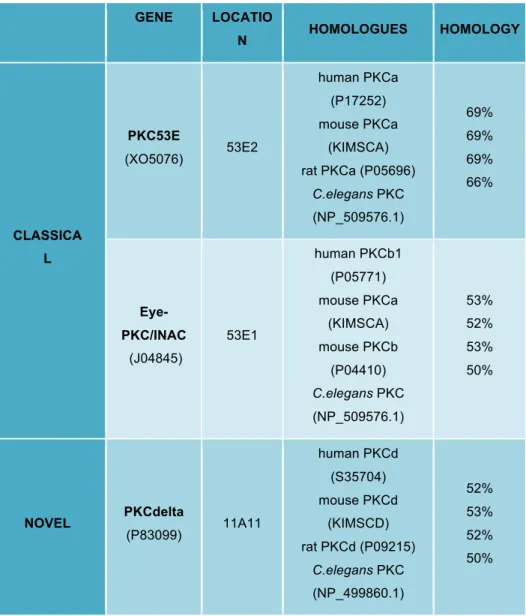
Table 1.1: PKC isoforms in Drosophila. ... 22Table 2.1: Nine complementation groups were isolated in the 2R maternal mutant screen. ... 70
List Of Abbreviations
1P-MYO: Monophosphorylated myosin. Also 1p-myosin
2P-MYO: Diphosphorylated myosin. Also 2p-myosin
A: Anterior
ABD: Actin binding domain
ABL: Abelson (abelson murine leukemia viral oncogene homolog)
AD: Adenoviruses
ADF: Actin depolymerizing factor. Often referred as cofilin
AJ: Adherens junction
AP: Anterior-posterior
APKC: Atypical protein kinase c
ARM: Armadillo. Also bcat
ARP2/3: Actin related protein 2/actin related protein 3
ATP: Adenosine triphosphate
BAC: Bacterial artificial chromosome
BAZ: Bazooka. Also par3
BBT: 1xPbs (pH 7.4) with 0.1% tween-20, 0.1% bovine serum albumin (BSA) and 5% donkey serum (DS)
BC: Border cells
BCAT: Beta-catenin. Also arm
BL: Bloomington Drosophila stock center
BSA: Bovine serum albumin
CAP: Cyclase-associated proteins
CC: Centripetal cell
CDC42: Cell division control protein 42
CG: Complementation group
CIP4: Cdc42-interacting protein 4
CP: Capping proteins
CPA: Capping proteins alpha
CPB: Capping proteins beta
CPI-17: Protein kinase c-potentiated inhibitor of 17 kDa
CPKC: Conventional Pkc
CRB: Crumbs
CTTN: Cortactin
D: Dorsal
DE-CAD: Drosophila epithelial cadherin. Also e-cad and shg
DF: Deficiency
DIA: Diaphanous-related formins. Also drf or diaph
DIC: Differential interference contrast
DNA: Deoxyribonucleic acid
DRHOGEF2: Drosophila rho guanine nucleotide exchange factor 2
DS: Donkey serum
DSHB: Developmental studies hybridoma bank
DV: Dorsal-ventral
E-CAD: Epithelial cadherin. Also de-cad and shg
E4ORF4: Early region orf4 protein
EBNA2: Epstein-barr virus (ebv) nuclear antigen 2
EC: Egg-chamber
ECL: Electrochemiluminescence or electrogenerated chemiluminescence
ECM: Extracellular matrix
EGTA: Ethylene glycol tetraacetic acid
EMLC: Essential myosin light chain subunit
EMS: Ethyl methanesulfonate
EMT: Epithelial to mesenchymal transition
ETF:QO: Flavoprotein:ubiquinone oxidoreductase
F-ACTIN: Filamentous/multimeric actin
FAK1: Focal adhesion kinase 1
FAND: Fandango. Also Drosophila ortholog of yeast syf1 and human xab2
FC: Follicle epithelial cell
FE: Follicle epithelium
FLP: Flipase
FLW: Flapwing, also pp1b
FRT: Flipase recombination target
G-ACTIN: Globular/monomeric actin
GAP: GTPase activating protein
GBE: Germband extension
GC: Germline cells
GDI: Guanine nucleotide dissociation inhibitors
GDP: Guanosine diphosphate
GEF: Guanine nucleotide exchange factor
GFP: Green fluorescent protein
GPCR: Guanylyl-nucleotide-binding protein (g protein)-coupled receptor
GSC: Germline stem cell
GTP: Guanosine triphosphate
GTPASE: Guanosine triphosphate hydrolase
H: Hours
HBV: Hepatitis b virus
HIG: Hikaru genki
HPIV-3: Human parainfluenza virus 3
HPV: Human papillomavirus
HR1: Heptapetide repeat 1
HS: Heat shock
HSV: Herpes simplex virus
JNK: C-jun n-terminal kinase
KD: Kinase domain
LCMS: Liquid chromatography-mass spectrometry
LGL: Lethal giant larvae
LIMK: Lim (lin-11, isl-1, mec-3) domain kinase
LUT: Lookup table
MBFC: Main body follicular cells
MBS: Myosin phosphatase target subunit, myosin binding subunit. Also mypt
MDCK: Madin-darby canine kidney
MET: Mesenchymal to epithelial transition
MIN: Minutes
MLCK: Myosin light-chain kinase
MRHC: Myosin regulatory heavy chain. Also zip
MRLC: Myosin regulatory light chain. Also sqh
MT: Microtubule
MTOC: Microtubule organizing center
MYPT: Myosin phosphatase target subunit, myosin-binding subunit. Also mbs
NC: Nurse cell
NCC: Nurse cell cluster
NIH: National institutes of health
NLS: Nuclear signal
O-FUT-1: O-fucosyltransferase 1
O: Oocyte. Also OC and OO
OC: Oocyte. Also O and OO
OO: Oocyte. Also OC and O
OR: OregonR
ORF: Open reading frame
P: Posterior
PAK: P21-activated kinase
PALS1: Protein associated with lin-7 1. Also sdt
PAR: Partition defective
PAR1: Partition defective 1
PAR3: Partition defective 3. Also baz
PAR6: Partition defective 6
PATJ: Pals1-associated tight junction protein
PB1: Protein binding domain 1
PBS: Phosphate-buffered saline
PBST: Phosphate buffered saline with 0.1% tween-20
PBST0.2%: 1x Pbs + 0.2% tween-20
PC: Polar cell
PCR: Polymerase chain reaction
PDK1: 3-phosphoinositide-dependent kinase 1
PEM: 1M pipes pH6.9 + 0.1M EGTA pH8 + 0.1M MgCl2
PFC: Posterior follicle cell
PGC: Pole germ cells
PHAL: Phalloidin
PI3K: Phosphoinositide 3-kinase
PIPES: Piperazine-n,n′-bis(2-ethanesulfonic acid)
PKC: Protein kinase c
PKN: Protein kinase n
PLD: Phospho lipase d
PP1: Protein phosphatase 1
PP1B: Catalytic subunit of protein phosphatase 1 (PP1). Also flw
PRK: Related pkc.
PRV: Pseudorabies virus
QGAP1: Iq motif containing GTPase activating protein 1
RAC: Ras-related c3 botulinum toxin substrate
RAC1: Ras-related c3 botulinum toxin substrate 1
RBD: Regulatory binding domain
RC: Ring cannals
RHO-A: Ras homolog gene family, member a. Also rho.
RHO: Ras homolog gene family, member. Also rho-a
RNA: Ribonucleic acid
RNAI: Rna interference
RNASE: Ribonuclease a
ROK: Rho-associated protein kinases. Also rock
ROCK: Rho-associated protein kinases. Also rok
RSV: Rous sarcoma virus
RT: Room temperature
SC: Stalk cell
SCRAPS: Anillin
SDS: Sodium dodecyl sulfate
SDT: Stardust. Also pals1
SHG: Shotgun. Also de-cad and e-cad
SQH: Spaghetti squash. Also myosin regulatory light chain
SRC: Proto-oncogene tyrosine-protein kinase, proto-oncogene c-src
STC: The stretched cells
SV40: Simian virus 40
SYF1: Synthetic lethal with cdc41. Also fand and xab2
TC: Terminal cell
TJ: Tight junction
TOCA1: Transducer of cdc42-dependent actin assembly 1
TS: Temperature sensitive
UBINLS-GFP: Ubiqutous nuclear signal – GFP (polyubiquitin promoter that drives ubiquitous nuclear green fluorescent protein)
V: Ventral
VV: Vaccinia virus
WASH: Was protein family homolog
WASP: Wiskott–Aldrich syndrome protein
WAVE: Verprolin-homologous protein
WGA: Wheat germ agglutinin
WT: Wild type
XAB2: Xpa binding protein 2. Also fand and syf1
ZIP: Zipper. Also mrhc
Abstract
For cells to form tissues and organs, they have to coordinate individual
cell shape with tissue morphogenesis. To achieve that, cellular adhesion and
actin-myosin cytoskeleton contractility need to be coordinated from the cell to
the tissue level. In my thesis I focused on the hypothesis that Protein Kinase
Cs (PKCs), has central RhoGTPase signaling effectors, are important
regulators of cell shape and tissue integrity during morphogenesis.
We devised a screen, using Drosophila as a model system, to isolate
novel candidate alleles that would be required for epithelial morphogenesis.
Among other mutants, we isolated novel alleles of PKC kinases: two alleles of
atypical Protein Kinase C (aPKC) and two of Protein Kinase N (PKN). In my
work, I addressed the mechanisms by which these kinases regulate cell shape
and tissue integrity during morphogenesis.
aPKC is a known regulator of epithelial polarity, a function that is
conserved among vertebrates. It had been suggested that aPKC could have
functions that would be independent from its binding partner, Partition
Defective 6 (Par6). This is different from what we observed from the
characterization of a Par6 binding deficient aPKC allele (apkc[pb1]). From our
work, all tested aPKC functions are Par6 dependent.
It had been previously suggested that, similarly to asymmetric cell
divisions, the aPKC-Par6 apical complex and its positive regulator Cdc42 are
also important for planar orientation of the mitotic spindle of dividing
mammalian cultured epithelial cells. Yet, in chicken neuroepithelial cells this
was described not to be the case. In order to investigate whether Drosophila
aPKC is required for spindle planar orientation, we took advantage of a
temperature- sensitive allele of aPKC (apkc[ts]) to modulate in vivo aPKC
activity. From our work we conclude that, similar to what has been reported in
mammalian tissue culture cells, Drosophila aPKC is required for spindle planar
cells. Our observations suggest that the spindle cortical cues are conserved
between Drosophila and mammalian cells, and we provide the first in vivo
evidence for a role of aPKC in spindle planar orientation. Also from the study
of the apkc[ts] allele, and in agreement with previous suggestions, we showed
that different tissues have different requirements for aPKC activity.
I also focused on the role of PKN in cell shape and tissue integrity.
Although the precise molecular function of PKN is still unknown, it has been
associated actin and adhesion regulation. In my thesis, I focused on the
hypothesis that PKN could be required for cell shape changes by the
regulation of cell adhesion and/or actin-myosin cytoskeleton. Different from
what had been suggested for mammalian PKN2, we observed that Drosophila
PKN is not required for cellular adhesion and b-catenin regulation at adherens
junctions. Instead, pkn mutants showed significant defects during Drosophila
oogenesis associated with actin-myosin activity. We observed that Pkn
behaves as a negative regulator of actin–myosin activity, and that this function
was conserved between epithelial tissues and the germline. pkn mutant
germline showed loss of tissue integrity and abnormally low nurse cell
cytoplasm transfer rates; while pkn mutant epithelial cells showed cell shape
defects. Both phenotypes are most likely the result of excessive contractility.
Our work shows that Drosophila Pkn is a negative regulator of actin–myosin
activity, whose function is most likely important for coordination of cellular
contractility during morphogenesis. We propose Pkn provides a negative
feedback loop to help avoid excessive contractility after local activation of
Sumário
Para que as células se organizem em tecidos e órgãos, tem que
coordenar a sua morfologia individual com os movimentos de morfogénese do
tecido. Para tal, adesão celular e a contractilidade do citoesqueleto de
actina-miosina precisam de ser coordenadas do nível celular ao nível do tecido. Na
minha tese foquei-me na hipótese de que PKCs, como efetores centrais de
sinalização de RhoGTPases, são reguladores importantes de morfologia
celular e integridade dos tecidos durante morfogénese.
Nós elaboramos um rastreio, usando Drosophila como organismo
modelo, para isolar novos alelos necessários para morfogénese epitelial.
Isolámos novos alelos de PKCs: dois alelos de aPKC e dois alelos de PKN.
No meu trabalho, eu foquei-me nos mecanismos pelos quais estas cinases
regulam morfologia celular e integridade do tecido durante morfogénese.
aPKC é um regulador conhecido de polaridade epitelial, e a sua função
é conservada em vertebrados, e requer a interação com Par6. Foi sugerido
que aPKC poderia funcionar de forma independente de Par6 em Drosophila, o
que é diferente do que nós observamos. A caracterização do alelo apkc[pb1],
(onde a associação a Par6 está potencialmente afectada) sugere que todas
as funções de aPKC por nós testadas dependem de Par6.
Estudos anteriores sugeriram que, à semelhança das divisões
assimétricas, o complexo apical aPKC-Par6 e o seu regulador positivo Cdc42
seriam também importantes na orientação planar do fuso mitótico de células
de epiteliais de mamíferos em cultura. Contudo, em células neuroepiteliais de
galinha foi descrito que o mesmo não se observa. De forma a investigar se
aPKC de Drosophila é necessária para orientação planar do fuso mitótico,
tomámos partido de um alelo de aPKC (apkc[ts]), para modular a actividade
de aPKC in vivo. Do nosso trabalho concluímos que, à semelhança do
observado em células de mamífero, em Drosophila aPKC é necessário para
simétrica de células epiteliais. As nossas observações sugerem que os sinais
corticais para orientação do fuso mitótico são conservados entre Drosophila e
mamíferos, são a primeira evidência in vivo do papel de aPKC na orientação
planar do fuso mitótico. A caracterização do alelo apkc[ts], e de acordo com
estudos prévios, permitiu ainda mostrar que tecidos diferentes têm
necessidades diferentes de atividade de aPKC.
No meu trabalho estudei também no papel de PKN na regulação de
morfologia celular e integridade epitelial. Os mecanismos moleculares através
dos quais PKN atua são desconhecidos, mas foram descritas funções na
regulação do citoesqueleto de actina e adesão celular. Na minha tese
foquei-me na hipótese de que PKN é necessário para a modular alterações de
morfologia celular, atuando ao nivel da adesão celular e/ou do citoesqueleto
de actina-miosina. Contrário ao que foi sugerido para PKN2 de mamíferos, o
nossos resultados indicam que em Drosophila, PKN não é necessário para a
regulação de adesão celular e de b-catenina nas junções aderentes.
Alternativamente, mutantes de pkn mostram defeitos significativos durante
oogénese, associados ao citoesqueleto de actina-miosina. Nós observámos
que Pkn atua como regulador negativo da atividade de actina-miosina, e que
esta função é conservada entre tecidos epiteliais e a linha germinal. Mutantes
de pkn apresentam um aumento de actina e miosina, levando a defeitos de
morfologia celular e a uma taxa anormalmente baixa de transferência dos
conteúdos citoplasmáticos. Nós propomos que os fenótipos resultam
provavelmente do excesso de contractilidade existente na ausência de Pkn. O
nosso trabalho mostra que Pkn, em Drosophila, regula negativamente a
atividade de actina-miosina, e que a sua função é provavelmente importante
para a coordenação de contração celular durante morfogénese. Em particular,
propomos que Pkn actua para evitar contração excessiva após ativação local
1.1. Tissue Morphogenesis
For cells to form tissues and organs they have to adhere and
communicate with their neighbours. Ultimately, cells adopt specific cell shapes
that will contribute to the overall tissue and organ shape. Finally, cells have to
receive, integrate and respond, to internal and external signals that will dictate
what to do and when to do it.
We use Drosophila as a model system to understand how cells adhere
and establish contacts. Moreover, how adhesion is regulated to accommodate
cell shape changes and how this is coordinated tissue-wide. The advanced
genetic and live cell biology tools make Drosophila a powerful animal model to
study morphogenesis. Further, many processes that occur in Drosophila
closely mimic what happens in mammals, including cell adhesion, migration,
division and shape-changes.
Cells adhere to their neighbours through adherens junctions, which are
multi-protein complexes. Adherens junctions connect the cell membrane to the
cytoskeleton, contributing to the regulation of overall cell shape. During
morphogenesis cells selectively adhere and detach from particular
neighbours, change their shape and contribute to tissue architecture, and can
also migrate to new locations. Better understanding of morphogenetic
processes requires the knowledge of how cell adhesion is integrated with
cytoskeleton regulation and signal transduction, both cell and tissue-wide, and
how this is disrupted in diseases.
In my PhD work I focused on the role of PKCs in tissue morphogenesis,
as RhoGTPase effectors. More specifically, I focused on the role of aPKC, a
known apical-basal polarity regulator, in mitotic symmetric spindle. This work
is described in Chapter 2 and 3 of this thesis and was partially published in
Development 139, 503-513 (Guilgur et al., 2012). I also focused on the role of
PKN as a regulator of actin-myosin cytoskeleton activity. This work is
Developmental Biology 394 (2014) 277–291 (Ferreira et al., 2014).
1.2. The Cytoskeleton Is A Dynamic Network Of
Filaments
The ability of cells to change their shape relies mostly on cortical forces
produced at cell surfaces and transmitted tissue-wide through cell interfaces
(He et al., 2014). Force generation greatly depends on the contractile
actin-myosin cytoskeleton, which is indispensable for most morphogenetic
processes (Lavayern and Lecuit, 2012; Vicente-Manzanares et al., 2009).
Although much is known on the components of the cytoskeleton and
how the basic architectures of the filamentous networks are achieved; little is
known on how its dynamics is regulated. How are the different cytoskeleton
architectures interconverted? How are cellular forces transmitted at the tissue
level in order to achieve long-range coordination? How tension balanced with
cell adhesion and polarity, to achieve morphogenesis without loss of tissue
integrity?
1.2.1. The Cytoskeleton And Its Components
The cytoskeleton is a network of structural and regulatory components
within the cellular cytoplasm. In eukaryotes, the cytoskeleton can be classified
based on its three major components (Figure 1.1): Microtubules (composed by
tubulin filaments), Intermediate filaments (composed by different structural
components, in a cell-specific manner) and Microfilaments (composed by actin
filaments) (Saraf et al., 2011).
1.2.1.1. The Microtubule Cytoskeleton
Microtubules are polymers of alpha and beta tubulin monomers (α
-tubulin, β-tubulin). Polarised filaments form in a GTP
Organising Centers (MTOCs), such as the centrosome. Microtubules regulate
cell shape and provide a circulation route for intracellular transport of cell
organelles and vesicles. As component of motile structures it regulates cell
motility, and as part of the mitotic spindle it regulates cell division (Hemphill et
al., 1992; Vieira et al., 2008).
Figure 1.1: Cellular components of the eukaryotic cytoskeleton.
(Left, Green) Microtubules are long hollow cylinders made of alpha and beta tubulin dimers. Microtubules are long, and typically have one end attached to a single
microtubule-organising center (MTOC). (Bottom) Micrograph of a microtubule
filament. (Middle, blue) Intermediate filaments are made of intermediate filament
proteins. They can be either cytoplasmic, and give mechanical strength to cells; or nuclear, where they form a meshwork called the nuclear lamina, beneath the inner
nuclear membrane. (Bottom) Micrograph of an intermediate filament. (Right, pink)
Microfilaments are actin-based filaments. They are flexible helices, organised into a variety of bundles and tridimensional networks. Bottom: Micrograph of microfilament. Adapted from Alberts et al (2009).
1.2.1.2. The Intermediate Filaments Cytoskeleton
Intermediate filaments are heterogeneous constituents of the
cytoskeleton, and are organised as unpolarised tetramers of two anti-parallel
helices. There are different types of intermediate filaments, depending on their
composition and/or cellular localisation (Chang and Goldman, 2004).
Intermediate filaments help cells sustain tension, regulate cell shape changes,
organelles and serving as structural components of the nuclear lamina.
Drosophila melanogaster lacks cytoplasmic intermediate filaments, although
some of the known components are expressed. The nuclear lamina exists in
all animals and all tissues (Wagner et al., 2007; Parry et al., 2007; Hadfield et
al., 2003).
1.2.1.3. The Actin Cytoskeleton
The actin cytoskeleton is required for many processes during
development, such as cell division, cell morphology and migration, contraction
and organ and boundary formation (eg: Verheyen and Cooley, 1994; Hudson
and Cooley, 2002; Alberts et al., 2009; Grosshans et al., 2005; Gates et al.,
2009; Monier et al., 2010). Overall, the actin cytoskeleton is required for most
morphogenesis events within a living organism.
In this work we focus on the actin component of the cellular
cytoskeleton. From hereafter, whenever we refer to cytoskeleton, we are
referring to the actin cytoskeleton.
1.2.1.3.A. The Structural Dynamics Of The Actin Cytoskeleton
Actin filaments (F-actin) are dynamic polymers that exist in equilibrium
with actin monomers (G-actin). Actin filaments are formed by the addition of
G-actin monomers to the fast growing end of pre-existing actin filaments,
known as the barbed end (reviewed in Pollard et al., 2000). Actin filaments
can then be cross-linked into filamentous networks (Hudson and Cooley,
2002; Schmidt and Hall, 1998) (Figure 1.2) that can be further bundled and
arranged to form multiple tridimensional structures (Figure 1.3).
1.2.1.3.B. The Actin Cytoskeleton And Its Regulators
The initial formation (nucleation) of actin filaments in vivo is an inefficient
process (Sept and McCammon, 2001). In vivo the actin concentration is not
subsequent addition of actin monomers. Spontaneous nucleation in vivo is
further suppressed by profilin (a G-actin binding protein that catalyses
nucleotide exchange and activation of actin monomers promoting actin
elongation) that in the absence of free barbed ends sequesters G-actin,
inhibiting its spontaneous nucleation (Pantaloni and Carlier, 1993; Pollard,
2007). As a result, cellular factors with the ability to overcome these kinetic
barriers and nucleate filaments (that then grow by barbed-end addition)
perform critical roles in specifying the timing and location of actin network
formation in cells.
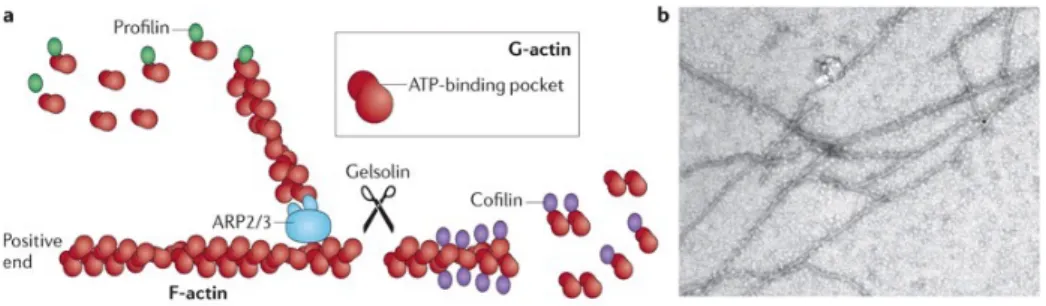
Figure 1.2: Actin filaments are formed by two parallel strands of head–tail polymers of actin monomers.
(A) Actin polymerisation is initiated by the ARP2/3 complex and stimulated by
cofactors such as Profilin. The direction of actin filaments is determined by the orientation of the monomers, with the positive end being defined as the opposite-end to the ATP-binding pocket. Actin depolymerisation can occur at either end of the filament. Cofilin interacts with actin dimers to promote disassembly, which can be initiated by the activity of Gelsolin. (B) Actin filaments that were polymerised in vitro and visualised under an electron microscope. Taken from: Wen et al. (2009) in Taylor et al. (2011).
Dynamic rearrangements of the actin cytoskeleton require rapid
inter-conversions between the G-actin and F-actin pools. Actin remodelling must be
triggered at the correct time and place, and this is tightly regulated by a large
number of actin-binding proteins.
Actin polymerisation is initiated by actin nucleators, which set the “seed”
for the formation of a new actin filament. New filaments can also be added to
branching. Branching filaments are typically present in lamellipodia-like
structures (Figure 1.4).
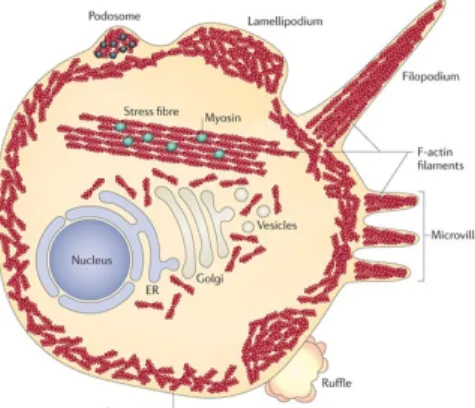
Figure 1.3: Within the cell actin filaments can be arranged to form multiple structures.
Stress fibres are large assemblies of actin filaments. The presence of myosin in stress
fibres enables contractility. Underneath the plasma membrane is the loosely organised network of actin filaments - termed cortical actin. Actin filaments can also organize to produce a range of cellular extensions (indicated in figure), including lamellipodia and filopodia, that contain several actin-binding proteins (black dots). ER: endoplasmatic reticulum. From Taylor et al. (2011).
Three main nucleators of actin assembly have been identified: Spire, the
Arp2/3 complex and formins (for review see Chhabra and Higgs, 2007).
Filament branching is achieved by the Arp2/3 complex (Machesky et al.,
1994, Mullins et al., 1998a; Mullins et al., 1998b). Arp2/3 generates filament
branching by binding to the side of an existing actin filament and nucleating
the formation of a new filament (Mullins et al., 1998a; Mullins et al., 1998b).
Arp2/3 is normally repressed, and requires a transient association with an
activator, like the members of the Wiskott family - Wasp and Wave. Both
usually require small G proteins of the Rho family for activation (Miki and
Takenawa, 2003).
Formins can also nucleate actin filaments, but do from the sides of
pre-existing filaments. They can stimulate linear actin polymerisation by binding
profiling-bound G-actin, adding the monomers to the growing end (for review see
Chabra and Higgs, 2007).
Spire can nucleate new filaments, but remains associated with the
slow-growing pointed end of the new filament. Spire contains four WASP homology
2 (WH2) domains, each of which binds an actin monomer (Quinlan et al.,
2005).
To prevent continuous F-actin elongation, the barbed end remains free
only transiently and is rapidly capped by a capping protein. Thus, the
cytoplasm contains mainly short and capped actin filaments. Capping protein
(CP) is an heterodimer composed by an alpha (CPa) and a beta (CPb) chain
that bind the barbed end of filaments and prevent further addition of
monomers, blocking their growth (Amandio et al., 2014). The ratio between
capping protein and proteins like Ena and Dia, that are thought to be
anti-cappers, determines the rate and duration of actin filament elongation.
In epithelial cells the actin cytoskeleton typically consists of a thin actin
meshwork bound to the inner cytosolic face of the plasma membrane (cortical
actin) and of a medial cytosolic actin meshwork (medial actin) (Kasza and
Zallen, 2011). The first is directly anchored to the adhesive contacts between
cells, while the second is connected to the cortical belt.
Figure 1.4: Actin binding proteins influence actin structure.
such as Arp2/3 promote de novo actin filament nucleation and branching. ADF (Actin-depolymerising factor homology domain)/Cofilin factors severe the filaments and promote dissociation of the actin monomers. Cyclase-associated proteins (CAP) sequester actin monomers preventing their incorporation into filaments. Capping proteins (CP) restrict access to the growing end, forming a cap that prevents further addition of actin monomers. From Disanza et al. (2005).
The structure of the cytoskeleton is critical for cell-cell adhesion, and
linear actin filaments are required to stabilise adhesion complexes (Pilot et al.,
2006; Cavey et al., 2008), and both help organising cell polarity, shape and
migration. Although it is known that disruption of the actin
cytoskeleton/adhesion leads to the disruption of the other (e.g., Cox et al.,
1996; Quinlan and Hyatt, 1999) how they are both dynamically regulated
remains more elusive.
In order to try and understand how regulation of the cytoskeleton and
adhesion are coordinated during morphogenesis, we proposed to find new
alleles that are required for tissue morphogenesis.
1.2.2. The Cytoskeleton And The Regulation Of
Contractility
The structure of cytoskeleton directly determines cell shape.
Additionally, contraction - achieved by the action of myosin (a motor protein
that moves along actin filaments) – further contributes to regulate cell shape.
Non-muscle myosin II (myosin, from hereafter) has been implicated as a
major player in apical cell constriction in Drosophila melanogaster (Drosophila
from hereafter; Young et al., 1991), Xenopus laevis (Xenopus from hereafter;
Lee and Harland, 2007) and Caenorhabditis elegans (C.elegans from
hereafter; Lee and Goldstein, 2003).
Myosin is a heterodimeric protein complex that crosslinks actin
filaments, making them slide across each other and thus allowing for the
Verkhovsky and Borisy, 1993; Verkhovsky et al., 1995). The association of
myosin to the actin cytoskeleton forms what is known as the actin-myosin
cytoskeleton.
Similarly to the cortical and medial actin meshwork, within a cell we can
distinguish the apical actin-myosin and the medial actin-myosin networks
(Kasza and Zallen, 2011; Grammont, 2007; Martin et al., 2009). The apical
actin-myosin belt associates to adherens junctions, and it is required to
regulate epithelial integrity and to transmit internally generated forces to the
surrounding cells or to the extracellular matrix (ECM) (Figure 1.5) (Morone et
al., 2006; Bray and White, 1988; Diz-Munoz et al., 2010; Sedzinski et al.,
2011). The medial network does not affect tissue integrity, but generates
lower-intensity forces that coordinate the pulsatile contractility pattern of cell
membranes (Fernandez-Gonzales and Zallen, 2011; Grammont, 2007; Martin
et al., 2009; Roh-Johnson et al., 2012; Martin et al., 2010; Vasquez et al., 2014). Myosin-generated tension in F-actin networks is also thought to drive
the clustering of actin-associated adherens junction complexes at nascent
junction engagement sites, to promote AJs formation and maturation (Shewan
et al., 2005, Yamada and Nelson, 2007).
Figure 1.5: Actin-myosin network organisation and cell adhesion, and force generation dynamics.
(A) Upon cell-cell contact, cells change their shape in response to mechanical forces
associated with actin-myosin contractility (green arrow) and adhesion (blue arrow). (B)
In epithelial tissues, adhesive contacts and the actin-myosin network are organised in belt-like structures at the apical domain of the cell. The arrangement of epithelial cells
A
B
C
D
E
at their apex is determined by actin-myosin contractility and cell-cell adhesion. (C)
Once coupled to adhesive contacts; pulsatile and centripetal flow of the apical actin-myosin network promotes apical cell constriction. In Drosophila mesodermal cells, the accumulation of apical actin-myosin is thought to stabilise cell shape changes between each pulse, leading to incremental reductions of the cell apical area. (D)
Pulsatile anisotropic flow induces junction shortening during cell intercalation. Resultant enrichment of actin-myosin at the junction stabilises junction length
reduction. (E) Basal myosin flow on a static-oriented actin network produces
anisotropic deformation of the base of the Drosophila follicular cells. (F) Continuous actin-myosin flow in the zebrafish yolk cell produces the mechanical force necessary for enveloping layer (EVL) spreading over the yolk cell during early zebrafish development. Adapted from Heisenberg and Bellaiche (2013).
Myosin is a hexamer of three different subunits: two heavy chain
subunits (MRHC, zipper in Drosophila), two regulatory light chain subunits
(MRLC, Spaghetti Squash in Drosophila) and two essential myosin light chain
subunits (EMLC) (Sellers, 2000) (Figure 1.6). The heavy chains have a
well-conserved N-terminal head domain that is responsible for ATP hydrolysis and
binding to F-actin.
Figure 1.6: Non-muscle myosin.
(a) Schematic diagram of a myosin II monomer, depicting the light and heavy chains.
The different parts of the heavy chain, including the motor, neck, coiled-coil and non-helical domains, are indicated. (b) Myosin II self-assembles into bipolar filaments through interactions of the C-terminus; the N-terminus binds to actin filaments. Activation of the myosin II motor domain leads to the pulling of actin filaments (in the direction of the arrows) to induce cortical tension. From Clark et al. (2007).
When myosin is activated, by phosphorylation in the MRLC subunit (at
serine 19 (S19) and/or threonine 18 (T18) in vertebrates; serine 21 (S21)
and/or threonine 20 (T20) in Drosophila) it binds and cross-links actin
tension (Figure 1.6) (Ikebe, 1988; Gardel, 2004; Jung, 2008, Verkhovsky and
Borisy, 1993; Verkhovsky et al., 1995; Amano et al., 1996; Kimura et al., 1996;
Winter et al., 2001). The phosphorylation status of myosin is a key
determinant of the cytoskeleton activity.
Rho and Myosin light chain kinases are known to phosphorylate and
activate MRLC. On the other hand, Myosin light chain phosphatase
dephosphorylates MRLC and inactivates it, and it can itself be inhibited by
Rho kinase phosphorylation (Amano et al., 1996; Kimura et al., 1996; Kawano
et al., 1999; Winter et al., 2001), thus creating a dynamic control of myosin
activity. MRLC can in further be inhibited by phosphorylation in serine 1/2
(S1/2) and threonine 9 (T9). This phosphorylation directly inhibits the
assembly of filaments and also decreases the affinity of MLCK for MRLC
activation (Beach et al., 2011). Actin-myosin contractility during cell shape
changes and morphogenesis needs to be coordinated with cellular and
tissue-wide regulation of adhesion and polarity, to ensure maintenance of integrity.
Actin-myosin contraction has negative (Sahai and Marshall, 2002) or positive
effects on cellular adhesion (Smutny et al., 2010); and adhesion also feeds
back to actin-myosin (Tsang et al., 2012; Kishikawa et al., 2008).
Understanding at which level affecting one structure impacts in the
other, and which are the molecular players that interconnect both, is crucial for
the understanding of tissue morphogenesis. We therefore decided to
characterise both adhesion and cytoskeleton integrity in the morphogenesis
mutants we isolated, and at which extent one or both structures are affected.
1.3. RhoGTPases And The Regulation Of The
Actin-Myosin Cytoskeleton
GTPases are the most common regulators of cytoskeletal reorganisation
and cell adhesion (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall,
Rho small GTPases are molecular switches that in general bind GTP
(Guanosine-5'-triphosphate) or GDP (Guanosine-5'-diphosphate) and have
GTP-hydrolyzing activities. They rapidly cycle between a GTP-bound active
state and a GDP-bound inactive state. In the active state, the active GTPase
interacts with diverse effector proteins, which trigger cytoskeletal and
adhesion rearrangements (Hall and Nobes, 2000; Garret et al., 1989; Johnson
and Pringle, 1990; Paterson et al., 1990; Rosa et al., 2015; Abreu-Blanco et
al., 2014; Mason et al., 2013).
The Rho family of GTPases consists of Rho, Rac, and Cdc42 proteins.
Classic studies in mammalian fibroblasts have implicated each type of Rho
GTPase in a different aspect of actin structure regulation. While Rho
stimulates the assembly of actin filaments into actin stress fibres
(Chrzanowska-Wodnicka and Burridge 1996); Rac stimulates lamellipodia
formation (Ridley et al., 1992, Nobes and Hall 1999) while Cdc42 induces
filopodia formation (Nobes and Hall, 1995, Nobes and Hall 1999; Li and Higgs,
2003; Fukata et al., 2001; Ridley and Hall, 1992) (Figure 1.7).
It is thought that the specificity of each RhoGTPase is conferred by the
interaction with specific members of the vast family of Rho regulatory proteins,
including: RhoGEFs (activators), RhoGAPs and RhoGDIs (inhibitors) (Simoes
et al., 2006; reviewed in Moon and Zheng, 2003). Perturbations of the activity
of these GTPases in yeast, flies and mice result in the disruption of many
biological processes (Figure 1.8), including yeast budding (Michelitch and
Chant, 1996), axon and dendrite outgrowth (Luo et al., 1994, Julian and Luo,
2004; Lundquist et al., 2001), myoblast fusion (Luo et al., 1994), cell migration
(Paladi and Tepass, 2004), cell and tissue shape, polarity and integrity
(Harden et al., 1999; Magie et al., 1999; Harden et al., 1995; Eaton et al.,
1995; Murphy and Montell, 1996; Strutt et al., 1997; Luo et al. 1997).
We proposed that by selecting mutants with defects in morphogenesis,
we would identify novel alleles of RhoGTPase regulators and/or effectors,
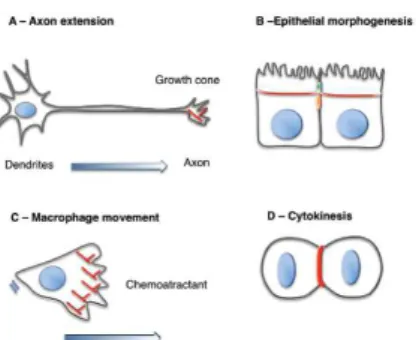
Figure 1.8: Cellular processes regulated by Rho GTPases and the actin cytoskeleton.
Rho GTPases are essential for the control of most cellular processes that require the assembly and reorganisation of the actin cytoskeleton. Examples of these processes
are: (A) Axon extension, (B) epithelial morphogenesis, (C) macrophage chemotaxis
and (D) cytokinesis. Actin polymerisation and contraction is required for the formation
of polarised epithelial cells (In (B) the actin circumferential belt that forms in polarised epithelial cells is shown). Macrophage movement in response to chemotactic factors
requires the formation of filopodia and lamellipodia at the front of the cell. Finally, (D)
cytokinesis is driven by the contraction of an actin myosin ring. Actin is in red, the nucleus is in blue, tight junctions are in green; adherens junctions are in orange.
Figure 1.9: Rho GTPases and epithelial morphogenesis.
Epithelial morphogenesis is the process through which a functional epithelium forms. Rho GTPases regulate several events during epithelial morphogenesis, including: the
formation of lamellipodia and filopodia that mediate the first cell-cell contacts (A); the
stabilisation of the cell contacts through the polymerisation of actin filaments (B), the
maturation of the junctions along the apical-basal axis, which is believed to require
actin myosin contractility and polarity (C); and the maintenance of the integrity of the
by Rho GTPases (D). Some examples of the players in each process are referenced. Actin is in red; the nucleus is in blue; tight junctions are in green; adherens junctions are in orange; apical junctions (tight junctions + adherens junctions) are in yellow.
1.3.1. Rho-GTP Effector Targets
In epithelial morphogenesis, Rho GTPases act over the actin-myosin
cytoskeleton to regulate different events that are required for the formation of
a functional epithelium (Figure 1.9) such as the establishment of the first
cell-cell contacts, maturation of primary cell-cell contacts into junctions and their
maintenance, and organisation of cellular polarity (Braga et al., 1997; Magie et
al., 2002; Bloor and Kiehart, 2002, Fox et al., 2005).
Rho-GTP signals to AJs and the cytoskeleton through its effectors, such
as Rho Kinase (ROK). ROK was initially implicated in Rho-mediated stress
fibre formation and myosin activation, and has been further associated to the
stabilisation of adhesion and induction of actin-myosin constriction (Shewan et
al., 2005; Winter et al., 2001; Kaliman et al., 2008).
ROK is the principal Rho-GTP downstream effector for myosin
regulation, but other kinases also contribute to final myosin activation levels
(for example, MLCK and ZIP Kinase - leucine zipper interacting kinase).
Myosin phosphatases de-phosphorylate and inactivate myosin (in most cases,
Protein phosphatase 1, PP1) (Kirchner et al., 2007; Mizuno et al., 2002; Tan et
al., 2003; Kirchner et al., 2007b; Mitonaka et al., 2007; Vereshchagina et al.,
2004) (Figure 1.10), counteracting the signalling of the activating kinases.
1.3.1.1. ROK Regulation Of Actin-Myosin Activity
ROK is a conserved effector of Rho-GTP signalling, and a positive
regulator of actin-myosin contractility. Rho-GTP binds to the Rho-binding
domain (RBD) of ROK to release it from auto-inhibition (Amano et al., 1999)
and promote ROK-dependent signals to the cytoskeleton. As previously
further by inhibiting myosin phosphatase (Kimura et al., 1996; Leung, 1996;
Jung, 2008) (Figure 1.10). ROK activity is required for many processes, since
the organisation of planar cell polarity in epithelia, to stress fibre formation, cell
shape regulation, wound healing, cytokinesis, cytoplasmic transfer and
migration (Simoes et al., 2010; Verdier et al., 2006; Winter et al., 2001; Dean
and Spudich, 2006; He et al., 2010; Hickson et al., 2006; Leung, 1996;
Vasquez et al., 2014; Abreu-Blanco et al., 2014).
Figure 1.10: Involvement of GTPases in the assembly and contractility of actin-myosin fibres.
Rho, Rac and Cdc42 interact with a variety of associated kinases (arrows). Myosin Light Chain Kinase (MLCK) is a calcium/calmodulin-responsive enzyme that maintains the myosin heavy chain (MHC)–myosin light chain (MLC) complex in an active state, but is negatively regulated by p21-Activated Kinase (PAK), which in turn is activated by Ras-related C3 botulinum toxin substrate 1 (RAC). ROK conversely blocks Protein Phosphatase (PP1), by phosphorylating the Myosin binding subunit (MBS), also referred to as the myosin phosphatase target subunit (MYPT). Phosphorylation of a central region of MBS results in direct inhibition of PP1 and a concomitant increase in phosphorylated MLC. ROK can also act via CPI-17 (protein kinase C-potentiated inhibitor of 17 kDa), an inhibitor of MBS/PP1, whose phosphorylation at Thr-38 potentiates its inhibitory activity. From Zhao and Manser (2005).
The balance of myosin activity is achieved by the concerted action of its
activators and inhibitors, and Protein phosphatase 1 (PP1) is one of the major
subunits: a regulatory subunit (MYPT, Myosin Binding Subunit (MBS) in
Drosophila), a catalytic subunit (PP1c, FLW in Drosophila) and a small 20KDa
non-catalytic subunit of unknown function. For an efficient myosin
de-phosphorylation, PP1 requires both the regulatory and the catalytic subunits
(Kirchner et al., 2007; Mizuno et al., 2002; Tan et al., 2003; Kirchner et al.,
2007b; Mitonaka et al., 2007; Vereshchagina et al., 2004). The tight control of
myosin activity is further achieved by the interplay of activating and inhibitory
pathways. For example, ROK can phosphorylate the MBS subunit of PP1,
reducing its ability to inhibit myosin activity. Alternatively, ROK can also
potentiate the inhibitory activity of the agonist CPI-17 (protein kinase
C-potentiated inhibitor of 17 kDa) that competes for the catalytic pocket of PP1
and prevents it from binding myosin (Zhao and Manser, 2005). By activating
myosin and repressing its inhibitors, ROK ensures that contractility is
generated in the proper time and place.
In our work, we characterised a novel kinase from the PKC family of
kinases (PKN, Protein Kinase N; see below for more details) and its role on
the regulation of actin-myosin contractility. We proposed PKN might be the
counterpart for ROK, since it inhibits both F-actin organisation and myosin
activation. We proposed that both kinases work in parallel to regulate
actin-myosin activity, ROK stimulating and PKN inhibiting it (Chapter 4).
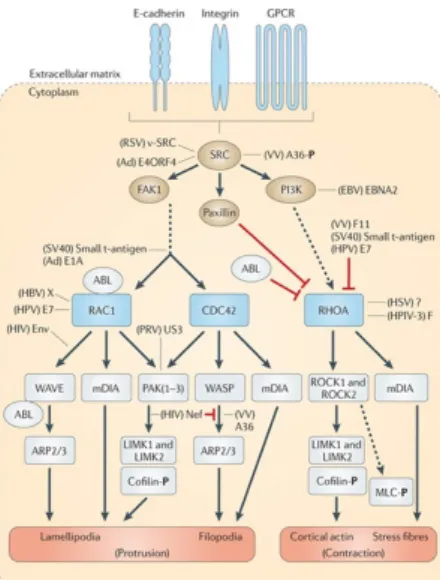
1.3.1.2. The PKC Family Of Kinases
Many of the Rho GTPase effector targets are kinases, predominantly
from the family of Protein Kinases C (PKCs) (Figure 1.11). The GTPases
signal through them to relay signals to the actin cytoskeleton, to modulate
adhesion and regulate polarity, during morphogenesis,
The function of protein kinases is defined according to their catalytic
domain (kinase domain), which mediates the phosphorylation of its