J. Braz. Chem. Soc. vol.22 número11
Texto
Imagem
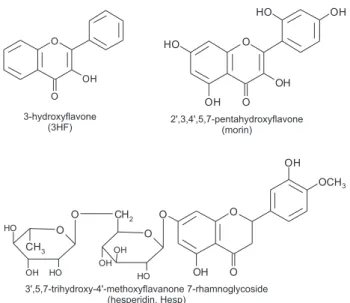

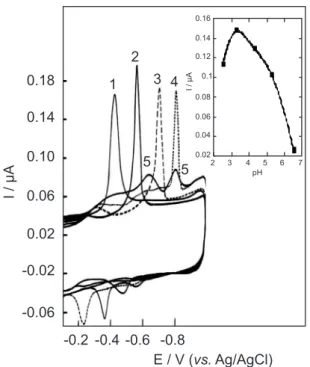

Documentos relacionados
The probability of attending school four our group of interest in this region increased by 6.5 percentage points after the expansion of the Bolsa Família program in 2007 and
Na hepatite B, as enzimas hepáticas têm valores menores tanto para quem toma quanto para os que não tomam café comparados ao vírus C, porém os dados foram estatisticamente
The present work describes the electrochemi- cal behavior of miconazole at mercury electrode in order to obtain an adsorptive stripping voltammetric method for determining the drug
Based on the adsorption behavior of nitazoxanide onto the mercury electrode surface, simple and highly sensitive linear sweep, differential pulse and square wave adsorptive
free linear sweep and square wave adsorptive cathodic stripping voltammetry (LS-AdCSV and SW-AdCSV, respectively) methods are described for trace quantitation of RLX in bulk form,
Therefore, the present work aimed to study the electroreduction of LV.HCl at the hanging mercury dropping electrode (HMDE) and to develop a simple and reliable square-wave
This adsorptive character of the molecule was used to develop a novel, fully validated, rapid, selective and simple differential pulse cathodic adsorptive stripping
47 Based on these considerations and on the thermodynamic data obtained, it is understandable that the adsorption of caffeine onto the surface of the carbon steel occurs


