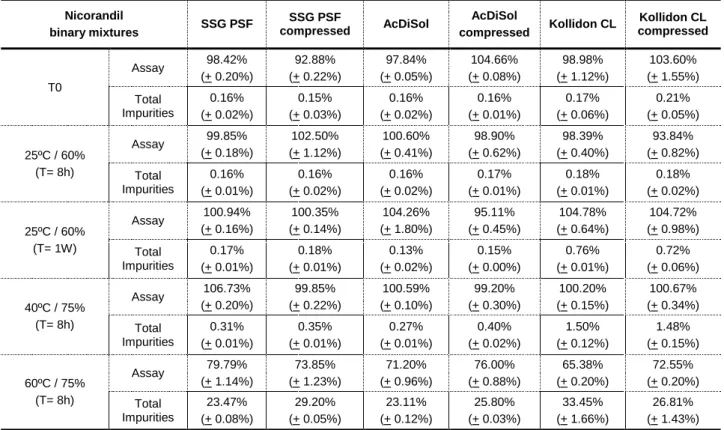
Development of Oral Tablets Containing Nicorandil
Texto
Imagem




Documentos relacionados
In recent years, an appropriate combination of excipients and techniques are applied to ODTs, which makes possible to obtain orally disintegrating sustained release tablets or
These prescription numbers were used to obtain copies of associated medical forms, and the forms were used to obtain the following information: each patient’s name
Although the majority of respondents agreed that generic drug are similar in quality to brand name drug (65%) with equal numbers of side effects (67%) and are equally safe (54%),
The optimized loating tablets excluding the drug and containing barium sulfate (50 mg per tablet) were prepared and studied for in vivo X-ray imaging study to establish the
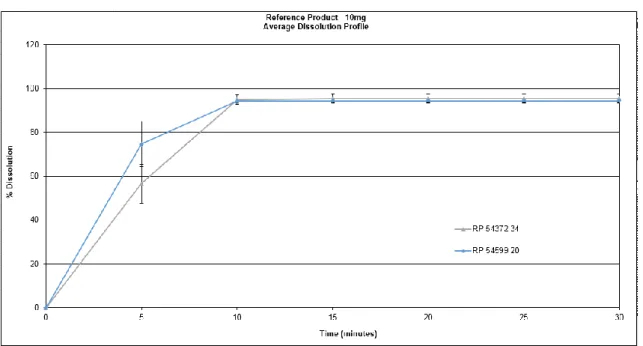
In this study, the dissolution proile of 20-mg prednisone tablets bioequivalent to the reference product and three test formulations were evaluated using stability testing.. During
Based on the results, the IDR could be used as a tool for classifying drugs in early drug development, which seems to be adequate since this method does not require a large amount
Our assumption is supported by the following evidence: first of all Optimus has been able to maintain its market share in recent years even facing a challenging environment