Financial support from Fundação para a Ciência e
a Tecnologia, Ministério da Educação e Ciência,
Portugal, through grant SFRH/BD/28519/2006
Work performed at the
Laboratory of Plant Molecular Biology
Instituto Gulbenkian de Ciência
Oeiras, Portugal
Supervisor
“é preciso dizer rosa em vez de dizer ideia é preciso dizer azul em vez de dizer pantera é preciso dizer febre em vez de dizer inocência é preciso dizer o mundo em vez de dizer um homem”
A
CKNOWLEDGEMENTSFirstly, I wish to thank Paula Duque, my supervisor, who guided me into the beauty of splicing research and its underneath, still unveiled, world. This journey comes to an end after innumerous long and pleasant discussions, hard but insightful defeats, and accomplished successes that we achieved together. I thank her also for precious advice and suggestions during the writing of this thesis.
I would like to thank Miguel Godinho-Ferreira and Jocelyne Demengeot, members of my thesis advisory committee, for guidance during this PhD, the tough scientific discussions and their kind support.
I thank Mathias Zeidler and Jutta Rösler, our collaborators in Germany, for receiving me in the lab to make use of the light experiments set-up. The very short period I spent in Giessen turned out to be an extraordinary time, during which we shared exciting scientific discussions.
The Plant Molecular Biology laboratory had several members during the time of my PhD. I thank Raquel Carvalho and Estelle Remy for the great discussions, support and all the fun shared at work. To the past members, I especially thank Inês Barbosa, Rita Saraiva and Teresa Maia, whom I had the chance to work with very closely. Finally, to Vera Nunes, our plant technician, for her endless care of our Arabidopsis plants and plant growth facilities.
Evolution Genomics group.
This work was financially supported by a grant from Fundação para a Ciência e a Tecnologia (SFRH/BD/28519/2006) and by the Instituto Gulbenkian de Ciência, a wonderful place providing excellent conditions to do science. I had the chance to meet several people who undoubtedly left a mark during the period of my PhD. Special thanks to Thiago Carvalho, for the great discussions and for reminding me that doing science always gets much easier when we keep smiling.
I would like to thank my former supervisor before I arrived at the Instituto Gulbenkian de Ciência, Filip Rolland, who opened my heart to plant research. Thank you also to two former professors during my undergraduate studies, Isabel Sá-Correia and Marília Mateus, who helped me in initiating this PhD.
I wish to thank Clube Futebol Benfica, and the Fofó players for sharing the best stress reliever therapy: soccer playing.
I thank Jorge and Linda for their endless care and support in every decision I have made.
For guiding me at every moment, thanks to Cata and Guida. Para a avó Dora e a avó Marta, por me mostrarem que todas as preocupações são relativas e que o passar do tempo ajuda sempre a encontrar soluções.
R
ESUMOO splicing do ARN é um passo essencial da expressão génica em eucariotas durante o qual os intrões são removidos com precisão do mARN precursor (pré-mARN) e os exões ligados entre si, originando uma molécula madura de mARN. A existência de vários exões por gene possibilita que a maquinaria de splicing processe o mesmo pré-mARN de modos diferentes e remova seletivamente sequências intrónicas distintas. Torna-se assim possível que se gerem vários transcritos, e possivelmente mais do que uma proteína, a partir de um único gene. Estas vias de splicing alternativo têm-se revelado um mecanismo-chave na geração de diversidade proteómica e complexidade funcional. A prevalência do splicing alternativo em muitos genomas, incluindo os de plantas superiores, sugere que este fenómeno desempenha um papel importante em processos biológicos.
De modo a adaptarem-se às alterações do meio ambiente, as plantas, enquanto organismos sésseis, desenvolveram elevados níveis de plasticidade de desenvolvimento e tolerância ao stress. Estes são, em última análise, regulados a nível do genoma e é provável que a excecional versatilidade associada à regulação génica pelo splicing alternativo desempenhe um papel proeminente na resposta das plantas ao meio ambiente. No entanto, a relevância biológica deste mecanismo de regulação pós-transcricional em plantas encontra-se ainda pouco estudada.
uma E3 ligase de ubiquitina envolvida na degradação proteica pelo proteassoma 26S. Numa segunda abordagem, descobriu-se a função in vivo e os alvos endógenos de um fator de splicing específico de plantas envolvido na regulação do splicing alternativo.
etileno. É de realçar que ensaios de complementação indicaram que ambas as variantes de splicing de XBAT35 têm atividade na regulação da curvatura do gancho apical mas, aparentemente, com uma contribuição diferencial para esta resposta. Em paralelo, iniciou-se também o isolamento de um mutante com perda de função de um homólogo de XBAT35, o XBAT34, de forma a investigar a possibilidade de existência de redundância funcional entre os dois genes.
epistáticas revelaram de forma inequívoca que a SCL30a atua na via do ABA. No entanto, não parece que a perda ou o aumento de função da SCL30a alterem os níveis de ABA em sementes. Notavelmente, plantas transgénicas sobreexprimindo SCL30a produzem sementes maiores e mais tolerantes ao stress salino e osmótico durante a germinação. Assim, a proteína SR SCL30a de Arabidopsis constitui um novo regulador da via de sinalização do ABA que modula características específicas das sementes, incluindo o tamanho, a dormência e os níveis de tolerância à salinidade elevada e à seca durante a germinação. Por último, para identificar alvos endógenos desta proteína de ligação ao ARN, efetuou-se uma análise global por deep sequencing ao transcritoma de sementes selvagens, mutantes em SCL30a e com sobreexpressão de SCL30a na presença de stress salino.
mecanismos moleculares que governam o modo de ação desta nova proteína de ligação ao ARN.
A
BSTRACTRNA splicing is an essential step in eukaryotic gene expression during which introns are precisely removed from the precursor-mRNA (pre-mRNA) and exons joined together to form the mature mRNA molecule. The presence of numerous exons per gene enables the splicing machinery to process the same pre-mRNA differently by selectively removing different intronic sequences, thus generating multiple transcripts, and eventually more than one protein, from a single gene. Such alternative splicing pathways have emerged as a key mechanism for generating proteome diversity and functional complexity. The prevalence of alternative splicing in many genomes, including those of higher plants, suggests that this mechanism plays crucial roles in biological processes.
To adapt to an environment in constant change, plants, as sessile organisms, have evolved high degrees of both developmental plasticity and stress tolerance, which are ultimately regulated at the genome level. The exceptional versatility associated with gene regulation by alternative splicing is likely to play a prominent role in plant responses to environmental cues, but the biological significance of this posttranscriptional regulatory mechanism in plants remains poorly understood.
approach, the functional relevance of an alternative splicing event in an E3 ubiquitin ligase gene involved in protein degradation by the 26S proteasome was investigated. As a second approach, we uncovered the in vivo function and endogenous targets of a plant-specific splicing factor implicated in the regulation of alternative splicing.
but appear to contribute differentially to this response. In addition, the isolation of a loss-of-function mutant for the close XBAT35 homolog, XBAT34, was initiated to assess functional redundancy between these two genes.
germination tolerance to high salinity and drought. Finally, endogenous targets of this RNA-binding protein were identified by means of global deep-sequencing transcriptome analysis of wild-type, SCL30a-mutant and SCL30a-overexpressing seeds under salt stress.
L
IST OFA
BBREVIATIONSaa amino acid ABA abscisic acid
ACC 1-aminocyclopropane-1-carboxylate ANK ankyrin
bp base pair
CDS coding sequence Da Dalton
DNA deoxyribonucleic acid DNG did not germinate ESE exonic splicing enhancer ESR exonic splicing regulator ESS exonic splicing silencer GUS β-glucuronidase
hnRNP heterogeneous ribonucleoprotein IAA indole-3-acetic acid
ISE intronic splicing enhancer ISR intronic splicing regulator ISS intronic splicing silencer KO knockout
LB left border
mRNA messenger RNA nt nucleotide
PTB polypyrimidine tract-binding Pre-mRNA precursor mRNA PTC premature stop codon
OX overexpression RH relative humidity
RING really interesting new gene RNA ribonucleic acid
RNAi RNA-interference RNP ribonucleoprotein RRM RNA-recognition motif RS arginine/serine
SCL SC35-like
snRNP small nuclear ribonucleoprotein SR protein serine/arginine-rich protein TAIR The Arabidopsis Information Resource UB ubiquitin
UTR untranslated region WT wild type
T
ABLE OFC
ONTENTSACKNOWLEDGEMENTS... vii
RESUMO... ix
ABSTRACT... xiii
LIST OF ABBREVIATIONS... xvii
TABLE OF CONTENTS... xix
CHAPTER 1: GENERAL INTRODUCTION...1
1.1. Pre-mRNA Splicing in Plants ...4
1.1.1. Structure of exons and introns ...4
1.1.2. The splicing reaction ...7
1.1.3. Alternative splicing ...13
1.1.4. The SR protein family ...21
1.1.4.1. The SCL30a SR protein ...29
1.2. Protein Ubiquitination in Plants ...31
1.2.1. E3 ligase classification and activity ...35
1.2.2. The XBAT family of RING E3 ligases ...38
1.2.3. Alternative splicing of E3 ligases...42
1.3. Thesis Outline...44
EXHIBITING DUAL TARGETING OF ITS SPLICE ISOFORMS, NEGATIVELY
REGULATES ETHYLENE-MEDIATED APICAL HOOK CURVATURE...63
2.1. Abstract ...65
2.2. Introduction...66
2.3. Results...69
2.3.1. Alternative splicing of the XBAT35 pre-mRNA excludes nuclear localization signal ...69
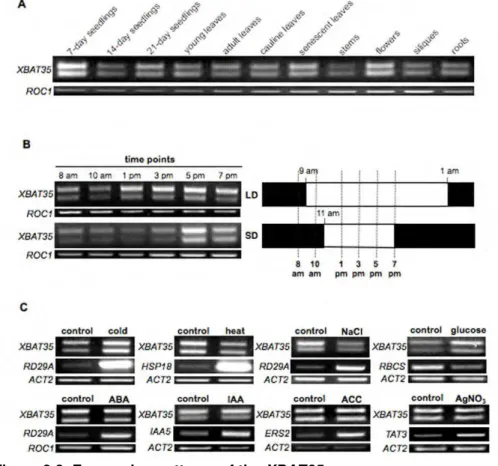
2.3.2. The XBAT35 gene is ubiquitously expressed in Arabidopsis...70
2.3.3. Exon skipping determines the subcellular localization of two XBAT35 isoforms ...73
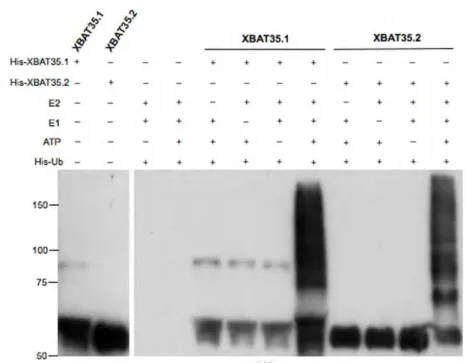
2.3.4. Both the nuclear and cytoplasmic XBAT35 isoforms are active E3 ubiquitin ligases...74
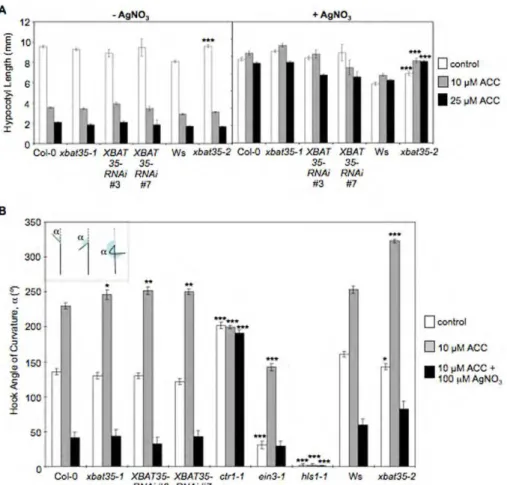
2.3.5. Loss of XBAT35 function causes hypersensitivity to ethylene-mediated control of apical hook curvature ...75
2.3.6. Both XBAT35 isoforms function in ethylene control of apical hook curvature...83
2.3.7. Four photosystem proteins and an unknown protein are putative XBAT35 interacting partners ...85
2.3.8. The XBAT35 and XBAT34 duplicated genes display overlapping expression patterns ...87
2.4. Discussion ...90
2.5. Materials and Methods...95
2.5.1. Plant materials and growth conditions...95
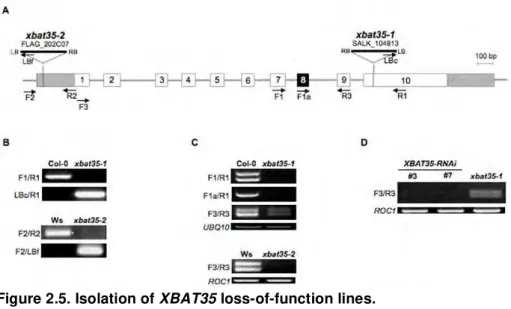
2.5.2. Generation of XBAT35-RNAi lines and complementation of the xbat35-1 mutant...96
2.5.3. Phenotypical analyses ...97
2.5.6. Expression of recombinant XBAT35.1 and XBAT35.2
proteins and in vitro ubiquitination assays...101
2.5.7. Yeast manipulations and two-hybrid assay ...101
2.6. References ...105
CHAPTER 3: THE ARABIDOPSIS SCL30aSR PROTEIN CONFERS ABA-DEPENDENT SALT AND OSMOTIC STRESS TOLERANCE DURING SEED GERMINATION...111
3.1. Abstract ...113
3.2. Introduction...114
3.3. Results...118
3.3.1. The SCL30a gene generates three splice variants and is markedly induced during seed germination...118
3.3.2. The scl30a-1 mutant displays seed-specific phenotypes and hypersensitivity to ABA, salt and osmotic stress during germination...122
3.3.3. SCL30a is a novel component of the ABA pathway ...128
3.3.4. SCL30a-overexpressing plants produce larger seeds exhibiting abiotic stress tolerance during germination ...133
3.3.5. SCL30a regulates ABA signaling under salt stress ...135
3.3.6. SCL30a affects the expression and splicing pattern of several Arabidopsis genes...136
3.3.7. The SCL33 and SCL30a duplicated gene pair displays similar expression patterns in Arabidopsis ...151
3.4. Discussion ...154
3.5. Materials and Methods...165
3.5.1. Plant materials and growth conditions...165
3.5.2. Generation of transgenic plants ...166
3.5.5. Determination of ABA content ...172 3.5.6. Deep-sequencing...172 3.5.6.1. Mapping reads to the Arabidopsis genome...173 3.5.6.2. Transcript prediction and expression level estimation...173 3.5.6.3. Comparison with TAIR annotation and testing for differential expression ...174 3.5.6.4. Splice junction analysis ...175 3.5.6.5. Transcript expression ratio analysis...175 3.6. References ...176
CHAPTER 4: CONCLUDING REMARKS AND FUTURE
PERSPECTIVES...185
4.1. Introduction...187 4.2. Alternative Splicing of the XBAT35 RING E3 Ligase ...188 4.3. Biological Roles of the Plant-Specific SCL30a SR Protein ...193 4.4. Conclusions ...198 4.5. References ...199
C
HAPTER1
Complex molecular signaling pathways control overall plant physiology and morphology and guide organismal growth throughout a life cycle. These pathways are precisely regulated, ensuring correct development, and at the same time relatively flexible, allowing for unique adaptive features of these sessile organisms, which confer the developmental plasticity and stress tolerance required to appropriately respond to environmental cues.
It is of fundamental interest to investigate the mechanisms that control plant growth. Besides the general biological interest, plant manipulation and the generation of transgenic plants more tolerant to certain adverse conditions can help feed the world population, whose resources are becoming limited due to its increase in number and to climate changes that affect crop yield.
Different plant species have been used as models for genetic studies to dissect plant developmental signaling pathways, but the mustard Arabidopsis thaliana has in recent years established itself as the most powerful system. It is small, easy to manipulate and transform by agroinfilitration, it has a short life cycle, it self-fertilizes or can be cross-pollinated, and its seed yield is high. In addition, a large number of mutant lines are publicly available, and the Arabidopsis genome sequence has been available to the research community for over ten years (The Arabidopsis Genome Initiative, 2000).
thesis focused on the biological relevance of a specific posttranscriptional regulatory mechanism, alternative pre-mRNA splicing.
1.1. Pre-mRNA Splicing in Plants
During DNA transcription, the precursor-mRNA (pre-mRNA) is formed. It is constituted by alternate sequences called exons and introns. As non-coding sequences, introns are removed from the pre-mRNA and exons, as coding sequences, are joined together in order to form the mature mRNA, which will be later translated into protein. The process of intron removal and exon ligation is called pre-mRNA splicing. It is a tightly regulated process that involves the activity of hundreds of proteins, ensuring that the correct sequences are spliced out from the pre-mRNA.
1.1.1. Structure of exons and introns
Splicing is conserved among eukaryotes and the basic splicing mechanism seems to be similar in plants and metazoans. However, in many aspects the splicing machinery diverges among these organisms, likely due to specific features of their intronic sequences.
are usually shorter than those found in vertebrates, with their size ranging from 60 to 10,000 kb, although around two thirds are shorter than 150 bp (reviewed in Lorkovic et al., 2000).
Exon/intron boundaries share consensus sequences, known as canonical splice sites, which are common to plants and vertebrates: the 5’ splice site is AG/GTAAGT and the 3’ splice site TGCAG/G, where the dash bars represent exon/intron junctions (reviewed in Brown and Simpson, 1998 and Lorkovic et al., 2000). The underlined base pairs (GT-AG) are the most conserved. In animals, two other intronic consensus sequences, that can be considered as part of the 3’ splice site, exist: the branch point, located 20 to 40 bp upstream of the 3’ junction, and a 10 to 15 bp-long polypyrimidine-rich sequence, the polypyrimidine tract, downstream of the branch point (reviewed in Lorkovic et al., 2000 and Hertel, 2008). Plant introns do not contain a polypyrimidine tract but instead a uridine-rich region. They also include a branch point, with a loose consensus sequence and an adenosine nucleotide conserved among plants, vertebrates and yeast. Despite the conserved sequences described, plant introns are often incorrectly processed in mammalian nuclear extracts (van Santen and Spritz, 1987; Reddy, 2001) and animal introns are not recognized in plant nuclei (Barta et al., 1986; van Santen and Spritz, 1987; Wiebauer et al., 1988), indicating that plant introns possess particular cis-acting sequences that are differently recognized by the splicing machinery.
their structure is conserved among vertebrates and plants but is absent from yeast (reviewed in Lorkovic et al., 2000).
Splice site recognition depends on the nucleotide length of exonic and intronic sequences. Two mechanisms have been proposed for exon and intron selection by the splicing machinery: the exon definition and the intron definition models (reviewed in Lorkovic et al., 2000; Ram and Ast, 2007; and Hertel, 2008). According to the exon definition model, splice sites flanking the exon are selected and splicing factors interact along the exon. In the intron definition model, splice sites at the 5’ and 3’ ends of introns are bound and splicing factors interact across the intron. In mammals, the much shorter exonic sequences when compared to introns probably evolved the spliceosome machinery to recognize short exonic sequences rather than intronic ones. Larger exons are usually skipped during splicing but are retained when the flanking introns are small, while shorter introns (up to 250 bp) promote the retention of exons with weak splice sites. As their introns are shorter, it is likely that in plants the intron definition model is preferred by the splicing machinery. Similarly, yeast possesses very short introns and likely prefers the intron definition model (reviewed in Ram and Ast, 2007). Thus, intron size provides important information on the tendency of the splicing machinery to include a particular exon. Furthermore, the upstream intron is usually more influent than the downstream one in the selection of the flanked exon, possibly due to the coupling of pre-mRNA splicing with DNA transcription (reviewed in Hertel, 2008).
Brown and Simpson, 1998; Simpson and Filipowicz, 1996; and Lorkovic et al., 2000). These U-rich sequences are important for correct splice site selection and intron processing and, unlike the polypirimidine tract, which is located downstream of the branch point in animals, they are distributed all along introns (Simpson et al., 2004).
Despite the clear definition of exon/intron junctions and the branch point, these splice sites sequences are short and degenerate and do not allow for precise fine-tuning of the splicing reaction (reviewed in House and Lynch, 2008 and Shepard and Hertel, 2010). Indeed, exons and introns contain additional cis-acting splicing enhancers and silencers, which serve as binding sites for essential splicing factors that promote accurate splicing events. While exons contain exonic splicing regulators (ESRs), intronic splicing regulators (ISRs) are found in introns. These sequence elements constitute binding sites for different families of splicing factors, which also interact with the splicing machinery and modulate its activity, thus helping define the splice sites.
1.1.2. The splicing reaction
phosphate group of the 3-hydroxyl group of the 5’ exon fragment. The two exons are then joined together and the released intron lariat is degraded for nucleotide recycling (reviewed in Brown and Simpson, 1998; and Fedor, 2008).
Figure 1.1. The process of intron removal.
(A) Schematic representation of 5’ and 3’ exons (black and gray boxes, respectively) flanking an intron, with indication of the 5’ and 3’ splice sites and the conserved adenosine residue at the branch point.
(B) The two-step reaction leading to intron removal and joining of exons. In the first step, the transesterification reaction occurs at the 5’ splice site and the intron lariat is formed, whereas in the second step the 3’ splice site is attacked, exons are ligated and the intron lariat released.
Adapted from Simpson and Filipowicz (1996) and Lorkovic et al. (2000)
Brown and Simpson, 1998). Furthermore, the pre-mRNA also undergoes conformation rearrangements essential for correct splice site selection (reviewed in Hertel, 2008).
The spliceosome is composed by more than 300 proteins (reviewed in Jurica and Moore, 2003). Its core components are five U-type (uridine-rich) small nuclear ribonucleoproteins (snRNPs) – U1, U2, U4, U5 and U6 – that are highly conserved from yeast to mammals and plants (reviewed in Brown and Simpson, 1998; and Lorkovic et al., 2000). The spliceosome complex is responsible for intron excision and exon joining, but a recent report has also described a role for the U1 snRNP in transcript stabilization independently of the splicing reaction (Kaida et al., 2010).
Additional non-U snRNP proteins can be found in the spliceosome, such as hnRNPs (heterogeneous RNPs), SR (serine/arginine-rich) proteins, and DEAD- or DEAH-box containing proteins (reviewed in Brown and Simpson, 1998). The DEAD- or DEAH-box containing proteins are RNA-dependent ATPases or ATP-dependent RNA helicases that mediate RNA conformational changes during spliceosome assembly and disassembly. hnRNPs and SR proteins bind the RNA and contain additional specific domains – rich in either glycine residues or serine/arginine repeats, respectively – which are involved in protein-protein interactions.
Splicing starts with a first approach to the pre-mRNA by the U1 and U2 snRNPs and other splicing regulators that initiate the recognition of exons and introns. The U1 snRNP binds first to the 5’ splice site. In mammals, the U2AF65 subunit of the U2AF (U2 auxiliary
of the splicing reaction (Fig. 1.2). Simultaneously, U2AF35 binds near the 3’ splice site and helps promote binding of U2AF65 to the
polypirimidine tract, by directly interacting with the second U2AF subunit, in a process that also involves the activity of SR proteins. Plants possess both subunits of U2AF but given their lack of polypirimidine tracts, binding of U2AF to the introns seems to rely on a conserved recognition of the 3’ splice site and stabilization of the large subunit at the branch point (reviewed in Brown and Simpson, 1998). The splicing reaction continues with the recruitment of the U2 snRNP to the branch point and the conversion of the E complex into the prespliceosomal A complex. The remaining three U snRNPs (U4, U5 and U6) assemble and join the A complex, resulting in formation of the B complex. The latter then undergoes conformational rearrangements to form complex C. Splicing is achieved at this moment and after lariat release and exon junction the spliceosome disassembles, with the mRNA being exported to the cytosol for translation into protein. Also at this stage, a nuclear surveillance mechanism detects aberrant transcripts, targeting them to degradation by nonsense-mediated decay (NMD).
Figure 1.2. Spliceosome activity in plants.
Schematic representation of U2-type spliceosome assembly, according to the preferred model in plants, the intron definition model. U snRNPs assemble sequentially along the pre-mRNA to form complexes E, A, B and C, after which the exons are ligated, the intron lariat is released and the spliceosome disassembles.
Adapted from Lorkovic et al. (2000), Jurica and Moore (2003), and House and Lynch (2008)
splicing at the first and last exons. Moreover, they can bind ESRs and/or ISRs in the pre-mRNA.
ESRs and ISRs can be either enhancers (ESEs and ISEs) or silencers (ESSs and ISSs). The hnRNP splicing factors usually bind silencers – when binding ESSs they inhibit spliceosome assembly by multimerization along the exon or by blocking snRNP recruitment to nearby splice sites (reviewed in Hertel, 2008). The polypyrimidine tract-binding (PTB) protein family, a class of hnRNPs, binds ISS motifs and represses inclusion of the adjacent exon in the splicing product.It is likely that binding to this region also affects the activity of U2AF65 at the same site. Arabidopsis possesses PTB-related proteins
whose activity during splicing has been shown (Stauffer et al., 2010). SR proteins usually bind ESEs or ISEs and aid in spliceosome assembly by recruiting the U1 snRNP to the 5’ splice site and U2AF to the 3’ splice site, eventually bridging an interaction between the components located at both splice sites (reviewed in Hertel, 2008 and Reddy and Ali, 2011). They also facilitate the incorporation of the U4/U6.U5 tri-snRNP in the spliceosome and promote U2 and U6 interactions (Roscigno and Garcia-Blanco, 2005).
Spliceosome assembly occurs simultaneously with transcription and the growing pre-mRNA is immediately bound to the splicing machinery (Prasanth et al., 2003; Das et al., 2006). Furthermore, some proteins acting during transcription also interact and/or co-localize with spliceosome-related factors, indicating a close connection between these two steps. For instance, in humans, localization of Cdc2-related kinase with RS domain, CrkRS, overlaps with that of RNA polymerase II and with splicing compartments (Ko et al., 2001). In Arabidopsis, the cyclin-dependent kinase 2 (CDKC2) phosphorylates RNA polymerase II and co-localizes with the SR34 SR protein and with cyclophilin CypRS64, whose activity has also been linked to the splicing machinery (Lorkovic et al., 2004b; Kitsios et al., 2008). Also, Cyp59 is a mutidomain cyclophilin that interacts with the SCL33 SR protein and with the C-terminus of RNA polymerase II (Gullerova et al., 2006). Besides its functional coupling with transcription, the splicing process has also been linked to chromatin remodeling and histone modifications (Zhang et al., 2011), transcriptional elongation (Das et al., 2006), and mRNA stabilization (Kaida et al., 2010), transport (Huang and Steitz, 2001; Delestienne et al., 2010) or translation (Sanford et al., 2004), suggesting the existence of common factors regulating different steps of gene expression.
1.1.3. Alternative splicing
targeted for translation, also several protein isoforms, upscaling the genome capacity. There are five common types of alternative splicing – exon skipping, alternative 5’ or 3’ splice site choice, mutual exclusion of exons and intron retention (Fig. 1.3). Intron retention is one of the most ancestral events and the most abundant (41%) in plants (Ner-Gaon et al., 2004; Barbazuk et al., 2008).
Figure 1.3. Common types of alternative splicing events.
Schematic representation of exons (boxes) and introns (horizontal lines) within a pre-mRNA structure, and representation of the selected splice sites in the most common types of alternative splicing events (left). Indication of the resulting mRNA structures (right).
The protein isoforms resulting from alternative splicing can have different specificities, such as binding properties, stability or intracellular localization. Moreover, it is also common to observe the inclusion of premature stop codons (PTCs) in alternative splice forms, which may trigger mRNA degradation by NMD. It was recently suggested that 13% of the Arabidopsis intron-containing genes are targeted for NMD (Kalyna et al., 2012). This number has been estimated to range from 20 to 30% in humans (Lewis et al., 2003). It was also recently reported that Arabidopsis splice variants resulting from intron retention are not NMD sensitive, in contrast with transcripts with alternative 5’- and 3’-splice sites at UTRs, which appear to be more prone to NMD (Kalyna et al., 2012).
The hundreds of proteins active during splicing can act synergistically or compete for a particular pre-mRNA binding site or for an interaction with another specific protein. Different combinations of all the components involved account for the occurrence of regulated alternative splicing. For instance, in zebrafish depletion of the U1C subunit of the U1 snRNP results in aberrant alternative splicing profiles, but it can be relatively compensated by an increase in the amount of some SR proteins (Rosel et al., 2011). Several splicing regulators display different tissue-, developmental- and stress-specific expression patterns, which can provide a means of regulating the splicing pattern of a particular gene target and concomitantly the activity of its encoded protein(s) (Stauffer et al., 2010).
(Pan et al., 2008; Wang et al., 2008). In plants, this number was recently estimated to be 42% (Filichkin et al., 2010). Differences in the estimates between organisms can be explained by the fewer expressed sequence data available in plants, as suggested by the fact that the estimated fraction of alternatively-spliced Arabidopsis genes was initially estimated to be only 5% (Brett et al., 2002) but tends to increase with every report released (Iida et al., 2004; Ner-Gaon et al., 2004; Haas et al., 2005; Nagasaki et al., 2005; Wang and Brendel, 2006; Filichkin et al., 2010). In addition, since alternative splicing in plants is known to be regulated by developmental and stress cues (Tanabe et al., 2006; Palusa et al., 2007), splice variants that are only expressed during a specific developmental stage or in response to a particular external signal are likely to be absent from a sample used in a genome-wide approach. However, it is also plausible that fewer genes undergo alternative splicing in plants due to differences at the level of splicing machinery activity or of exonic and intronic structures. In the context of this hypothesis, it is interesting to note that, until recently, alternative splicing was thought to be absent from yeast. However, in Saccharomyces cerevisiae the ubiquitin-like molecule Hub1, which helps in the assembly of the tri-snRNP complex prior to formation of complex B, has been found to affect splice site selection and therefore alternative splicing (Mishra et al., 2011). This indicates that novel splicing factors can emerge in the future and supports the notion that the poor knowledge on plant alternative splicing is probably just a consequence of fewer studies in these organisms.
alternatively-spliced transcripts (Penfield et al., 2010). The full-length splice variant encodes a transcription factor harboring also a phytochrome-interacting domain, whereas the second transcript is shorter and does not include the DNA-binding domain. Interestingly, transgenic plants overexpressing each of the alternative transcripts revealed that the shortest splice variant accounts for a role of PIF6 in the control of seed dormancy in Arabidopsis, whereas both isoforms are active in light regulated-hypocotyl growth, especially under red light (Penfield et al., 2010). In another recent study, the identification of a novel splicing factor, suppressor of abi3-5 (SUA), allowed the annotation of a developmentally-regulated alternative splicing event in the ABA signaling ABI3 gene, a transcription factor that positively regulates seed dormancy and represses germination (Sugliani et al., 2010). The ABI3 splicing event generates two transcripts, one encoding the full-length protein and the shortest encoding only two of the four functional domains. Importantly, the latter isoform accumulates at the end of seed maturation and counteracts the effect of the full-length protein, resulting in the initiation of seed germination (Sugliani et al., 2010). In yet another example, alternative splicing of the Arabidopsis inderminate domain 14 (IDD14) gene also results in two splice forms. While the full-length transcript encodes the IDD14α
transcription factor associated with the promotion of starch accumulation, the shortest, IDD14β, lacks the DNA-binding domain
and interacts with IDD14α, inhibiting its activity (Seo et al., 2011). The
expression of this shortest splice variant is induced by cold stress and allows for controlled starch accumulation under these conditions (Seo et al., 2011).
responses to stress cues, this issue has also been recently addressed. Two major lines of evidence support a role for plant alternative splicing under stress conditions (reviewed in Duque et al., 2011). Arabidopsis genes related to stress responses are more prone to undergo alternative splicing (Zhou et al., 2003, Wang and Brendel, 2006) and stress conditions exert a huge impact on the splicing profile of alternatively-spliced plant genes (Iida et al., 2004, Filichkin et al., 2010). An additional interesting observation relating splicing with plant stress responses is the fact that several genes with roles in splicing display stress-responsive splicing patterns, with the SR protein gene family being a good example (Palusa et al., 2007; reviewed in Reddy and Ali, 2011). Moreover, the activity of splicing regulators has been linked to stress responses. For instance, a mutation in the U6 snRNP-specific Sm-like protein LSM4 results in hypersensitivity to salt stress during germination in Arabidopsis (Zhang et al., 2011), and correct splicing of the nad2 subunit 2 of mitochondrial complex I, promoted by the pentatricopeptide ABO5, is needed for proper ABA signal transduction (Liu et al., 2010).
rapid responses to unfavorable external conditions (Matsukura et al., 2010). Other reports still fail to provide extensive insight into the functional relevance of alternative splice forms. For instance, different splicing profiles have been identified in members of the rice sulphate transporter family under sulphate starvation, suggesting the involvement of alternative splicing in the control of sulphur status, which is essential for protein and lipid structures as well as for many cofactors and coenzymes during plant development and stress responses (Kumar et al., 2011). Also in rice, the OsBWML1 MAPK kinase gene generates three splice variants that display differential relative expression levels in various tissues and upon stress induction, with the resulting three isoforms being differently localized in the cell (Koo et al., 2007). In maize, ZmrbohB, encoding a nicotine adenine dinucleotide phosphate oxidase with a role in the generation of reactive oxygen species, undergoes tissue-, developmental- and stress-regulated alternative splicing (Lin et al., 2009). Moreover, cold acclimation in cotton involves alternative splicing of phospholipase Dα, resulting in altered levels of the intermediate signaling molecule
splice variant arises from an intron retention event that introduces a PTC and it was suggested that the resulting shorter isoform facilitates rapid defense responses (Del Carratore et al., 2011).
Together, the examples described above clearly demonstrate the potential that alternative splicing may have on controlling many biological processes in several plant species, underscoring the importance of further research exploring this hypothesis.
1.1.4. The SR protein family
SR proteins are highly conserved splicing factors present in both metazoans and plants. They display a consensus structure consisting of one or two N-terminal RNA-recognition motifs (RRMs) and a C-terminal RS domain rich in arginine and serine residues. The RRM binds the pre-mRNA at ESRs or ISRs and therefore determines pre-mRNA recognition specificity. The RS domain is responsible for the interaction with other proteins during spliceosome recruitment to splice sites and also helps in pre-spliceosome assembly by binding the 5’ splice site and the branch point (reviewed in Ram and Ast, 2007; Barta et al., 2010; and Duque, 2011). As ESRs are found in both constitutively and alternatively-spliced exons, SR proteins are thought to play roles during both constitutive and alternative splicing (Golovkin and Reddy, 1999; Lorkovic et al., 2004b; Tanabe et al., 2006; reviewed in Hertel, 2008; Solis and Patton, 2010).
al., 2004; Delestienne et al., 2010). Interestingly, recent data also suggest a link between alternative splicing and DNA methylation, which is likely to involve the activity of SR proteins (Li et al., 2008; reviewed in Reddy and Ali, 2011). In support of this notion, a report on the analysis of the Human Epigenome Project data indicates that DNA methylation appears to be increased in sequences containing multiple ESEs (Anastasiadou et al., 2011).
Knowledge on the mode of action of SR proteins stems mainly from studies in animal cells but it has been considered to be similar to that operating in plants, since several plant SR proteins have been identified as U1-70K interactors (Golovkin and Reddy, 1999; Lorkovic et al., 2004b; Tanabe et al., 2006). Although, the establishment of an efficient in vitro splicing reaction for plants has hitherto failed, a few plant SR proteins have efficiently complemented HeLa S100 mammalian splicing-deficient cell extracts (Lopato et al., 1996; Lopato et al., 1999a; Ali et al., 2007; Barta et al., 2008).
and attenuating erroneous splice site choices (reviewed in Ram and Ast, 2007).
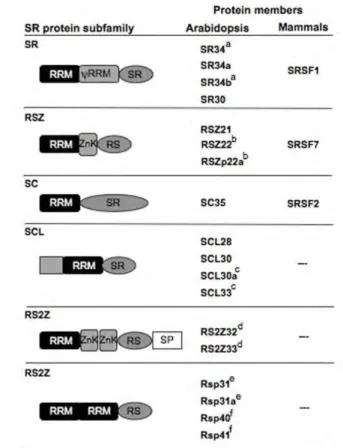
Plant SR proteins can be divided into subfamilies, according to their structural domain organization (Barta et al., 2010). In Arabidopsis, six subfamilies (SR, SC, RSZ, SCL, RS2Z and RS) can be identified, three of which (SCL, RS2Z and RS) are specific to plants and the other three true orthologs of human SR proteins (Fig. 1.4). Within each subfamily, the size and number of exons and introns of the protein-encoding genes are also conserved (Kalyna and Barta, 2004). In addition, some members have arised from gene duplication events, raising the possibility of functional redundancy (Kalyna and Barta, 2004). However, several duplicated gene pairs show different tissue and/or developmental-specific expression patterns, suggesting specific roles for each pair member (Palusa et al., 2007).
phosphorylate SR33, SR45, RSZ21 and RSZ22 (Golovkin and Reddy, 1999), while in tobacco the LAMMER kinase PK12 phosphorylates several SR proteins (Savaldi-Goldstein et al., 2000). Also, a microarray-based proteomic approach in Arabidopsis has identified several SR proteins as possible targets for mitogen-activated protein kinases, MAPKs (Feilner et al., 2005).
Figure 1.4. The Arabidopsis SR protein family.
Schematic representation of the structure of the 18 Arabidopsis SR proteins and their classification into six subfamilies. Same letters indicate duplicated gene pairs, and the nomenclature of the mammalian orthologs is shown. RRM: RNA-recognition motif. ψRRM: RRM with an additional SWQDLKD
motif. RS: arginine/serine-rich. SR: serine/arginine dipeptides rich. SP: serine/proline-rich. The SCL subfamily contains an additional N-terminal domain rich in arginine, proline, serine, glycine and tyrosine residues. ZnK: zinc-knuckle.
Phosphorylation is a fundamental mechanism to regulate overall SR protein family activity. However, other specific protein domains contained within each subfamily can account for additional regulatory mechanisms. For instance, the SC35-like subfamily contains residues rich in arginines, prolines, serines, glycines and tyrosines in its N-terminus. Methylation at arginine residues by protein arginine methyltransferases (PRMTs) is a regulatory mechanism that has been described for RNA-binding proteins, including SR proteins, which may affect their recruitment to splice sites, activity and/or mobility (reviewed in Yu, 2011). In fact, two human SR proteins, SFRS9/SRp30c and SF2/ASF, have been shown to be methylated as a means of controlling their role in mRNA stabilization and export to the cytosol (Bressan et al., 2009; Sinha et al., 2010). In yeast, Hmt1 methylates the U1 Snp1 specific protein and the levels of Snp1 methylation affect its association with the SR-like protein Np13 and splicing efficiency (Chen et al., 2010). Finally, in Arabidopsis PRMT5/SKB1 methylates the U6 snRNP-specific Sm-like protein LSM4, and mutations in PRMT5/SKB1 result in severe defects in splicing (Zhang et al., 2011).
compartments, or in the nucleoplasm, a feature slightly divergent from their animal counterparts (Lorkovic at al., 2004a; Tillemans et al., 2005; Tanabe et al., 2006). Speckles are clusters of interchromatin used as storage, assembly and modification sites for SR proteins when transcription rates are low. SR proteins diffuse to the nucleoplasm upon transcription, in order to associate with the pre-mRNA and participate in its splicing, and shuttle back to the nuclear speckles when transcription slows down (Huang and Steitz, 2001; Fang et al., 2004; Tillemans et al., 2006). Some observations suggest particular shapes for nuclear speckles depending on the cell type or the SR protein analyzed (described for RSp31, SR34, RSZ22 and RS2Z33), but no explanation for this has been proposed (Tillemans et al., 2005; Lorkovic et al., 2008). Shuttling between cellular compartments is also dependent on the phosphorylation status of SR proteins (Rausin et al., 2010; Sanford et al., 2005; reviewed in Stamm, 2008). Indeed, the mobility of these RNA-binding factors is tightly related to transcription and phosphorylation (Ali and Reddy, 2006; Kitsios et al., 2008; reviewed in Reddy and Ali, 2011). In support of their role in mRNA transport and stability during translation, several animal SR proteins have been observed in the cytoplasm (Caceres et al., 1998; Delestienne et al., 2010). The Arabidopsis RSZ22 is so far the sole such case described in plants (Rausin et al., 2010).
cells, the PKA C subunit, involved in the transcriptional activation of cAMP-responsive elements, co-localizes with SC35, phosphorylates SR proteins and is involved in splicing regulation (Kvissel et al., 2007). In Arabidopsis, SR34 colocalizes with TGH, a putative RNA-processing protein, whose loss of function results in several developmental defects (Calderon-Villalobos et al., 2005).
protein gene expression regulation, including the inclusion of PTCs in alternative splice forms to control SR protein activity, may contribute to their ability to select for correct splice sites of a given set of target genes.
The bias of plant alternative splicing to stress-related genes and the existence of a higher number of SR proteins in plants suggest that SR proteins are important mediators of stress responses in plants. In agreement, the splicing pattern of different SR proteins is not only developmentally-regulated but also responsive to external stress stimuli (Tanabe et al., 2006; Palusa et al., 2007). Moreover, SR protein phosphorylation can also be regulated by external cues, pointing to a mechanism of relaying stress signals to these splicing factors. For instance, the MAPK kinases MPK3 and MPK6, which phosphorylate SR proteins, are important mediators in stress signaling (Feilner et al., 2005), and the LAMMER kinase PK12 is involved in ethylene signaling (Savaldi-Goldstein et al., 2000). Furthermore, SR protein dynamics and re-localization have also been shown to be affected by external stress signals (Delestienne et al., 2010; Rausin et al., 2010; reviewed in Lorkovic and Barta, 2004).
overexpression of SR30 and RS2Z33 cause changes in alternative splicing of other SR protein genes and general morphological and developmental abnormalities (Lopato et al., 1999b; Kalyna et al., 2003). In addition, heterologous expression of portions containing the RS domain of two Arabidopsis SR-like proteins (RCY1 and SRL1) has been found to confer salt stress tolerance in yeast (Forment et al., 2002). Disruption of the SR-related SR45 gene results in aberrant growth (Ali et al., 2007) and altered responses to sugars and ABA (Carvalho et al., 2010).
1.1.4.1. The SCL30a SR protein
The Arabidopsis SCL30a belongs to the plant-specific SC35-like (SCL) subfamily of SR proteins (Barta et al., 2010) (Fig. 1.5). Together with SCL33 it arose from a genomic interchromosomal duplication (Kalyna and Barta, 2004) (see Figure 1.4). The SCL30a sequence harbors an RRM similar to the one found in the Arabidopsis and the mammalian SC35 factors. However, its N-terminal end contains an extension rich in arginine, proline, serine, glycine and tyrosine residues, which places it, together with the other SCL members, in a plant-specific subfamily (Barta et al., 2010; Manley and Krainer, 2010). Importantly, the mammalian SC35 (recently renamed SRSF2; Manley and Krainer, 2010) has been shown to regulate alternative splicing in vivo (Merdzhanova et al., 2008; Shi et al., 2008).
speckles and has been reported to interact with itself and many other SR proteins, such as SCL28, SCL30, SC35, RSZ21, SR30, SR34 and RS2Z33 (Lopato et al., 2002; Lorkovic et al., 2008). Intriguingly, SCL30a does not appear to interact with the fourth member of the SCL subfamily, SCL33, which is its duplicated gene pair and interacts with SCL28 and SCL30. The SCL30a SR protein also co-localizes partially with RS2Z33 and SC35 (Lorkovic et al., 2008).
Figure 1.5. The SCL30a SR protein.
Schematic representation of the annotated genomic structure of the SCL30a
gene (boxes represent exons and lines introns, with the coding sequence shown in black) and the corresponding encoded protein. RRM: RNA-recognition motif. SR: serine/arginine dipeptides rich.
Adapted from www.arabidopsis.org and Barta et al. (2010)
1.2. Protein Ubiquitination in Plants
Ubiquitin is a highly conserved small protein present in all eukaryotes (Hershko and Ciechanover, 1986). The covalent attachment of ubiquitin to other molecules is known as protein ubiqutination. It allows specific proteins to be targeted for degradation and constitutes the major proteolytic system in plants (reviewed in Smalle and Vierstra, 2004). Protein degradation is essential for removing abnormal and short-lived regulatory proteins and for amino acid recycling for further protein synthesis, but it also allows the regulation of protein activity in response to stimuli that direct growth and development and/or responses to stress.
The covalent attachment of polymers of the 76-amino acid ubiquitin protein to a specific substrate is performed by three groups of enzymes (E1, E2, and E3) through a conjugation cascade (reviewed in Hershko and Ciechanover, 1998). The ubiquitin molecule has the same amino acid sequence in all higher plants, differing in only two residues in yeast and in three residues in animals, and is ubiquitously expressed as its name indicates (reviewed in Smalle and Vierstra, 2004). Target substrates can be localized in several cellular compartments, such as the cytoplasm or the nucleus, they can be membrane-localized or even found in the endoplasmic reticulum (reviewed in Smalle and Vierstra, 2004 and Haglund and Dikic, 2005). Substrate ubiquitination can be regulated by different signals, via the stimulation of cell surface receptors by external ligands or by protein phosphorylation (reviewed in Hicke and Dunn, 2003 and Haglund and Dikic, 2005).
C-terminus to a cysteine residue in E1 via a thioesther linkage – see Figure 1.6 (reviewed in Hershko and Ciechanover, 1998 and Smalle and Vierstra, 2004). The activated ubiquitin molecule is then transferred to an E2 ubiquitin-conjugating enzyme by a transesterification reaction. Lastly, the ubiquitin-E2 intermediate delivers the ubiquitin molecule to the target substrate using the activity of an E3 ubiquitin-protein ligase. Here, the glycine residue at the C-terminal end of ubiquitin is attached to a lysine residue in the target protein.
Figure 1.6. The ubiquitination/26S proteasome pathway.
Schematic representation of the attachment of a ubiquitin monomer (UB) to a target substrate, through a conjugation cascade performed by the E1, E2 and E3 enzymes. The cycle repeats itself when polyubiquitination is needed. If the chain gets attached at lys-48, the target substrate will be recognized by the 26S proteasome and subsequently degraded.
Adapted from Pickart (2001) and Vierstra (2003)
destruction in the lysosome/vacuole or serve as a signaling intermediate in the control of other processes, such as DNA repair and methylation, histone modifications or transcription (Kraft et al., 2008; reviewed in Hicke and Dunn, 2003 and Haglund and Dikic, 2005). However, it has been more common to observe several repetitions of the ubiquitination cycle, during which a lysine residue in the last ubiquitin molecule attached is used as acceptor. After at least four repeats of the cascade, a polyubiquitin chain will be attached to the substrate (Thrower et al., 2000). Seven lysine residues – lys-6, lys-11, lys-27, lys-29, lys-33, lys-48 and lys-63 – can be used as acceptors but the last two have been the most described (reviewed in Haglund and Dikic, 2005). If the chain is bound at the ubiquitin molecule’s lysine residue 63, activation of downstream events related to protein kinase activation, stress responses or DNA damage repair will occur. In contrast, attachment of the polyubiquitin chain at lysyl-48 results in recognition of the target protein by the 26S proteasome, which will degrade the substrate in an ATP-dependent manner, releasing in the end the ubiquitin monomers (reviewed in Hershko and Ciechanover, 1998). Attachment at lys-48 is the best characterized polyubiquitin mechanism. It is likely that only a subset of the polyubiquitin chain is recognized by the downstream components, which may explain how the number of ubiquitin monomers in the chain and the lysine residues used for ubiquitin binding affect recognition by the corresponding regulated pathways (Thrower et al., 2000).
habit of plants, and their need to rapidly adapt growth and development to changing external conditions, resulted in an ubiquitination pathway with a larger spectrum of action. In support of this notion, disruption of ubiquitination pathway-related components results in dramatic effects on plant growth and development under stress conditions (reviewed in Moon et al., 2004).
2004, Smalle and Vierstra, 2004, and Dreher and Callis, 2007). However, substrates for most of the E3 ligases remain to be identified. Future research in this field may uncover additional biological processes involving the activity of E3 ligases and the ubiquitination pathway.
1.2.1. E3 ligase classification and activity
In Arabidopsis, analysis of the structure of E3 ligase-encoding genes has allowed clustering into nine subfamilies (reviewed in Mazzucotelli et al., 2006). The homology to E6-AP C-terminus (HECT), plant U-box (PUB), CULLIN (CUL), Arabidopsis Skp1-related (ASK), bric-a-brac, tramtrack and broad complex (BTB), CULLIN4-damaged DNA-binding protein (CUL4-DDB) and anaphase promoting complex (APC) are the less represented subfamilies, ranging from five (CUL4-DDB) to 81 (BTB) gene members. The other two subfamilies – really interesting new gene (RING) and cyclin F proteins (F-box) – possess around 500 and 700 members, respectively. The U-box members are related to those harboring RING domains and it is acceptable to consider a single subfamily (RING/U-box) comprising both motifs (Aravind and Koonin, 2000).
particular E3 ligase generally defines its mode of action, since RING E3s generally function as adaptors between the E2 and the target protein, whereas HECTs form a covalent bond with the ubiquitin molecule before transferring it to the substrate. In addition, RING/U-box E3s, in contrast to the HECTs, can form multisubunit complexes with other ligases in order to form an active E3 ligase. These multidomain complexes can be either CULLIN- or RING-based, depending on the motif identifiable in the catalytic module, the domain responsible for E2 binding. The catalytic module of E3 ligases binds the second domain found in these complexes – the adaptor module, responsible for substrate recognition. A single catalytic module can bind different adaptors, increasing the specificity of the E3 ligase complexes. Furthermore, a single E3 ligase can target different substrates, depending for instance on target protein availability. For example, the Arabidopsis AIP2 E3 ligase targets the germination regulator transcription factor ABI3 for degradation, but both genes display low overlap of tissue- and developmental-specific expression patterns (Zhang et al., 2005). Whereas AIP2 is mostly expressed in vegetative and reproductive tissues, ABI3 is preferentially seed-specific, suggesting that AIP2 may have other non seed-related protein targets (Zhang et al., 2005). On the other hand, ubiquitination of a single substrate may depend on the activity of multiple E3s. Degradation of the kip-related protein 1 (KRP1), involved in the control of cell cycle progression, is dependent on both SCFSKP2b and
the RING RKP E3 ligases (Ren et al., 2008).
binding the target substrate to be ubiquitinated (Stone et al., 2005; reviewed in Smalle and Vierstra, 2004).
The best characterized E3 ligase in Arabidopsis is the RING-motif-containing COP1. COP1 is a negative regulator of photomorphogenesis whose activity, based on a microarray analysis, seems to affect direct or indirectly more than 20% of the genome, probably through its activity on the controlled degradation of several transcription factors, thus influencing their target gene expression (Ma et al., 2002; reviewed in Moon et al., 2004). cop1 mutant seedlings exhibit short hypocotyls and photosynthetic activity when developing in the dark, both characteristics of light-grown seedlings (Deng et al., 1991). Under dark conditions, COP1 is found in the nucleus, where it targets for degradation activators of photomorphogenesis, such as the transcription factor long hypocotyl 5, HY5, and the photoreceptor A, phyA (Osterlund et al., 2000; Seo et al., 2004). In the light COP1 shuttles back to the cytoplasm, and the nuclear inducers of light responses get stabilized. It should be noted that COP1 has also been widely studied in humans, where it degrades the tumor suppressor p53 protein (Dornan et al., 2004).
1.2.2. The XBAT family of RING E3 ligases
XBAT E3 ligases contain a RING-HCa domain at the C terminus preceded by an additional recognizable domain at the N-terminus with ankyrin repeats – see Figure 1.7B (Stone et al., 2005). BLAST searches have revealed the existence of this protein structure in humans, mouse, C. elegans, Drosophila and Xenopus (Stone et al., 2005). Further analyses revealed similar proteins in several plant species, including Artemisia desertorum, Lilium longiflorum, Medicago truncatula, Oryza sativa, Populus trichocarpa, Solanum tuberosum, and Vitis vinifera (Prasad and Stone, 2010). The RING-HC type is the second most represented within Arabidopsis RING ligases and also includes COP1. Whereas the RING domain is responsible for E2 binding, the protein-protein interaction ankyrin repeats motif appears to be involved in substrate recognition (Bork, 1993; Lorick et al., 1999). According to Stone and coworkers (2005), the family of RING-HCa domain E3 ligases containing ankyrin repeats comprises seven members – five of which, as mentioned above, compose the XBAT family. The other two members, At3g28880 and At5g13530 (keep on going, KEG), share the XBAT structure but whereas the KEG gene contains an additional serine/threonine kinase domain at the N-terminus (Stone et al., 2005), At3g28880 encodes a protein with a low level of similarity (less than 20%) to the XBAT members (www.arabidopsis.org).
In vitro ubiquitination capacity has been shown for XBAT32, XBAT33, XBAT35 and KEG (Nodzon et al., 2004; Stone et al., 2005; Stone et al., 2006). Negative results were reported for XBAT31 and XBAT34, although this could reflect the use of inappropriate ubiquitination components for these particular ligases in the in vitro assays (Stone et al., 2005).
Figure 1.7. The XBAT family of RING E3 ligases.
(A) Phylogenetic tree of XBAT family members, according to their predicted
amino acid sequences.
(B) Schematic representation of the conserved structure of the XBAT family
of RING E3 ligases. ANK: ankyrin repeats (the number of repeats varies between XBAT members). RING: zinc-finger domain.
Adapted from Nodzon et al. (2004) and Stone et al. (2005)
proteins can act in different hormone signaling pathways, thus controlling multiple physiological processes. In agreement with this notion, XBAT32 also appears to be involved in ethylene-mediated responses to salt stress (Prasad and Stone, 2010).
The involvement of the rice XB3 in innate immunity and of XBAT32 in ethylene and auxin signaling, two hormones not only implicated in plant growth but also in disease resistance, raises the hypothesis of conserved functionalities between XBAT members and their orthologous proteins (Nodzon et al., 2004). XBAT32 expression was also found outside the root system, such as in leaves, stems and anthers, and disruption of its activity results in delayed growth of aerial organs, suggesting a role for XBAT32 in other processes during plant development (Nodzon et al., 2004). Interestingly, the Lily ankyrin repeat-containing protein (LIANK), homologous to the XBAT family, possesses in vitro E3 ligase activity and is required for pollen germination and tube growth (Huang et al., 2006). Huang and coworkers (2006) refer to the XBAT32 expression in anthers, an observation that was not explored by the group reporting it (Nodzon et al., 2004), underscoring the importance of considering conserved functionalities. Surprisingly, LIANK is associated with membrane-enclosed organelles but possesses no amino acid sequence pointing to its localization in transmembrane regions. It was proposed that the ankyrin repeat domain interacts with proteins specific to these organelles, thus targeting the protein to the observed intracellular localization (Huang et al., 2006).
in contrast to what is observed for XBAT32 (Prasad et al., 2010). XBAT34 and XBAT35 are the closest members in the XBAT family (68% identity, 73% similarity) and homologous proteins exist in Oryza sativa, Populus trichocarpa and Vitis vinifera, but none has been characterized (Nodzon et al., 2004; Prasad and Stone, 2010). Interestingly, XBAT34 and XBAT35 may have arisen from a genome duplication event (Nodzon et al., 2004; Stone et al., 2005), which appears to be common within the RING family, and although functional redundancy can be expected, the possibility of unique roles for duplicated genes should not be excluded.
1.2.3. Alternative splicing of E3 ligases
genes, which has been barely addressed in Arabidopsis. Interestingly, from the XBAT family, both XBAT31 and XBAT35 genes undergo alternative splicing (www.arabidopsis.org), but its functional significance has not been investigated.
The human RBCK1 interacts with protein kinase C and possesses E3 ligase activity, but its activity is inhibited when interacting with RBCK2, its alternative splice variant that lacks the RING domain (Tatematsu et al., 2008). In Drosophila, the E3 ligase D-Cbl gene, which plays a role in eye development, also generates two splice variants by alternative splicing (Wang et al., 2010). Despite the fact that the encoded isoforms possess similar structures, the longer isoform, D-CblL, is involved in restricting Epidermal Growth Factor Receptor (EGFR) signaling, whereas D-CblS is associated with Notch signaling restriction (Wang et al., 2010). More recently, alternative splicing of the rat gene encoding the RING-CH 10 E3 ligase was shown to generate two isoforms, both of which are expressed in elongating and elongated spermatids, but while the longer protein is associated with microtubules, the shortest is cytoplasmic (Iyengar et al., 2011). Moreover, the microtubule-associated protein is a functional E3 ligase, whereas the second isoform acts most likely as an adaptor or scaffold protein, as it lacks the RING-finger domain (Iyengar et al., 2011).
Interestingly, the COP1b isoform has a dominant-negative effect on the protein activity of the full-length COP1, thus interfering with the suppression of photomorphogenesis in the dark (Zhou et al., 1998).
1.3. Thesis Outline
The work presented in this thesis aimed at exploring the biological relevance of alternative splicing in higher plants. To this end, a two-fold approach was undertaken, using the model organism Arabidopsis thaliana. Firstly, the physiological significance of an exon skipping event, the least common type of alternative splicing in Arabidopsis, in an E3-ligase-encoding gene was investigated. Secondly, we have functionally characterized an RNA-binding protein belonging to the SR protein family, which plays key roles in alternative splicing, and identified some of its endogenous targets.
In addition to this introductory Chapter 1, this thesis comprises three more chapters:
Chapter 2. XBAT35, a novel Arabidopsis RING E3 ligase
exhibiting dual targeting of its splice isoforms, is involved in
ethylene-mediated regulation of apical hook curvature
this E3 ligase in ethylene control of apical hook formation in etiolated seedlings. Both XBAT35 isoforms are functional in this response, but appear to differentially contribute to apical hook exaggeration in the dark. Finally, using a yeast two-hybrid assay, we isolated five putative XBAT35 ubiquitination substrates.
Chapter 3. The Arabidopsis SCL30a SR protein confers
ABA-dependent salt and osmotic stress tolerance during seed
germination
This Chapter reports the first functional characterization of a loss-of-function mutant for a plant SR protein. Loss- and gain-of-function approaches, as well as epistatic analyses, uncovered a role for the Arabidopsis SCL30a as a negative regulator of the ABA signaling pathway under salt and osmotic stress during seed germination. Furthermore, SCL30a was found to regulate seed size and dormancy, as well as light responses during early seedling development. Importantly, endogenous transcripts targeted by the SCL30a SR protein were identified by means of deep-sequencing-based global transcriptome analysis of wild-type, SCL30a-mutant and SCL30a-overexpressing seeds germinated under salt stress.
Chapter 4. Conclusions and future perspectives
Most of Chapter 2 is the reproduction of the following publication: Carvalho, S.D., Saraiva, R., Maia, T.M., Abreu, I.A., and Duque, P. (2012). XBAT35, a novel Arabidopsis RING E3 ligase exhibiting dual targeting of its splice forms, is involved in ethylene-mediated regulation of apical hook curvature. Mol Plant (DOI: 10.1093/mp/sss048).
A substantial part of Chapter 3 constitutes a manuscript currently under preparation.
1.4. References
Ali, G.S., and Reddy, A.S. (2006). ATP, phosphorylation and transcription regulate the mobility of plant splicing factors. J Cell Sci 119, 3527-3538.
Ali, G.S., Palusa, S.G., Golovkin, M., Prasad, J., Manley, J.L., and Reddy, A.S. (2007). Regulation of plant developmental processes by a novel splicing factor. PLoS One 30, e471.
Anastasiadou, C., Malousi, A., Maglaveras, N., and Kouidou, S. (2011). Human epigenome data reveal increased CpG methylation in alternatively spliced sites and putative exonic splicing enhancers. DNA Cell Biol 30, 267-275.
Aravind, L., and Koonin, E.V. (2000). The U box is a modified RING finger – a common domain in ubiquitination. Curr Biol 10, 132-134.