am struck by a profound gratitude to all those people who have contributed in one way or another to this work. A few words at the beginning of a thesis cannot hope to do justice to the wonderful people who have surrounded me during this period, but I hope to at least bring attention to some of them.
Immense thanks are due to:
All of my family for being so supportive when I came up with this idea of moving so far away; for always being there when I needed an ear or just a reminder of home; and for making the effort to see what my life here is like;
My friends and flatmates in Lisbon – without you, none of this would have been bearable. I can’t thank you all by name here, but it would be remiss of
me not to give mention to Vivek, Diogo, Bruno, Mert, Luis, Catarina, Jens, Bobby, Kirsten, Jimmy, Julia, Rita and Danbee;
My friends back in the UK, particularly Eddy, Justin and Amy; The FLiACT fellows and faculty, who provided amazing opportunities in the
fly world, gave me a peer group, and created friends for life around the world;
All members, past and present, of the Ribeiro lab, for creating a welcoming and stimulating environment that nourishes great science. Your support has been invaluable personally, and scientifically you have fuelled all of the insights in this thesis through discussions, and have always been ready to lend a hand when I needed it. In particular: Kathrin, with whom I worked closely on the data presented in Chapter 2 of this thesis; Pasha, who just made things work; Ana Paula, Célia and Margarida, who were always there
to carry me through when my fly work got too much; and Samantha, Verónica and Patrícia, for countless lunches and lanches;
Carlos, my biggest cheerleader in science, who gave me this opportunity, guided me along the way, and in so doing showed me how to mentor by always making the effort to think about how to support each person as an
individual;
My thesis committee, Eugenia and Zach, for providing valuable insights when my head was stuck in the details;
Portugal, for welcoming me with open arms and being so flipping beautiful; And Sofia, my light, for always helping me to be a better person.
The best sauce for food is hunger
- Socrates
N
EURONAL MECHANISMS OF NUTRIENT SELECTION
IN
D
ROSOPHILA
This dissertation is the result of my own work and any work done in collaboration is explicitly indicated in the text. It has not been previously submitted, in part or whole, to any university of institution for any degree, diploma, or other qualification.
The work presented in this thesis was funded largely by the Marie Curie FP7 programme FLiACT.
Os animais utilizam informação sobre o seu estado corporal para guiar comportamentos. A informação sobre esses estados internos é particularmente importante para que os animais controlem a quantidade de comida que devem ingerir, bem como o tipo de alimentos que devem consumir. Por sua vez, estas decisões alimentares são críticas de forma a optimizar a sua sobrevivência e reprodução. Para atingirem homeostase nutricional, muitos animais desenvolvem ‘apetites específicos’, procurando e consumindo certo tipo de alimentos em resposta a estados internos que impõem requisitos nutricionais particulares. No entanto, a forma como estados internos modulam a actividade neuronal para gerar apetites específicos é ainda uma questão em aberto. Nesta tese, apresento resultados que me permitiram identificar mecanismos, em circuitos neurais, que estão na base de apetites para dois recursos nutricionais cruciais em Drosophila. Mostro que os estados nutricionais e reprodutivos das moscas interagem para modular os apetites para levedura, a sua fonte mais rica de proteínas, e sal. Estes dois apetites são mediados por populações específicas de neurónios sensoriais, e a forma como estes neurónios respondem é regulada de forma oposta pelo estado interno proteico da mosca. Perante uma situação de deprivação de proteína ocorre simultaneamente um aumento de apetite para levedura e uma redução de apetite para sal. Em contraste, o estado reprodutivo da mosca não afecta a resposta dos neurónios sensoriais ao sal ou levedura. Isto sugere que o acasalamento modula o processamento do sabor a jusante nesde circuito neuronal de forma a desencadear apetites para nutrientes específicos. Por fim, identifico um circuito neuronal que modula nutrição, de uma forma independente das necessidades nutricionais da mosca, antecipando os requisitos nutricionais associados à produção de ovos. Em geral, estes resultados demonstram que a modulação de canais gustativos específicos pode estar na base do mecanismo que regula apetites específicos. Adicionalmente, estes resultados identificam elementos neuronais com valencia sensorial, mas também neurónios que reflectem estados internos, demonstrando que a modulação sinergética do comportamento por diferentes estados internos pode ser implementada a diferentes níveis durante o processamento sensorial.
ABSTRACT
Animals use information about the state of their body to guide behaviour. Such internal state information is particularly important to inform decisions about both how much to eat and which foods to consume, which are critical to optimising survival and reproduction. To achieve nutritional homeostasis, many animals develop so-called ‘specific appetites’, seeking out and consuming specific foods in response to internal states that impose specific nutritional demands. How such states modulate neuronal processing to generate specific appetites, however, is poorly understood. In this thesis, I will present data dissecting neural circuit mechanisms underlying appetites for two key nutritional resources in Drosophila. I show that nutritional and reproductive states interact to modulate flies’ appetites for salt and for yeast, flies’ major source of protein. These two appetites are mediated by specific populations of sensory neurons, and the gain of these sensory neurons is oppositely regulated by the fly’s internal protein state, mirroring the increased yeast appetite and decreased salt appetite induced by deprivation from protein. Reproductive state, in contrast, does not affect the gain of sensory neuron responses to salt or yeast, suggesting that mating modulates downstream taste processing to elicit nutrient-specific appetites. Finally, I identify a neuronal circuit that modulates nutrition in a need-independent manner to anticipate the nutritional demands of egg production. Sex Peptide transferred from the male during copulation acts through a specific ascending neural circuit to induce appetites for both salt and yeast, both of which are important for egg production. Overall, these data demonstrate modulation of specific gustatory channels as a potential mechanism underlying specific appetites, identify circuit elements carrying internal state and sensory signals, and show that synergistic modulation of behaviour by different internal states can be implemented by neuronal modulation at different levels of sensory processing.
Imagine a table laden with food arrayed in front of you. Chances are, if you haven’t eaten for a while, you’ll be drawn to the food and it will taste delicious. But faced with that same table just after having eaten, you might react very differently. How does the brain change its reaction to this same situation depending on the state of your body? This is the question that runs throughout my thesis.
It turns out that like humans, fruit flies will change their food preferences depending on the state of their body. If a fly hasn’t eaten any protein for a few days, they will eagerly devour a protein source that is presented, whereas if they have had plenty of protein already, they will eat much less – and for flies, their favourite protein source is yeast. What’s more, after a female fly mates with a male, she will start eating much more yeast, in order to provide the protein she needs to produce eggs. So the state of the body affects nutrient intake in different ways. What do we need to know to begin to understand how feeding is controlled according to the state of the body? Well, first we need to know how the animal detects that the right kind of food is available. Next, we need to understand how the brain detects the state of the body. Finally, we need to know how these signals are integrated – that is, how the brain’s responses to food stimuli are changed depending on the state of the body. In this thesis, I attempt to tackle these three questions, comparing how flies regulate their intake of two key nutrients, yeast and salt (yes, it turns out flies have a lust for salt just like humans, which is enhanced after females mate).
My results show that different taste receptor cells on the fly’s tongue detect different nutrient sources. In fact, the response of these taste receptors is changed depending on the fly’s internal nutritional state, so that foods actually taste different when the fly is hungry for protein. Mating also affects how the brain processes taste information, but in this case it doesn’t affect the taste receptor cells themselves, but somewhere deeper in the brain. So different bodily states change flies’ tastes in different ways. But how does a female know when she has mated? Well, it turns out that the male transfers a particular protein signal to the female during copulation, and the female uses this signal to induce changes in food preference. In this way, the female can anticipate the nutrients that she will need to produce eggs, and start
consuming them once she is mated. Together, these results help us to understand how the state of the body changes the brain in order to allow animals to eat a balanced diet.
C
ONTENTS
1 GENERAL INTRODUCTION ... 1 1.1NUTRITION AND HEALTH ... 1
1.2NUTRIENT-SPECIFIC APPETITES ... 4
1.2.1 Salt appetite 4
1.2.2 Protein hunger 8
1.3STATE-DEPENDENT MODULATION OF SENSORY PROCESSING ... 12
1.3.1 Behavioural state 12
1.3.2 Reproductive state 13
1.3.3 Hunger state 15
1.3.4 Nutrient-specific modulation 17
1.4NEURONAL REGULATION OF APPETITE IN DROSOPHILA ... 18
1.4.1 Drosophila chemosensation 19
1.4.2 Postingestive nutrient sensing 33
1.4.3 Central circuits controlling feeding and nutrition 36
1.5CONCLUSIONS:NEURONAL MECHANISMS OF NUTRIENT SELECTION ... 45
2 THE GUSTATORY BASIS OF PROTEIN HOMEOSTASIS IN
DROSOPHILA ... 47
2.1SUMMARY ... 47
2.2INTRODUCTION ... 48
2.2.1 Regulation of protein intake across species 48
2.2.2 Taste in Drosophila 50
2.2.3 Chemosensory detection of yeast 51
2.3RESULTS... 53
2.3.1 Specific appetites for protein and sugar in Drosophila 53
2.3.2 Identification of sensory neurons underlying yeast appetite 55
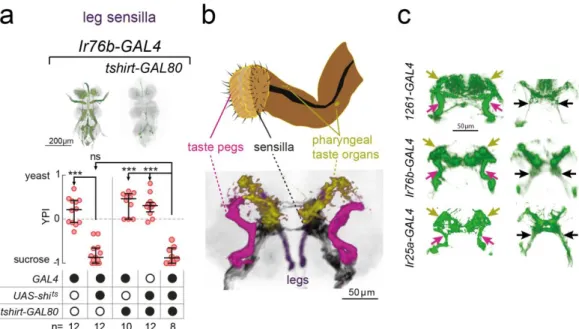
2.3.3 1261-, Ir76b- and Ir25a-GAL4 label common neurons required for yeast feeding 59 2.3.4 Taste receptor neurons within the identified lines are required for yeast feeding 62 2.3.5 Proboscis gustatory receptor neurons in distinct locations act in parallel to support
yeast feeding 65
2.3.6 Ir76b labellar GRNs respond to yeast taste 71
2.3.7 The response of yeast GRNs is modulated by internal amino acid state 74
2.3.8 Internal state competition 78
2.3.9 Second-order taste neurons sustain yeast feeding 80
2.4DISCUSSION ... 87
2.4.1 Resource-specific modulation of sensory neurons 87
2.4.2 Nutrient balancing and motivational state competition 88
2.4.4 Second-order taste neurons sustain yeast feeding bursts 92
2.4.5 Yeast taste neurons are regulated by internal amino acid state 95
2.5MATERIALS &METHODS ... 95
2.5.1 Fly husbandry 95
2.5.2 Drosophila stocks and genetics 96
2.5.3 Neuronal silencing experiments 97
2.5.4 Two-colour food choice assays 97
2.5.5 flyPAD assays 97
2.5.6 Immunohistochemistry, image acquisition and 3D rendering 98
2.5.7 Calcium imaging 99
2.5.8 Calcium imaging analysis 100
2.5.9 Statistics 101
2.6ACKNOWLEDGEMENTS ... 101
3 MATING DRIVES SALT APPETITE TO SUPPORT REPRODUCTION103 3.1SUMMARY ... 103
3.2INTRODUCTION ... 104
3.3RESULTS ... 106
3.3.1 Mating specifically increases the behavioural response to sodium taste 106
3.3.2 Mating increases intake of salt 111
3.3.3 Dietary salt supports egg production 113
3.3.4 A subset of sweet-sensing neurons is necessary for sodium appetite 114
3.3.5 Sweet-sensing GRNs respond to salt taste 116
3.3.6 Protein deprivation modulates neuronal and behavioural responses to salt 117
3.3.7 Effects of dietary sodium on Drosophila salt appetite 119
3.4DISCUSSION ... 121
3.4.1 Reproductive state modulation of taste responsiveness 121
3.4.2 Dietary sodium supports reproduction 122
3.4.3 Sweet-sensing neurons mediate attractive salt taste through Ir76b 124
3.4.4 Nutritional state regulation of salt taste responses 126
3.4.5 Mating drives a specific appetite for sodium 128
3.5MATERIALS &METHODS ... 128
3.5.1 Fly husbandry and stocks 128
3.5.2 Proboscis extension response assays 129
3.5.3 CAFE assay 130
3.5.4 flyPAD assays 130
3.5.5 Egg laying and viability assays 130
3.5.6 Calcium imaging experiments and analysis 131
3.5.7 Statistics 131
3.6ACKNOWLEDGEMENTS ... 131
4.1SUMMARY ... 133
4.2INTRODUCTION ... 134
4.3RESULTS... 137
4.3.1 Postmating salt appetite is need-independent 137
4.3.2 Male-derived Sex Peptide increases salt responses 138
4.3.3 Sex Peptide Receptor acts in specific neurons of the reproductive tract to drive
nutrient-specific appetites 139
4.3.4 Inhibition of SPSNs or ascending SAG neurons drives salt and yeast appetites 142
4.3.5 Octopamine dissociates postmating salt and yeast appetites 147
4.3.6 Mating modulates taste processing downstream of sensory neurons 154
4.4DISCUSSION ... 156
4.4.1 Inhibition of postmating circuitry drives appetites for salt and yeast 156
4.4.2 Circuitry mediating distinct postmating behaviours 157
4.4.3 Mating modulates taste processing downstream of GRNs 159
4.4.4 Postmating circuitry modulates taste processing 160
4.5MATERIALS &METHODS ... 160
4.5.1 Fly husbandry and stocks 160
4.5.2 Germline manipulation 161
4.5.3 Behavioural assays 161
4.5.4 Calcium imaging experiments and analysis 162
4.5.5 Statistics 162
4.6ACKNOWLEDGEMENTS ... 162
5 GENERAL DISCUSSION ... 164 5.1IMPLICATIONS FOR TASTE PROCESSING ... 165
5.1.1 Diverse roles of Ir76b-expressing neurons 165
5.1.2 Distinct internal states modulate different levels of sensory processing 166 5.2OUTSTANDING QUESTIONS ... 167
5.2.1 How are internal states translated into a variety of behavioural outputs? 167
5.2.2 How broad are the effects of internal states on the brain? 169
5.2.3 How many nutrient-specific appetites are there? 170
5.2.4 What behaviours are recruited to regulate nutrition? 172
5.2.5 How is taste processed in the Drosophila brain? 172
5.3PREDICTIVE REGULATION OF NUTRITION ... 173
5.3.1 Regulation of nutrient intake 173
5.3.2 Reaction and prediction 175
5.3.3 Food for the next generation 177
5.3.4 Prediction beyond reproduction 181
5.3.5 Prediction beyond nutrition 182
5.3.6 Conclusions 183
6 REFERENCES ... 185 7 TABLES ... 226
L
IST OF
T
ABLES
TABLE 1 – GAL4 LINES SCREENED FOR YEAST PREFERENCE PHENOTYPE BY THERMOGENETIC SILENCING ... 226
TABLE 2 – NUMBER OF CELL BODIES LABELLED ON EITHER SIDE OF THE INTERNAL SENSE ORGANS BY THE INDICATED LINES ... 230
L
IST OF
F
IGURES
FIGURE 1.1 – SCHEMATIC OF OLFACTORY STRUCTURES ON THE ANTENNA OF
DROSOPHILA ... 20
FIGURE 1.2–ANATOMY OF THE OLFACTORY SYSTEM IN THE BRAIN ... 21
FIGURE 1.3–THE PERIPHERAL GUSTATORY ORGANS OF DROSOPHILA ... 25
FIGURE 1.4–PROJECTION PATTERNS OF GRN AXONS IN THE CNS ... 30
FIGURE 1.5–CENTRAL NEURONS THAT REPRESENT HUNGER/SATIETY STATE IN THE DROSOPHILA BRAIN ... 38
FIGURE 1.6 – NEURONAL POPULATIONS THAT MODULATE SPECIFIC BEHAVIOURS ACCORDING TO HUNGER STATE ... 42
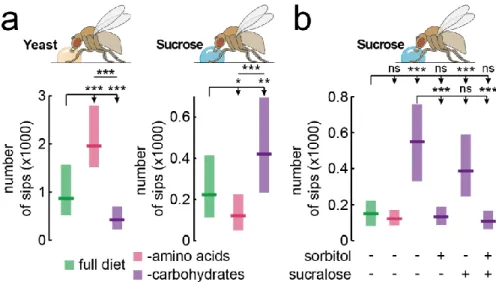
FIGURE 2.1 –NUTRIENT-SPECIFIC APPETITES FOR SUGAR AND YEAST ... 54
FIGURE 2.2–FEEDING ON YEAST METABOLITES ... 55
FIGURE 2.3 – SILENCING CARBONATION OR GLYCEROL GRNS DOES NOT AFFECT YEAST PREFERENCE ... 56
FIGURE 2.4–SCHEMATIC OF THE EXPERIMENTAL DESIGN FOR SCREENING GRNS .... 56
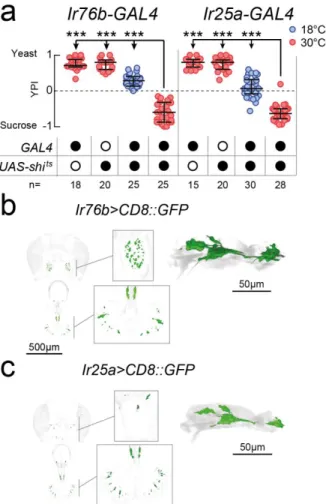
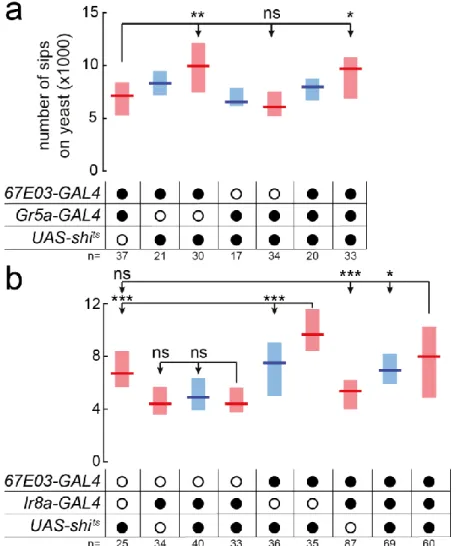
FIGURE 2.5–1261-GAL4 NEURONS REQUIRED FOR YEAST PREFERENCE ... 57
FIGURE 2.6–IR-EXPRESSING NEURONS REQUIRED FOR YEAST PREFERENCE ... 58
FIGURE 2.7–1261-GAL4 SILENCING AFFECTS YEAST, NOT SUCROSE, FEEDING ... 59
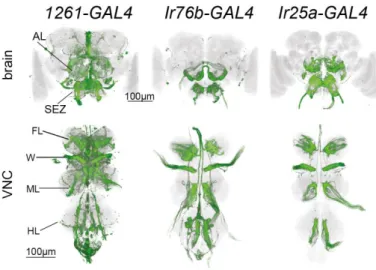
FIGURE 2.8–CENTRAL EXPRESSION PATTERNS OF IR76B-,IR25A- AND 1261-GAL4 . 60 FIGURE 2.9–OVERLAPPING NEURONS ARE REQUIRED FOR YEAST PREFERENCE ... 61
FIGURE 2.10–AMINO ACID TASTE DOES NOT EXPLAIN YEAST FEEDING ... 62
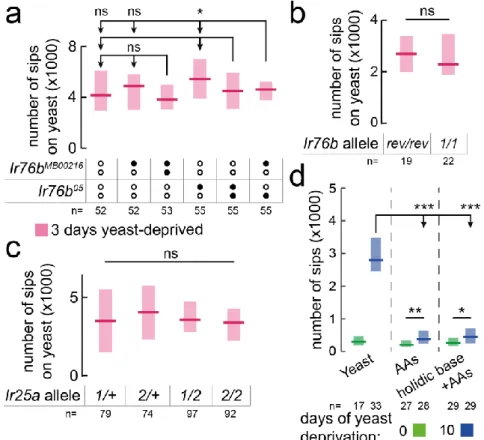
FIGURE 2.11 – TASTE NEURONS IN THE IDENTIFIED LINES CONTRIBUTE TO YEAST FEEDING ... 63
FIGURE 2.12–OLFACTORY MANIPULATIONS DO NOT AFFECT YEAST PREFERENCE ... 64
FIGURE 2.13–NEURONS IN THE HEAD MEDIATE THIS SILENCING PHENOTYPE ... 65
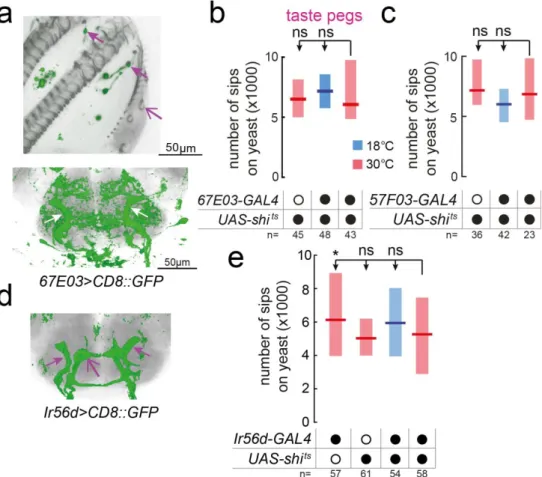
FIGURE 2.14–EXTERNAL SENSILLAR GRNS ARE DISPENSIBLE FOR YEAST FEEDING 67 FIGURE 2.15–TASTE PEG GRNS ARE DISPENSIBLE FOR YEAST FEEDING. ... 68
FIGURE 2.16–SILENCING TASTE PEG GRNS IN COMBINATION WITH OTHER SENSORY NEURON SUBSETS DOES NOT REDUCE YEAST FEEDING ... 69
FIGURE 2.17–PHARYNGEAL GRNS ARE DISPENSIBLE FOR YEAST FEEDING ... 70
FIGURE 2.18 – SCHEMATIC OF CALCIUM IMAGING PREPARATION AND DATA PROCESSING PIPELINE ... 72
FIGURE 2.19–LABELLAR IR76B GRNS RESPOND PREFERENTIALLY TO YEAST ... 73
FIGURE 2.20–YEAST RESPONSES ARE SHARPLY ALIGNED TO STIMULUS TIMING ... 73
FIGURE 2.22–YEAST DEPRIVATION INCREASES GRN RESPONSES TO YEAST ... 75
FIGURE 2.23–SWEET GRNS ARE NOT MODULATED BY YEAST DEPRIVATION ... 76
FIGURE 2.24–AA DEPRIVATION INCREASES YEAST GRN GAIN ... 77
FIGURE 2.25– SUCROSE DEPRIVATION REDUCES YEAST FEEDING IN AA-DEFICIENT FLIES ... 78
FIGURE 2.26–INTERNAL STATE COMPETITION IS NOT IMPLEMENTED IN YEAST TASTE NEURONS ... 80
FIGURE 2.27 – AA DEPRIVATION AFFECTS DIFFERENT COMPONENTS OF FEEDING MICROSTRUCTURE... 81
FIGURE 2.28 – IN1 NEURONS ARE DISPENSIBLE FOR REGULATION OF FEEDING ON 20MM SUCROSE FOLLOWING STARVATION ... 82
FIGURE 2.29 – IN1 NEURONS ARE REQUIRED TO INCREASE YEAST FEEDING BURST LENGTH FOLLOWING YEAST DEPRIVATION ... 83
FIGURE 2.30–IN1 NEURONS ARE DISPENSIBLE FOR NUTRIENT-SPECIFIC REGULATION OF 20MM SUCROSE FEEDING ... 84
FIGURE 2.31 – IN1 NEURONS ARE REQUIRED TO INCREASE YEAST FEEDING BURST LENGTH FOLLOWING AA DEPRIVATION ... 85
FIGURE 2.32 – CUMULATIVE PROBABILITY DISTRIBUTIONS OF NUMBER OF SIPS PER FEEDING BURST ON YEAST WITH IN1 SILENCING ... 86
FIGURE 2.33–MODEL OF POTENTIAL IN1 FUNCTION IN FEEDING BEHAVIOUR ... 93
FIGURE 3.1–REGULATION OF SUGAR TASTE RESPONSES BY MATING. ... 107
FIGURE 3.2–EFFECTS OF MATING ON BITTER TASTE SENSITIVITY ... 108
FIGURE 3.3 – MATING SPECIFICALLY INCREASES THE BEHAVIOURAL RESPONSE TO SODIUM TASTE IN FEMALES ... 109
FIGURE 3.4–MATING ALSO INCREASES SALT TASTE RESPONSES IN MALES ... 111
FIGURE 3.5 – MATING INCREASES SALT INTAKE BY MODULATING FEEDING BURST INITIATION ... 112
FIGURE 3.6–DIETARY SALT SUPPORTS EGG PRODUCTION ... 113
FIGURE 3.7–IR76B IS REQUIRED FOR SALT TASTE RESPONSES. ... 114
FIGURE 3.8–SWEET-SENSING NEURONS ARE REQUIRED FOR SALT PER... 115
FIGURE 3.9–LABELLAR SWEET GRNS RESPOND SPECIFICALLY TO SODIUM ... 116
FIGURE 3.10–PROTEIN DEPRIVATION DECREASES SALT PER ... 117
FIGURE 3.11–PROTEIN DEPRIVATION DECREASES GRN RESPONSES TO SALT... 118
FIGURE 3.12–EFFECTS OF DIETARY SODIUM ON SALT PER ... 119
FIGURE 4.1–POSTMATING SALT APPETITE IS NEED-INDEPENDENT ... 138
FIGURE 4.2–SEX PEPTIDE DRIVES SALT APPETITE FOLLOWING MATING ... 139
FIGURE 4.4–SPR IS ALSO REQUIRED FOR POSTMATING YEAST APPETITE ... 141 FIGURE 4.5–SPR ACTS IN SPSNS TO MODULATE YEAST APPETITE ... 142 FIGURE 4.6– SILENCING SPSNS MIMICS EFFECTS OF MATING ON YEAST AND SALT
APPETITE ... 143 FIGURE 4.7–SILENCING SAG NEURONS MIMICS EFFECTS OF MATING ON YEAST AND
SALT APPETITE ... 144 FIGURE 4.8–TARGETED SILENCING OF SAG NEURONS RECAPITULATES POSTMATING
EFFECTS ... 145 FIGURE 4.9 – EFFECTS OF MANIPULATIONS OF POSTMATING CIRCUITRY ON YEAST
FEEDING MICROSTRUCTURE ... 146 FIGURE 4.10 – NEURONAL SILENCING SCREEN TO IDENTIFY NEURONS MEDIATING
POSTMATING EFFECTS ON SALT RESPONSES ... 147 FIGURE 4.11 –OCTOPAMINERGIC NEURONS REGULATE SALT RESPONSE LEVEL, BUT
ARE DISPENSIBLE FOR POSTMATING REGULATION ... 148 FIGURE 4.12 –OCTOPAMINE IS REQUIRED FOR REGULATION OF YEAST FEEDING BY
MATING, BUT NOT BY NUTRITIONAL STATE ... 150 FIGURE 4.13–OCTOPAMINERGIC NEURONS ARE REQUIRED FOR POSTMATING YEAST
APPETITE ... 151 FIGURE 4.14–SIMULTANEOUS SILENCING OF OCTOPAMINERGIC AND SAG NEURONS
REDUCES YEAST FEEDING ... 152 FIGURE 4.15 – MATING AND PROTEIN STATE SYNERGISTICALLY REGULATE YEAST
FEEDING ... 154 FIGURE 4.16–MATING DOES NOT AFFECT GRN RESPONSES TO YEAST ... 155 FIGURE 4.17–MATING DOES NOT INCREASE GRN RESPONSES TO SALT ... 156 FIGURE 4.18–PROPOSED MODEL FOR THE REGULATION OF SALT AND YEAST TASTE
PROCESSING BY NUTRITIONAL AND REPRODUCTIVE STATES ... 157 FIGURE 5.1–EXAMPLES OF CONTROL-THEORETIC MODELS FOR ACHIEVING NUTRIENT
REGULATION ... 176 FIGURE 5.2–ADVANTAGES OF USING FEEDFORWARD CONTROL TO REGULATE INTAKE
OF NUTRIENTS FOR EGG PRODUCTION ... 178 FIGURE 5.3 – A COMMON PATHWAY STIMULATES BOTH EGG PRODUCTION AND
L
IST OF
A
BBREVIATIONS AND
A
CRONYMS
A - antenna: 59
AA - amino acid: 9, 11, 49, 52-54, 59, 61, 77-81, 86-95, 118, 120, 159, 160, 172
AKH - adipokinetic hormone: 41 AL - antennal lobe: 20-22, 31, 60 AMPK - adenosine
monophosphate-activated protein kinase: 41 AMS1 - anterior maxillary sensory 1:
66, 71, 73, 159
AstA - allatostatin A: 39, 40 ATP - adenosine triphosphate: 89 Bam - Bag-of-marbles: 138 CAFÉ - capillary feeder: 111, 112,
130, 131
CNS - central nervous system: 25, 31, 32, 39, 152, 158
CS - Canton-Special: 106, 107, 109, 110
cVA - 11-cis-vaccenyl acetate: 14 D2R - type 2 dopamine receptor: 16 DA - dopamine: 9, 10, 90, 166 DCSO - dorsal cibarial sense organ:
25
Dh44 - diuretic hormone 44: 34, 41 Dilp2 - Drosophila insulin-like
peptide 2: 38
Dop1R1 - D1-type dopamine
receptor 1: 10
Dop1R2 - D1-type dopamine
receptor 2: 10
DopEcR - dopamine/ecdysone
receptor: 16
Dsk - Drosulfakinin: 38
dsx - doublesex: 149, 158 EAA - essential amino acid: 36 FB - fan-shaped body: 88 FIT - female-specific independent of
transformer: 10, 49, 88, 166
flyPAD - fly proboscis activity detector: 53, 58, 59, 79, 97, 98, 101, 112, 130-132, 161-163 GABA - γ-aminobutyric acid: 16, 22,
29
GCN2 - general control nonderepressible 2: 36 GFP - green fluorescent protein: 98
Gr - gustatory receptor: 21, 26, 27,
30, 34, 50, 51, 68, 69, 76, 77, 115, 116, 118, 125, 126, 155, 166 GRN - gustatory receptor neuron:
15-17, 24-33, 43, 47, 50-52, 59, 60, 62, 63, 65-71, 73, 75-78, 80, 82, 87, 90-94, 105, 108, 110, 115, 116, 118, 121, 124-126, 128, 134, 137, 139-141, 154, 155, 156, 159, 165-167 hr - hour: 95, 128, 130, 131, 160 IN1 - ingestion neurons: 45, 82-86,
92-94, 101, 168
IPC - insulin-producing cell: 38, 39
Ir - ionotropic receptor: 20, 27, 28,
30, 52, 57, 60, 61, 64, 65, 68, 69, 71, 73, 75, 80, 90-93, 101, 105, 110, 114, 116, 125, 140, 141, 159, 165, 166
ISN - interoceptive SEZ neuron: 41, 44, 89 KC - Kenyon cell: 22, 23, 32 KCl - potassium chloride: 28, 110, 116, 120 Kir2.1 - inwardly-rectifying potassium channel 2.1: 142-144, 161
LAL - lateral accessory lobe: 88 LH - lateral horn 23, 24 Lk - leucokinin: 41, 42 LN - local interneuron: 21 LSO - labral sense organ: 25, 30, 60,
66
MB - mushroom body: 22-24, 32, 35, 43
MBON - mushroom body output neuron: 22
MIP - myoinhibitory peptide: 39 NaCl - sodium chloride: 7, 28,
109-118, 120, 125, 126, 156 NPF - neuropeptide F: 15, 16, 23, 39, 40, 42, 43 NPY - neuropeptide Y: 15, 16, 39 OA - octopamine: 136 Or - olfactory receptor: 20, 64
Orco - olfactory co-receptor: 64, 91
ORN - olfactory receptor neuron: 14, 15, 19-21, 24, 26, 59, 63-65, 69 P:C - protein:carbohydrate: 2 PBS - phosphate-buffered saline: 98, 161 PBST - phosphate-buffered saline with Tween 20: 98
PER - proboscis extension response: 106, 108, 110, 114, 115, 118, 120, 124, 129, 131, 148, 149, 161, 162 PI - pars intercerebralis: 38, 39 PMS4 - posterior maxillary sensory
4: 66, 71, 73
PN - projection neuron: 21- 24
Poxn - pox-neuro: 62, 66, 96, 171 ppk - pickpocket: 26, 27, 141 RNAi - ribonucleic acid interference:
140, 161
ROI - region-of-interest: 100 RT - room temperature: 98
S6K - S6-kinase 9
SAG - Sex Peptide abdominal ganglion: 133, 134, 136, 137, 143-145, 153, 157-160, 164, 167-170 SEM - standard error of the mean:
101, 131, 162
SEZ - subesophageal zone: 15, 16, 29, 31, 32, 41, 44, 51, 60, 66, 71, 87, 99, 139, 167, 169, 172, 173 SFP - seminal fluid protein: 136, 180 sNPF - short neuropeptide F: 15, 16 SP - Sex Peptide: 11, 136, 137, 139, 140, 141, 142, 144, 152, 158, 161 SPR - Sex Peptide Receptor: 11, 136,
139, 140, 141, 144, 157, 159 SPSN - Sex Peptide sensory neuron:
136, 139--145, 157, 164
Tdc2 - tyrosine decarboxylase 2: 148-150, 153, 158
TNT - tetanus toxin: 83 TOR - target of rapamycin: 9 TRP - transient receptor potential: 26
TβH - tyramine β-hydroxylase: 149, 150, 152, 158
upd1 - unpaired 1 39, 40 VCSO - ventral cibarial sense organ:
25, 60
VNC - ventral nerve cord: 31, 60, 124
WED - wedge: 9, 10, 88, 90, 166 YBM - yeast-based medium: 95, 97 YPI - yeast preference index: 97
OVERVIEW
The general introduction outlines the topics that will be discussed throughout this thesis, and provides background that is relevant to these topics. To do so, the introduction brings together knowledge from both insects and vertebrates, and encompasses both classical work and recent advances. It outlines how intake of specific nutrients is regulated, presents some examples of how internal states can modulate processing of sensory information, and describes what is known about the neuronal control of feeding in Drosophila, from chemosensory neurons to central hunger circuits.
The subsequent three chapters comprise the body of my research. Chapter 2 focuses on identifying neuronal substrates of protein-specific appetite in Drosophila. The results presented demonstrate that a subset of taste receptor neurons mediates yeast feeding and that the gain of these sensory neurons is modulated by the nutritional state of the fly; and suggests that a population of second-order taste neurons regulates a specific aspect of feeding behaviour. In Chapter 3, I describe a previously unknown nutrient-specific appetite for sodium that is induced by mating in Drosophila, and I identify sweet-sensing neurons as important mediators of salt taste. Chapter 4 then investigates the neuronal substrates that detect mating state and induce nutrient-specific appetites for salt and yeast to support reproduction. Finally, I show data suggesting that in contrast to nutritional state, this reproductive state modulates taste processing downstream of sensory neurons. In Chapter 5, I present a general discussion that aims to synthesise these findings and to place them into the context of the field. I also suggest how these findings could be taken forward in future work, and present a framework for understanding how different control systems contribute to nutritional homeostasis across species.
1 G
ENERAL
INTRODUCTION
1.1 Nutrition and health
Nutrition is key to the health and fitness of all animals. While energy consumption is essential to life, excessive intake of calories is a major global public health concern due to its link with obesity and metabolic diseases (Caballero, 2007). However, in addition to overall caloric intake, dietary composition also has a striking impact on human health: the quality and ratio of nutrients in the diet have been linked with various health outcomes. Micronutrient deficiencies, for example, contribute to a significant number of deaths in low- and middle-income countries (Black et al., 2008). Sodium is an essential micronutrient, and low plasma sodium has been linked with chronic fatigue syndrome and reduced fertility (Moinier and Drüeke, 2008). However, in all countries for which relatively recent data are available, average dietary sodium intake is much higher than physiologically necessary (Elliott and Brown, 2007); rather, excessive intake of sodium is a worldwide concern due to its link with hypertension and associated cardiovascular diseases (Graudal et al., 2017; World Health Organization, 2012). Likewise, protein is essential for growth and maintenance of body tissues, but
increasing dietary protein levels have been associated with an increased risk of mortality in the under-65 population, and particularly increased risk of cancer mortality (Levine et al., 2014). Thus, maintaining intake of specific nutrients within tight bounds is important for human health and well-being, but how the brain controls intake of specific nutrients is poorly understood. As in humans, dietary macronutrient composition has been shown to strongly affect fitness in species ranging from Drosophila to mammals (Simpson et al., 2017). In mice, increasing dietary protein:carbohydrate (P:C) ratio is associated with increased mortality (Solon-Biet et al., 2014), and protein restriction may underlie at least some of the healthspan-extending effects of dietary restriction (Gallinetti et al., 2013; Pamplona and Barja, 2006; Youngman, 1993). In the fly, the beneficial effects of caloric restriction on lifespan have been specifically attributed to reduction in amino acid intake (Grandison et al., 2009). Consistent with this, increasing P:C ratio decreases lifespan, while on the other hand increasing reproductive output (Lee et al., 2008). Because of these different effects of dietary composition, regulating nutrient intake towards a balance that optimises life history outcomes would provide an evolutionary advantage to animals, and as such we might expect animals to be able to regulate their nutrient intake. Indeed, when given a choice between diets varying in their P:C ratio, female Drosophila preferentially feed from the diet that optimises lifetime egg production (Lee et al., 2008; Wong et al., 2017). Likewise, other insects have been shown to select dietary P:C ratios that optimise various life history traits (Ponton et al., 2011; Povey et al., 2014; Rodrigues et al., 2015). Furthermore, when given a diet with imbalanced ratios of nutrients, many animals will overconsume one nutrient in order to obtain adequate amounts of another (Simpson and Raubenheimer, 2012). This phenomenon has led to the so-called protein leverage hypothesis, which posits that many species prioritise achieving their target intake of protein over other nutrients. Evidence for protein leverage has been demonstrated in species from locusts to humans (Felton et al., 2009; Gosby et al., 2011; Martens et al., 2013; Raubenheimer and Simpson, 1997). Indeed, it has been proposed that protein leverage could contribute to the current obesity epidemic across the world: the availability of high-energy, low-protein foods could lead to
overconsumption in order to reach a target intake of protein (Simpson and Raubenheimer, 2005). Thus, understanding the mechanisms controlling intake of specific nutrients, particularly protein, is relevant to understanding and potentially combating human obesity.
Animals use a number of physiological and behavioural adaptations to maintain relatively constant and optimal internal conditions, or homeostasis. To maintain a constant supply of nutrients to the body, one of the key adaptations is the regulation of nutrient intake. Since the nervous system ultimately controls behavioural outputs including feeding, what are the tasks the brain needs to perform to regulate nutrient intake in accordance with physiological needs? First, it needs to have access to a representation of the physiological states of the body that are associated with needs for a nutrient. This can be a measure of the levels of that nutrient in the body, or can be a state that is associated with future demand. Second, the brain needs to have access to information about the nutrient(s) that are available in the environment, through direct sensing of the nutrient, or through cues associated with that nutrient. Third, it needs to produce appropriate behavioural responses to that cue only when in a state of need. In order to do this, the mapping between sensory stimuli and behavioural outputs must be altered depending on internal state.
In this introduction, I will address what is known about how the brain performs these tasks, and highlight the key questions that remain to be addressed. First, I will outline the phenomenon of nutrient-specific appetites, providing evidence from diverse species that animals can regulate their intake of individual nutrients quite apart from total food intake. Second, I will discuss how sensory processing can be modulated by internal states, distilling possible mechanisms for how state-specific behavioural responses could be generated from known cases of neuronal modulation in different species. Finally, I will describe in some detail the neuronal populations in the Drosophila brain that detect and represent external cues associated with food, and internal states that modulate ingestive behaviour. Together, these concepts set the stage for identifying how nutrient-specific appetites are implemented at the circuit level.
1.2 Nutrient-specific appetites
In order to regulate nutritional intake, many animals show so-called nutrient-specific appetites, seeking out and consuming a nutrient-specific nutrient in response to a need for that nutrient. Nutrient-specific appetites are widespread in insects, and innate appetites for some nutrients are also common in mammals. Nutrient-specific appetites can also be acquired by learning associations between sensory cues predictive of foods that restore nutritional imbalances (Gibson and Booth, 1986; Hughes and Wood-Gush, 1971; Simpson and White, 1990; Villalba et al., 2008). In either case, the necessary conditions for a nutrient-specific appetite are that the behavioural response to sensory cues associated with a specific nutrient is modulated by a physiological need state for that nutrient, in a way that favours acquisition and ingestion of the necessary nutrient (Schulkin, 1991).
One example of a specific nutritional deficit that induces consumption of the deficient nutrient is thirst, which is ubiquitous throughout the animal kingdom. Much is known about the sensory pathways and central circuits that guide thirst in both mammals and Drosophila (Cameron et al., 2010; Jourjine et al., 2016; Matsuda et al., 2017; Oka et al., 2015; Zimmerman et al., 2016, 2017; Zocchi et al., 2017). However, in this thesis I will largely focus on appetites for food nutrients, rather than water. In this section, I will contrast salt appetite, about which much is known in mammals, and protein appetite, which has been more extensively studied in insects. I will leave aside other specific appetites, notably that for calcium, which has been observed behaviourally in rodents, and its basis in calcium taste (Richter and Eckert, 1939; Schulkin, 2001; Tordoff, 2001; Tordoff et al., 2012).
1.2.1 Salt appetite
1.2.1.1 Deficiency-induced sodium appetite
Arguably the best-studied nutrient-specific appetite across species is that for sodium. A specific appetite for sodium was first demonstrated by Curt Richter, who observed that upon removal of the adrenal glands, rats would show a voracious appetite for salt (Richter, 1936). This appetite was specific to sodium, since rats would increase their intake of sodium salts, but not
other salts (Richter and Eckert, 1938); and was innate, since sodium intake was increased the first time rats had access to sodium chloride, within a few seconds (Richter, 1939; Wolf, 1969). Adrenalectomy leads to a dramatic insufficiency of sodium due to loss in the urine (Rubin and Krick, 1933), and can cause death if a source of sodium is not available (Richter, 1936). Thus, this specific appetite for sodium was a compensatory response to a bodily deficiency of sodium.
Subsequent studies over the next few decades demonstrated that a variety of manipulations that decrease bodily sodium levels induce a specific appetite for sodium (De Luca Jr. et al., 2010; Jalowiec, 1974; Stricker et al., 1991). The sodium appetite produced by these manipulations is characterised by its specificity to sodium, along with an upward shift in the behavioural dose-response curve, such that lower concentrations are ingested, and high sodium concentrations, which usually elicit rejection, become accepted. Moreover, sodium deficiency induces alliesthesia, a shift in the hedonic properties of a stimulus (Cabanac, 1971): hypertonic saline switches valence from highly aversive to rewarding (Berridge et al., 1984), and rats will display motivated behaviours in order to obtain sodium (Robinson and Berridge, 2013; Wolf, 1969). These characteristic behavioural features provide a point of comparison for sodium appetite, as well as other motivational states, in species outside of mammals.
Thus, rats and many other mammalian species (including humans) show a specific appetite for sodium when in a sodium-deficient state (Baldwin, 1969; Bell and Sly, 1979; Blair-West et al., 1968, 1997; Denton and Sabine, 1961; Denton et al., 1985, 1990, 1993; Houpt et al., 1991; Kochli et al., 2005; Schulkin et al., 1984; Takamata et al., 1994). This appetite is thought not to be stimulated by decreased plasma sodium concentration; rather, a number of physiological factors drive sodium appetite, largely as a response to decreased plasma volume (Geerling and Loewy, 2008). This is because sodium concentration is normally regulated within tight bounds by physiological mechanisms that alter extracellular fluid volume, and restoration of this volume can only be achieved by ingestion of both salt and water.
Much is known about the physiological and hormonal factors that influence salt appetite in mammals. In particular, sodium depletion elevates circulating
levels of both aldosterone and angiotensin II (Bojesen, 1966; Okubo et al., 1997; Stricker et al., 1979), which are thought to act synergistically to drive sodium appetite (the ‘synergy hypothesis’: Epstein, 1982; Fluharty and Epstein, 1983; Krause and Sakai, 2007), by activating distinct populations of neurons in the nucleus of the solitary tract and subfornical organ, respectively (Geerling et al., 2006; Jarvie and Palmiter, 2017; Matsuda et al., 2017; Nation et al., 2016; Resch et al., 2017; Simpson and Routtenberg, 1973). Both of these populations project to the ventral bed nucleus of the stria terminalis, suggesting that this synergism may be implemented in this area. In addition to circulating hormones, the nervous system also integrates other systemic signals to control sodium appetite. Baroreceptors, which monitor blood volume by detecting arterial stretch, can inhibit salt appetite (Thunhorst et al., 1994; Toth et al., 1987), as can hyponatraemia, which is detected by glia in the brain (Hiyama et al., 2002; Matsuda et al., 2017; Shimizu et al., 2007; Watanabe et al., 2000). In this way, the nervous system integrates multiple systemic signals to positively and negatively regulate salt intake according to physiological needs. Thus, the representation of the internal state of sodium deficit is somewhat understood in mammals. However, the mechanisms through which these signals modulate responses to sensory cues predictive of sodium are poorly understood (see Section 1.3.4).
1.2.1.2 Salt intake and reproduction
Nutritional needs are not static, but vary depending on internal physiological processes. Nutrient intake must therefore be dynamically modified to fulfil these needs. Intake of sodium in many species is sexually dimorphic, with females generally ingesting higher levels of sodium (Krause and Sakai, 2007). Moreover, in a range of wild and laboratory mammals, females show a strong and specific appetite for sodium during pregnancy and lactation (Barelare and Richter, 1937; Denton and Nelson, 1971, 1980; Faas et al., 2010; Friend and Wolynetz, 1981; McBurnie et al., 1999; Richter and Barelare, 1938). This salt appetite is evident within a few days of mating, while embryos are still small, suggesting that it might not be driven by sequestration of sodium by the embryo, but rather by maternal hormones. In agreement with this, reproductive sodium appetite in wild rabbits is not
preceded by a drop in sodium excretion or any change in sodium balance (Denton and Nelson, 1971). In fact, this pattern of increased NaCl intake can be mimicked by the sequential administration of around 4-5 reproductive hormones (Denton and Nelson, 1978, 1980). This suggests that pregnancy might increase salt intake in anticipation of increased sodium requirements, but conclusive evidence of how a need-independent appetite could be implemented is lacking.
Why might pregnant animals need more sodium? During pregnancy and lactation, isosmotic fluid, containing high levels of sodium, is retained by the developing foetus and associated tissues (Churchill et al., 1980; Gray and Plentl, 1954), and is lost in milk. As mentioned previously, extracellular fluid volume is tightly coupled to sodium levels, so any expansion of this fluid will require intake of sodium. Reproductive sodium appetite may help to fulfil this requirement. Indeed, environmental sodium levels have been shown to limit population growth in several environments (Aumann and Emlen, 1965; Pennisi, 2014), and sodium intake is correlated with litter size in rats (Denton and Nelson, 1980). Consistent with this, reducing dietary sodium reduces reproductive output in species including mice, chickens and voles (Batzli, 1986; Chou et al., 2014; McBurnie et al., 1999; Moinier and Drüeke, 2008; Whitehead and Shannon, 1974). Thus, while sodium intake impacts reproduction in many species, the mechanisms through which the brain ensures an adequate supply of sodium for reproduction is poorly understood. Furthermore, how the brain could anticipate the demands of reproduction and induce an anticipatory appetite for sodium is unclear. Studies in insects may help to shed light on these matters, since insects have less complex nervous systems, but still possess a strong reproductive drive and are able to regulate their intake of specific nutrients.
1.2.1.3 Sodium intake in insects
The detection of salt through taste receptors is not restricted to mammals, but is widespread throughout the animal kingdom. Salt taste has been observed in mammals, birds, fishes, freshwater and soil invertebrates, and insects (Dethier, 1977). This taste likely evolved due to the relative scarcity of sodium in certain environments, and particularly in herbivorous diets. However, salt taste is found in both herbivorous and carnivorous mammals;
and in insects with such diverse diets as caterpillars (Dethier, 1973), mosquitoes (Elizarov and Sinitsina, 1974), blowflies (Hodgson and Roeder, 1956) and fruit flies (Stocker, 1994). This taste underlies animals’ innate preference for low salt concentrations (and rejection of high concentrations) even in a state of salt balance (Chandrashekar et al., 2010; Oka et al., 2013; Zhang et al., 2013). However, physiological modulation of salt appetite has not been demonstrated in insects, either in response to a lack of sodium or to reproductive demands. During desiccation, some insects, including Drosophila, lose sodium (Folk and Bradley, 2003), and so a selective pressure could have favoured the development of feedback control of sodium appetite in insects in order to recover from desiccation. Whether such an appetite exists in any insect is unknown.
Although it is not known whether insects regulate salt intake, appropriate levels of dietary sodium are essential for most insects. Furthermore, in some insects, sodium has been shown to affect reproductive success. Males of some butterfly species, for example, exhibit a behaviour known as puddling, or flocking to puddles containing nutrients that are not obtained from their diet of nectar (Downes, 1973; Molleman, 2010). Sodium is a major stimulus for puddling (Arms et al., 1974), and males use the sodium acquired in this way to produce a “nuptial gift”: they transfer up to 32% of their body sodium to the female during copulation (Pivnick and McNeil, 1987). This sodium is used by the female to produce eggs (Smedley and Eisner, 1996), and increases both fertility and egg drought resistance (Pivnick and McNeil, 1987). This highlights how sodium may be important for reproduction in insects, just as in mammals; but whether insects are able to regulate their sodium intake to optimise reproductive success is unknown.
1.2.2 Protein hunger
1.2.2.1 Deprivation-induced protein appetite
Protein is an essential macronutrient, and must be ingested in much greater quantities than sodium. However, compared to sodium, much less is known about how animals regulate their intake of protein. The ability to regulate intake of protein has been demonstrated in both humans (Griffioen-Roose et al., 2012; Martens et al., 2013) and rodents (Deutsch et al., 1989; DiBattista
and Campbell, 1998; Leib and Knight, 2015). Several studies have suggested the involvement of learning in mammalian protein appetite: rats are able to associate specific flavours with the postingestive consequences of protein ingestion, and show a preference for this flavour specifically in a protein-deprived state (Baker et al., 1987; Gibson and Booth, 1986). In fact, a similar ability has been demonstrated in locusts: protein-deprived locusts show preference for an odour previously paired with a high-protein diet over one paired with a high-carbohydrate diet (Simpson and White, 1990). Thus, unlike salt appetite, protein appetite may involve learned associations between specific flavours and their postingestive consequences.
Like mammals, insects are able to regulate their intake of protein towards an optimal level (Lee et al., 2008; Simpson and Raubenheimer, 2012; Waldbauer and Friedman, 1991). In several species, dietary protein deficiency drives an innate compensatory appetite for proteinaceous foods (Barton Browne and Kerr, 1986; Dethier, 1961; Mayntz et al., 2005; Ribeiro and Dickson, 2010; Robacker, 1991; Simmonds et al., 1992). For most drosophilids, the main source of dietary protein in the wild is yeast (Baumberger, 1919; De Camargo and Phaff, 1957; Phaff et al., 1956), and Drosophila melanogaster flies compensate for deprivation from yeast by shifting their preference from sucrose to yeast (Ribeiro and Dickson, 2010; Vargas et al., 2010). This yeast appetite is driven by lack of essential amino acids (AAs), but not other dietary constituents or non-essential AAs (Leitão-Gonçalves et al., 2017; Piper et al., 2013). In fact, Drosophila can specifically select not only yeast, but also a holidic diet containing AAs over the same diet containing sucrose when in an essential AA-deprived state (Leitão-Gonçalves et al., 2017).
Recent studies have begun to investigate the mechanistic basis of this protein appetite in D. melanogaster. At the molecular level, detection of internal AA availability may involve direct sensing by the nervous system through the TOR/S6K signalling pathway. Genetic manipulations that increase or decrease TOR activity in the nervous system lead to an increased appetite for yeast (Ribeiro and Dickson, 2010; Vargas et al., 2010). To what extent this pathway underlies the protein appetite induced by AA deprivation, however, remains unclear. At the circuit level, this protein appetite is driven at least in part by activation of a small group of dopaminergic neurons, DA-WED (Liu
et al., 2017). The activity of DA-WED is increased by deprivation from protein; and this activity is causally related to protein appetite: activation of these neurons leads to a gain of yeast appetite in sated flies, whereas their inhibition blunts the yeast appetite induced by protein deprivation. DA-WED neurons are thought to signal through a group of neurons expressing the dopamine receptor Dop1R2 that innervate the fan-shaped body and lateral accessory lobes to induce protein appetite. In parallel, the activity of DA-WED also suppresses intake of sugars, through Dop1R1-expressing neurons in the posterior lateral protocerebrum. This highlights the specialisation of this circuit to balancing the intake of protein and carbohydrates.
Beyond the nervous system, hormonal regulation also plays a role in Drosophila protein appetite. Female-specific independent of transformer (FIT) is a hormone secreted from the fat body (a distributed tissue that performs functions analogous to the liver and adipose tissue of mammals) in response to feeding on protein. FIT acts as a protein-specific satiety hormone – that is, it specifically inhibits ingestion of protein-rich foods, and not sugars. It exerts this action through insulin-producing cells in the pars intercerebralis of the brain, which secrete Drosophila insulin-like peptide 2 in response to protein intake in a FIT-dependent manner. Thus, multiple neuronal and systemic signals are likely involved in the control of protein appetite in Drosophila.
1.2.2.2 Regulation of protein appetite by reproduction
As mentioned above, nutritional requirements vary as animals’ physiology changes, and one particularly relevant factor for nutrition is reproduction, which imposes a large nutritional burden on mostly female animals (Barton Browne, 1995). In some insect species, the nutrients required for adult reproduction are acquired during larval development; but for most, at least some nutrients must be obtained through adult feeding. Since eggs have a high protein content, dietary protein intake is essential to sustain egg production (Drummond-Barbosa and Spradling, 2001; Friend et al., 1965; Piper et al., 2013; Williams et al., 1979). For this reason, females of many insect species show a higher ingestion of protein-rich foods than males (Greenberg, 1959). Within females, this protein appetite is not constant, but
is regulated in accordance with requirements for egg development. In several species with ovarian cycles, females show their highest intake of nutrients that are important for reproduction during the early part of each cycle (Barton Browne, 1995).
In other insect species, including drosophilids, production of eggs is stimulated only following mating (Bloch Qazi et al., 2003; Riemann and Thorson, 1969). In order to provide nutrients for egg development, mated females will consume greater quantities of food than virgins (Carvalho et al., 2006; Clifford and Woodring, 1986; Slansky, 1980), and specifically protein-rich foods that support egg production (Greenberg, 1959; Ribeiro and Dickson, 2010; Uchizono et al., 2017; Vargas et al., 2010). Interestingly, this postmating protein appetite is thought to be driven not by feedback through depletion of AA reserves due to egg production, since females that do not produce eggs still show increased yeast preference following mating (Ribeiro and Dickson, 2010). Rather, the event of mating elicits an increased appetite for protein in anticipation of the nutritional demands of egg production, before any deficit occurs – thus, this postmating appetite is driven by a feedforward or non-demand-mediated signal (Barton Browne, 1995; Walker et al., 2017). In Drosophila, this signal is mediated at least in part by seminal fluid proteins transferred from the male to the female during copulation, principally Sex Peptide (SP), acting through its receptor Sex Peptide Receptor (SPR) in the nervous system (Ribeiro and Dickson, 2010) (see Chapter 4). However, the neural circuit through which this SP signal alters nutrient preference remains unknown.
Thus, multiple internal state signals, including nutritional and reproductive states, act through specific neural circuits to influence animals’ appetites for nutrients such as sodium and protein. How could these states change animals’ behavioural responses to foods? At some level, these states must change the mapping of sensory inputs to behavioural outputs, and this could be achieved by modulating neuronal responses to sensory stimuli that are associated with specific foods. How could this modulation be implemented? In the following section, I will present examples of how internal states can modulate sensory processing in different systems, and discuss how these mechanisms could be applied to regulate intake of food in general, and particularly of specific nutrients.
1.3 State-dependent modulation of sensory
processing
From the evidence presented above, it is clear that nutrient-specific appetites emerge in accordance with the internal physiological state of the animal. This internal state changes the behavioural response of the animal to nutrient-related sensory stimuli in order to increase intake of the necessary nutrient. This illustrates a general principle of neural processing: the way in which the brain processes sensory information and generates motor outputs can be modulated depending on the state of the brain and body in order to produce adaptive behaviours. How could internal states alter processing within the brain to adjust behavioural outputs? Examination of how this modulation is implemented in a variety of sensorimotor systems in different species can provide us with possible mechanisms that could be tested in the context of nutrient balancing.
State-dependent modulation of neural processing has been demonstrated in a variety of sensory systems across animal phyla. Neuronal responses can be modulated not just by physiological state, but also by other brain states such as behavioural state (e.g. walking or flying) and arousal state (e.g. sleep and specific arousal states such as aggressive or sexual arousal). In this section, I will present some examples of this state-dependent modulation, and outline some neuronal mechanisms that implement state-specific modulation across species.
1.3.1 Behavioural state
The brain state that has the most wide-ranging impact on neural processing, and which we experience every day, is sleep. Sleep acts as a gate on sensory processing, such that the threshold for inducing behavioural responses is strongly increased (Campbell and Tobler, 1984; Hendricks et al., 2000). Indeed, sleep has been shown to act through neuromodulatory systems to alter neural processing of sensory information in several species (Fontanini and Katz, 2008; Shea and Margoliash, 2010; Tsuno and Mori, 2009). Even in awake animals, however, sensory processing can be dramatically altered by states such as ongoing behavioural state and attention (Albergaria et al., 2017; Dubner, 1988; Fontanini and Katz, 2008; Reynolds and Chelazzi,
2004). In primary visual cortex, for example, locomotion increases the response of most neurons to visual stimuli, independently of arousal (Ayaz et al., 2013; Niell and Stryker, 2010; Vinck et al., 2015), through modulation of local interneuron activity by long-range input from neuromodulatory systems (Fu et al., 2014; Polack et al., 2013). In auditory cortex, in contrast, locomotion suppresses auditory responses (Schneider et al., 2014; Zhou et al., 2014). This locomotor input comes from motor cortex and likely functions to counteract responses to sounds induced by self-movement (so-called efference copy). Efference copy is an effective mechanism to cancel out self-induced sensory signals in many systems (Eliades and Wang, 2003; Lee et al., 2013; Poulet and Hedwig, 2006; Requarth and Sawtell, 2011). In Drosophila, as in mammals, visual processing is modulated by behavioural state. Visual responses of 4th-order visual neurons, tangential cells of the lobula plate, are increased during both flight and walking (Chiappe et al., 2010; Maimon et al., 2010), and these cells also receive graded motor-related input (Fujiwara et al., 2017). As in mammalian visual cortex, neuromodulatory input plays an essential role in this modulation during flight (Suver et al., 2012). Motor signals perform different functions in flying and walking flies: opposing visual and motor signals perform an efference copy function during flight (Kim et al., 2017a), whereas coherent visual and motor inputs are thought to maintain a straight course in walking flies (Fujiwara et al., 2017). Thus, motor-related signals are integrated into higher levels of sensory processing systems in both insects and mammals in a way that implements behaviourally relevant functions.
1.3.2 Reproductive state
In order to adapt to the changing needs of the body, animals’ behavioural responses to sensory cues are altered by not just behavioural, but also physiological states. Reproduction is a state that has particularly relevant effects on animals’ needs and priorities, and as such changes animals’ behaviour. These behavioural changes can be implemented by changes in neuronal responses at early stages of sensory processing. In mice, for instance, the response of females to male pheromones varies across the oestrus cycle, and this is mirrored by a decrease in the response of specific
peripheral vomeronasal sensory cells to male major urinary proteins, mediated by the steroid hormone progesterone (Dey et al., 2015).
In many insects, mating induces a shift away from receptivity to mates and towards identifying nutrients and oviposition sites to increase reproductive output. In concert with this, mating alters the behavioural response of these insects to odour stimuli, particularly those representing host plants and sex pheromones (Fernandez and Klowden, 1995; Jang, 1995; Landolt, 1989; Mechaber et al., 2002; Phelan and Baker, 1987; Tingle et al., 1989; Wiesenborn and Baker, 1990). In both male and female cotton leafworm moths, postmating changes in behavioural attraction to the odours of cotton leaf, lilac flower and female pheromone are mirrored by odour-specific modulation of olfactory receptor neuron (ORN) responses at the peripheral level (Kromann et al., 2015; Saveer et al., 2012). In noctuid moths, in contrast, while mating similarly inhibits male attraction to sex pheromone, peripheral odour responses are not affected (Barrozo et al., 2010, 2011; Gadenne et al., 2001). Instead, mating drastically reduces the response of 2nd-order olfactory neurons to sex pheromone, suggesting that in this species, mating acts within the nervous system on either primary sensory terminals or 2nd-order neurons to modulate pheromone responsiveness.
As in moths, mating affects chemosensory responses in Drosophila. Mating decreases female attraction to the male pheromone cVA by decreasing 2nd -order, but not ORN, cVA responses (Lebreton et al., 2014). This modulation is mediated by a parallel set of olfactory receptor neurons that are activated only upon long-term exposure to cVA, after it is deposited by the male during copulation, and inhibit the acute cVA response. Meanwhile, mating also modulates the response to polyamines, shifting olfactory-mediated positional attraction towards higher concentrations and enhancing taste-mediated oviposition aversion (Hussain et al., 2016a). At the neuronal level, mating transiently increases both olfactory and taste receptor neuron responses to polyamines, but this modulation does not persist for as long as the behavioural effect of mating, suggesting that this sensory neuron modulation cannot explain the effects of mating on behaviour.
Reproductive state therefore affects behavioural responses to chemosensory cues in many species, through neuronal modulation at the peripheral or 2nd
-order level. However, the mechanisms of this modulation are diverse and in many cases unclear, and moreover whether reproductive state modulates sensory processing to bring about changes in behaviour, including food preference and nutrition, is unknown.
1.3.3 Hunger state
Like reproduction, nutritional state has profound effects on neural processing and behaviour. As well as increasing food intake, starvation modulates behavioural responses to odours in species from C. elegans to Drosophila, rats and humans (Aimé et al., 2007; Colbert and Bargmann, 1997; Janowitz and Grossman, 1949; Palouzier-Paulignan et al., 2012; Prud’homme et al., 2009; Root et al., 2011). Many of these changes are induced by hormonal and neuropeptidergic mechanisms. In rats, hunger/satiety-related hormones such as leptin, insulin and orexin can modulate olfactory sensory neuron responses (Savigner et al., 2009), as well as responses in the olfactory bulb (Prud’homme et al., 2009). Likewise, the orexigenic peptide neuropeptide Y (NPY) can modulate olfactory neuron response amplitude in species as diverse as rats and axlotl (Mousley et al., 2006; Negroni et al., 2012). Whether these changes are physiologically recruited by hunger and satiety, however, is unclear. In Drosophila, both insulin and neuropeptides are involved in starvation-driven modulation of olfactory responses. In starved flies, reduced insulin signalling increases behavioural attraction to food odours through presynaptic facilitation of food-odour sensing ORNs (Ko et al., 2015; Root et al., 2011). Reduced insulin elevates expression of receptors for the neuropeptides sNPF and tachykinin in distinct subsets of ORNs. Through these receptors, sNPF signalling increases the response of attractive-coding ORNs, while tachykinin decreases the response of aversive-coding ORNs that are usually activated at high odour concentrations, so that behavioural attraction is ultimately increased across a wide concentration range.
As in the olfactory system, starvation bidirectionally modulates responses of attractive and aversive gustatory receptor neuron (GRN) terminals through parallel neuromodulatory mechanisms in Drosophila. Short periods of starvation increase neuropeptide F (NPF) signalling, which promotes release of dopamine within the subesophageal zone (SEZ), the primary taste
processing centre, at least in part from a single dopaminergic neuron (Inagaki et al., 2012, 2014; Marella et al., 2012). Dopamine acts presynaptically on its receptor DopEcR in sweet GRNs to increase their responsiveness, and thus increase behavioural responses to sweet taste (Inagaki et al., 2012). Dopamine also acts on a distinct receptor, D2R, in unknown neurons to increase sweet taste responsiveness (Marella et al., 2012). Longer periods of starvation, in contrast, elevate glucagon-like hormonal signalling, which activates a pathway from sNPF neurons, through sNPFR1 in GABAergic neurons (Inagaki et al., 2014). This pathway is thought to mediate the starvation-induced decrease in activity of octopaminergic neurons in the SEZ (OA-VL), which reduces the response of bitter GRNs, and thereby increases flies’ tolerance to feeding deterrents (LeDue et al., 2016). This parallel modulation of GRNs may participate in adjusting feeding behaviour to energetic requirements. Interestingly, starvation also modulates responses to low sugar concentrations at the peripheral level, through an unknown mechanism that also involves NPF (Wang et al., 2016).
In addition to innate chemosensory responses, hunger also modulates the neuronal response to cues previously associated with food. In mice, hunger greatly increases responses to visual cues associated with food in the insular cortex (Livneh et al., 2017). This modulation is mediated by a neuronal pathway emanating from hypothalamic hunger neurons expressing NPY, through the thalamus and amygdala to the insular cortex. A similar pathway emanating from NPF-expressing neurons gates responses in the Drosophila mushroom body to odours associated with sugar according to hunger state (Krashes et al., 2009).
Thus, distinct hormonal and neuropeptidergic mechanisms increase responsiveness to innate and learned food-related sensory stimuli, while decreasing sensitivity to deterrents, in hungry flies. This modulation acts at multiple levels, from peripheral dendrites and primary axon terminals, to higher-order neurons such as those in the mushroom body. Whether similar mechanisms contribute to regulating intake of specific nutrients, however, is unclear.