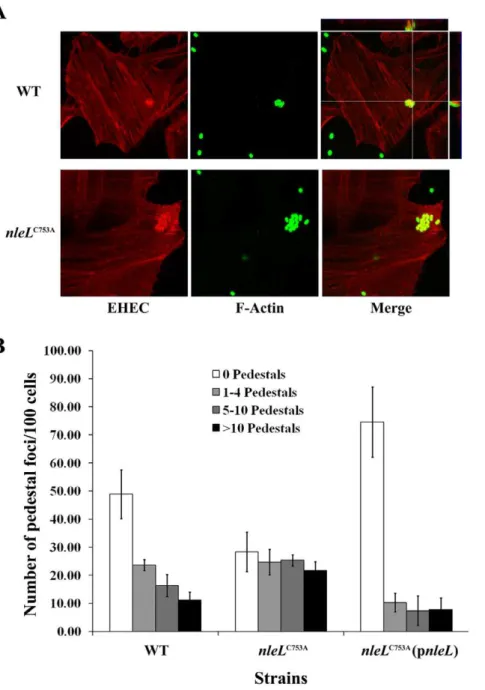
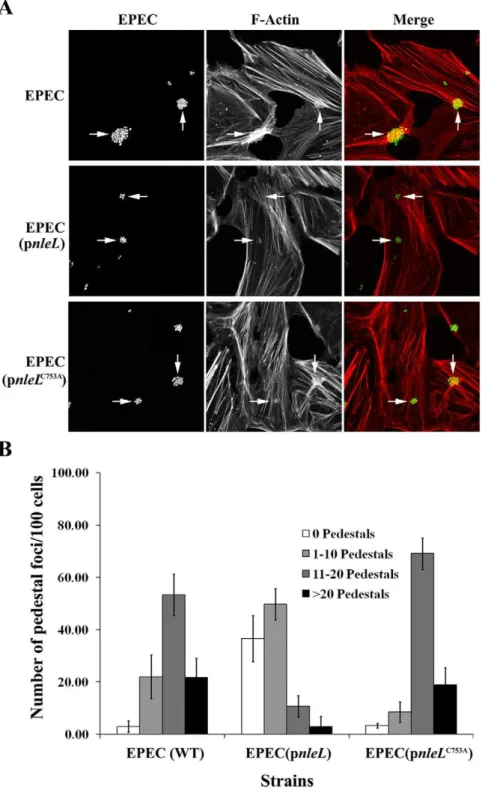
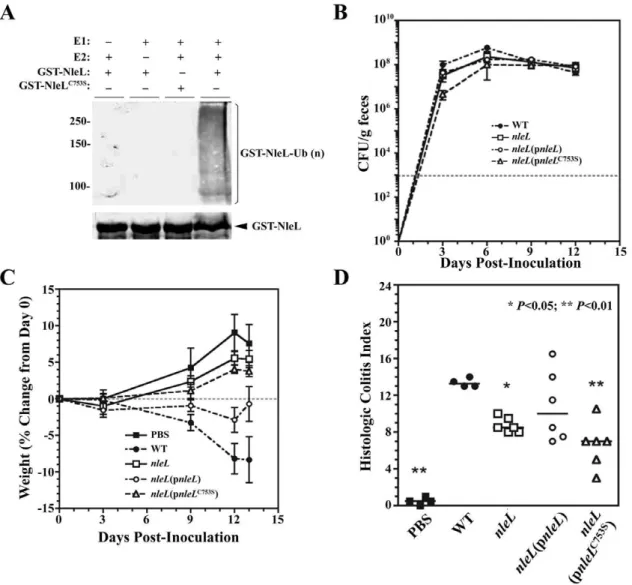
The EHEC type III effector NleL is an E3 ubiquitin ligase that modulates pedestal formation.
Texto
Imagem
Documentos relacionados
Cullin serves as a scaffold protein for the assembly of the multisubunit ubiquitin ligase complex that contains a RING domain protein at its C-terminus and a cullin-specific
Riddley - Notes on the botany of Fernando Noronha.. Desmidiaeeae "in" Symbolae
Human Scribble (Vartul) Is Targeted for Ubiquitin- Mediated Degradation by the High-Risk Papillomavirus E6 Proteins and the E6AP Ubiquitin- Protein Ligase. A strategy for
These genetic and biochemical results suggest that the Rtt101 Mms22 E3 ligase counteracts a function of Mrc1 that is linked to the replicative helicase at stalled replication forks,
When considered together with the failure of ANBR to rescue the Roc1a mutant, these results suggest that, within the context of the male germ line- specific Cul3 E3 ligase complex,
To determine whether the intracellular tail of ICAM-1 is required for the shift of clustered ICAM-1 to the immobile fraction, we used an ICAM-1-GFP mutant that lacks the
Recent- ly, GRAIL (the E3-ubiquitin ligase gene related to anergy in lymphocytes) was shown to be responsible for the Th2 hypore- sponsiveness in a mouse model of
In the susceptibility test (where the diameter of the growth inhibition disc produced by an antibiotic is used to assess whether a strain exhibits complete