UNIVERSIDADE FEDERAL DO CEARÁ CENTRO DE CIÊNCIAS
PROGRAMA DE PÓS-GRADUAÇÃO EM QUÍMICA TESE DE DOUTORADO
EDUARDO JOSÉ JUCÁ MALLMANN
GREEN SYNTHESIS OF SILVER NANOPARTICLES TROUGH REDUCING SUGARS AND THEIR ANTIMICROBIAL POTENTIAL
EDUARDO JOSÉ JUCÁ MALLMANN
GREEN SYNTHESIS OF SILVER NANOPARTICLES TROUGH REDUCING SUGARS AND THEIR ANTIMICROBIAL POTENTIAL
Tese apresentada ao curso de Doutorado em Química do Pós-Graduação em Química do Centro de Ciências da Universidade Federal do Ceará, como parte dos requisitos para obtenção do Título de Doutor em Química. Área de Concentração: Físico-Química.
Orientador: Prof. Dr. Pierre Basílio Almeida Fechine
Ao meu amigo Afrânio, autor da inesquecível frase “daqui a dez anos
seremos todos doutores”.
AGRADECIMENTOS
Um doutorado não é construído sozinho, de modo que várias pessoas contribuem para a realização deste projeto. Cabe então destacar aqueles que, de modo relevante, ajudaram na execução da pesquisa.
Primeiramente, agradeço a Deus, a grande fonte de força e inspiração para continuar seguindo em frente.
Agradeço a meus pais pelo incentivo contínuo aos estudos, e por todo o apoio dado a mim, o que é um alicerce tremendo que me permite trilhar caminhos mais ousados.
À minha noiva, Auriana, por sempre me apoiar, dar forças, estar presente, compreender, estudar comigo, me ouvir e ser também uma grande amiga nas horas em que mais preciso.
Ao professor Pierre, como orientador, sempre buscando mostrar o caminho certo, aconselhando e repreendendo nos momentos propícios. Incontáveis foram os e-mails com artigos para se estudar, prazos a cumprir, sugestões de atividades... É um espelho de inspiração e alguém de admirável conhecimento e caráter. Desde que fui seu aluno de físico-química para farmácia, percebi sua grandeza intelectual e como ser humano, e tive a sorte de tê-lo como orientador no mestrado e no doutorado. Como amigo, sei que posso confiar e esperar lealdade, e deixo a recíproca registrada como verdadeira.
Ao meu amigo e colega de doutorado Afrânio Cunha, alguém por quem tenho uma admiração enorme e como modelo de professor desde quando fui seu aluno na graduação. Sua contribuição para a execução deste trabalho de doutorado foi imensurável e certamente sem seu apoio as dificuldades pareceriam fatalmente intransponíveis. Com seu espírito solidário e sua imensa energia e potência de trabalho, suas incansáveis lições me inspiram e fazem continuar e dar o melhor no magistério. Seus intermináveis compartilhamentos de ideias, suas ligações telefônicas nos mais variados horários com propostas de trabalho e de pesquisa sensacionais, suas eternas cobranças o transformam em um verdadeiro modelo de pesquisador a ser seguido.
tardes de estudo, dividindo toda a evolução no caminhar desta disciplina sensacional.
Ao professor da referida disciplina, Dr. Luiz Constantino (“Luizão”) por todo o conhecimento compartilhado não somente técnico e acadêmico, mas verdadeiramente filosófico e como farol orientador para a vida.
À professora Selma Mazzetto por sempre manter abertas as portas de seu laboratório (LPT) para o que quer que precisemos. Além disso, sua ajuda foi importantíssima para uma conquista pessoal minha ao longo desta jornada doutoral. Ainda, ao seu orientando e colega de doutorado Eufrázio Júnior, que sempre se demonstra solícito e ágil para contribuir com análises termográficas.
Da mesma forma, agradeço aos professores Dr. Luiz Gonzaga e Drª Idalina Carvalho por, em um momento inicial e outro projeto de pesquisa do doutorado, ter disponibilizado seu laboratório, material e pessoal para que a pesquisa fosse desenvolvida, além de manterem sempre abertas as portas para as incontáveis análises de espectroscopia de infravermelho. É também por este espírito colaborador que faz com que nosso programa de pós-graduação em Química seja tão bem conceituado.
À professora Drª Nágila Ricardo, pois assim como os professores supracitados, abriu as portas de seu laboratório para que uma pesquisa inicial envolvendo polímeros fosse realizada sob sua supervisão e auxílio. Sou eternamente grato por todo seu suporte e carinho. Da mesma forma, à professora Drª Cristiane Pinto.
Agradeço também ao professor Dr. Antoninho Valentini, pessoa extremamente solícita, que sempre deixou abertas as portas de seu laboratório para que as análises espectroscópicas fossem lá realizadas. Também manifesto minha gratidão aos colegas de doutorado Moacir Júnior e Bárbara Sales, que sempre estiveram disponíveis para ajudar nas análises.
Minha gratidão à colega de doutorado Clara, que muito contribuiu para minha pesquisa realizando as análises de DLS, assim como meu muito obrigado à professora Drª Judith, por ceder espaço para as mesmas.
Ao professor Dr. Ayala e sua aluna Silmara Alves pela ajuda e contribuição nas análises de raios-x e infravermelho.
Às professoras Drª Wladiana Matos e Drª Gisele Lopes, assim como a seu aluno Luan, pelas amostras de análise de espectroscopia de absorção atômica – ICP-OES.
Toda minha gratidão à minha colega de bancada e aluna de iniciação científica, Valdirene Oliveira. As sínteses preliminares do doutorado não seriam as mesmas sem sua companhia, ajuda e otimismo, repetindo sempre o mantra “vai dar certo” a cada tentativa e etapa realizada. Muito obrigado Val!
Agradeço à minha amiga Jéssica Miranda pelo auxílio durante as mesmas sínteses preliminares citadas anteriormente, colaborando no que foi necessário com instruções técnicas e fornecimento de material, além do mais importante: seu tempo.
Meus agradecimentos ao meu amigo Davino, companheiro de caracterização de estabilidade e tamanho de amostras. Sempre solícito e atencioso, devo bastante à sua contribuição.
Minha profunda gratidão ao meu amigo Rafael que sempre ouviu e aconselhou em momentos chave da pesquisa, além de ter sido meu companheiro de estudo e preparação para o concurso de docente da UECE. Sua força e contribuição foram fundamentais e essenciais para que lograsse êxito nesse certame.
Ao meu amigo Victor, companheiro de bancada da primeira etapa de projeto de doutorado, dividindo experiências, expectativas, frustrações, alegrias, conhecimento... Sou grato por toda sua contribuição para este trabalho e desenvolvimento no doutorado.
A todos os integrantes do GQMAT, que formam uma grande família em que todos se apoiam, se ajudam, compartilham momentos diversos e estão sempre contribuindo uns com os outros para o crescimento como pesquisador e como ser humano. Vocês são sensacionais!
com novas possibilidades e novas resoluções de impasses e contratempos, além de novas parcerias firmadas para a caracterização de nossos materiais. Muito obrigado, craques da química!
À Central Analítica do Departamento de Física, onde as análises de microscopia foram cuidadosamente realizadas.
Ao professor Dr. Everardo por, desde minha graduação em Química, permitir o uso de seu Laboratório de Microbiologia da Faculdade de Farmácia da UFC. Sem dúvidas, um grande diferencial nesse doutorado foi seu companheirismo com o desenvolvimento da pesquisa.
A toda equipe de professores do curso de Química da Universidade Estadual do Ceará – Campus Itapipoca pela compreensão e apoio no desenvolvimento deste trabalho de pesquisa doutoral. Em especial, à minha amiga professora Doutora Edinilza Maria Anastácio Feitosa, que deu todo o suporte necessário na coordenação do PIBID, compreendendo a divisão de tempo e de tarefas entre o programa e o desenvolvimento deste trabalho doutoral. Sem este suporte, conciliar o trabalho com o doutorado seria ainda mais árduo, esgotante e beiraria a barreira do intransponível. Ainda ao meu amigo Walber, alguém de uma inteligência ímpar e companheiro de viagem, de ideias e de projetos.
Agradeço ainda a todos os meus alunos que sempre demonstraram entusiasmo em aprender sobre minha pesquisa e desta forma me incentivavam sempre a continuar crescendo acadêmica e intelectualmente para melhor servi-los. O corpo discente do curso de Química da UECE Itapipoca tem minha eterna gratidão e admiração.
Agradeço aos meus amigos externos às Instituições, pois sempre se demonstraram compreensivos quanto ao andamento do meu trabalho e minhas constantes ausências em momentos de confraternização, além de serem sempre interessados no objeto de minha pesquisa. Cabe citar nomes de grande relevância como Helder, Cássio, Roberto, Paulo André, Marcelo, Renar e Airton.
Agradeço à CAPES pela bolsa concedida nos anos iniciais de doutorado e à Universidade Federal do Ceará pela oportunidade de realizar este crescimento profissional, retornando à casa agora como aluno de doutorado.
A todos que, embora não tenham sido citados nestes agradecimentos, contribuíram direta ou indiretamente para a confecção deste trabalho. Sem a ajuda e o apoio de cada um, teria sido bem mais difícil!
RESUMO
Nanopartículas de prata (AgNPs) são materiais promissores em diversas áreas da ciência e tecnologia. Métodos tradicionais de síntese geram resíduos tóxicos e indesejáveis. A síntese verde de AgNPs a partir de açúcares redutores surge como uma opção viável de obtenção deste material. Neste trabalho foram utilizados os açúcares arabinose, glicose, ribose e xilose para obtenção de AgNPs, além dos sais de sódio citrato e dodecil sulfato como agentes estabilizantes. A caracterização destes materiais foi realizada por meio das técnicas de espectroscopia de absorção na região do UV-Vis – identificando a banda plasmônica – difração de raios X, espalhamento dinâmico de luz, potencial zeta, microscopia eletrônica de varredura, microscopia de força atômica e espectroscopia de absorção na região do infravermelho. As soluções apresentaram boa estabilidade (potencial zeta médio de -46,4mV), partículas com tamanhos que variaram de ±10nm a ±100nm e diferentes morfologias de grãos. A partir das AgNPs sintetizadas, materiais – filmes de ágar e gel de carbômero – foram obtidos para serem caracterizados e aplicados. A aplicação das soluções de AgNPs e destes materiais obtidos foi majoritariamente na verificação de atividade microbiológica contra fungos de diferentes espécies de
Candida spp e Aspergillus spp, além das bactérias patogênicas Escherichia coli, Staphylococcus aurus e Pseudomonas aeruginosa. Buscou-se ainda investigar os efeitos de associação entre as AgNPs e o Itraconazol, um medicamento disponível para tratamento de infecções fúngicas. Todas as nanopartículas que foram aplicadas tiveram atividade antibiótica eficaz contra os microrganismos, bem como os materiais desenvolvidos também apresentaram resultados satisfatórios. As AgNPs apresentaram ainda resultados que apontam para a potencialização do antifúngico Itraconazol, quando utilizado combinado com as nanopartículas. A relação custo x benefício da síntese de AgNPs, suas características e suas aplicações tornam o material desenvolvido nesta pesquisa um potencial coadjuvante no combate aos microrganismos multirresistentes.
ABSTRACT
Silver nanoparticles (AgNPs) are promising materials in many areas of Science and technology. Traditional methods of synthesis generate undesirable and toxic residues. The green synthesis of AgNPs starting from reducing sugars emerges as a viable option of obtaining this material. At this present work, there were used the sugars arabinose, glucose, ribose and xylose to obtain AgNPs, as well as sodium citrate and sodium dodecyl sulfate as capping agents. The characterization of these materials were done trough the UV-Vis absorption – identifying the plasmon band – X-ray diffraction, dynamic light scattering, zeta potential, scanning electron microscopy, atomic force microscopy and infrared spectroscopy techniques. The solutions showed a good stability (mean zeta potential -46.4mV), particles size ranging from ±10nm to ±100nm and different grains morphology. Starting from synthesized AgNPs, materials – agar films and carbomer gel – were obtained to be characterized and applied. The application of AgNPs solutions and derivate materials was predominantly at verifying of microbiological activity against fungi of different species of Candida spp and
Aspergillus spp, as well as pathogenic bacteria such Escherichia coli,
Staphylococcus aureus and Pseudomonas aeruginosa. It was also investigated the effects of association between AgNPs and Itraconazole, a medicine to be used against fungal infections. All the nanoparticles applied showed antibiotic activity against the microorganisms, as well as the developed materials. The AgNPs showed also results that aim to the potentiation of Itraconazole, when combined with nanoparticles. The relation cost x benefits of the synthesis of AgNPs, its characteristics and applications turn the developed material on this research a potential adjunctive in the battle against multi-resistant microorganisms.
LIST OF ILLUSTRATIONS
Chapter 1
Figure 1.1 – Different scales of materials and organisms 20 Figure 1.2 –Evolution of “nanoresearch” along 15 years 21 Figure 1.3 – Silver nanoparticles solutions: different sizes show different
colors 24
Figure 1.4 – Chemical structure of sugars monomers: gliceraldehyde (a),
glycogen (b), starch (c) e cellulose (d) 27
Figure 1.5 – L-arabinose (a), D-ribose (b) and D-xylose (c) 28 Figure 1.6 – Last fifteen years of publishing works involving the term silver
nanoparticles 30
Figure 1.7 – Physical and chemical methods of nanoparticles synthesis 32 Figure 1.8 – Stabilization: electrostatic (a), steric (b) and electrosteric (c) 34 Figure 1.9 – Stabilization of AgNP trough polymer (a), surfactant (b) and
ligand (c) 35
Figure 1.10 – Sodium citrate (a) and sodium dodecyl sulfate (b) 35 Figure 1.11 – Silver nanoparticles with capping agent (a) and without it
(b) 36
Figure 1.12 – Plasmon band 37
Chapter 2
Figure 2.1 – AgNP synthesis: illustrated steps (citrate series). 54 Figure 2.2 – Solution before (left) and after (right) centrifugation. 55 Figure 2.3 –Aspergillus fumigatus (a), A. terreus (b) and A. niger (c). 55 Figure 2.4 – UV-Vis absorption of AgAC-01, AgAC-02 and AgAC-03. 57 Figure 2.5 – UV-Vis absorption of AgRC-01, AgRC-02 and AgRC-03. 58 Figure 2.6 – UV-Vis absorption of 01, 02, 03,
AgXC-04, AgXC-05 and AgXC-06. 59
Figure 2.13 – Zeta potential of AgXC-06 sample. 67 Figure 2.14a – SEM images from AgRC-01 at 78,022x. 69 Figure 2.14b – SEM images from AgRC-01 at 424,973x. 70 Figure 2.15a – SEM images for AgRC-02 at 100,000x. 71 Figure 2.15b – SEM images for AgRC-02 at 187,802x. 71 Figure 2.16a – SEM images for AgRC-03 at 100,000x. 72 Figure 2.16b - SEM images for AgRC-03 at 200,000x. 73 Figure 2.17 – Histogram of AgRC-01 (a), AgRC-02 (b) and AgRC-03 (c)
samples. 74
Figure 2.18 –Aspergillus fumigatus: control (a), with AgRC-01 (b),
AgRC-02 (c) and AgRC-03 (d). 75
Figure 2.19 –Aspergillus terreus: control (a), with AgRC-01 (b), AgRC-02
(c) e AgRC-03 (d). 76
Figure 2.20 – Aspergillus niger: control (a), with AgRC-01 (b), AgRC-02
(c) e AgRC-03 (d). 77
Figure 2.21 – Growing of A. niger along six days. 79 Figure 2.22 – Growing of A. terreus along six days. 80 Figure 2.23 – Growing of A. fumigatus along six days. 81 Chapter 3
Figure 3.1 – Identification of C. tropicalis (a) and C. albicans (b) on
chromogenic medium. 87
Figure 3.2 – AgNP solution. 88
Figure 3.3 – UV-Vis specter of AgNP. 89
Figure 3.4 – Size distribution by volume (DLS). 89 Figure 3.5 – Fungicidal activity of AgNP: marked as R (AgNP synthesized by ribose), marked as G (AgNP synthesized by glucose – for comparative purposes, only) and marked as A (amphotericin B). 91 Chapter 4
Figure 4.1 – AgFilm. 102
Figure 4.2 – UV-Vis spectroscopy of AgNP and AgFilm. 103
Figure 4.3 – DLS. 104
Figure 4.4 – FTIR spectroscopy. 105
Figure 4.6 – SEM of AgFilm (a), EDX of AgFilm (b) and histogram for
AgNP base on SEM (c). 108
Figure 4.7 – AgFilm AFM (a), AgFilm AFM on topographic mode (b) and
histogram for AgNP based on AFM. 109
Figure 4.8 – Halos around AgFilm (a) and (b) and halo measurement
average (c). 111
Chapter 5
Figure 5.1 – Obtaining the gel functionalized with silver nanoparticles. 120 Figure 5.2 – UV–VIS absorption spectra of AgNPs. 123 Figure 5.3 – XRPD patterns of the AgNPs and diffraction peaks from JCPDS (04-0783) used for identification and comparison. 124 Figure 5.4 – SEM image of the AgRC-03 at an amplification of
100,000X(a) and 238,000X (b) and size distribution histogram (c). 125 Figure 5.5 – Shear stress (a), viscosity x deformation (b) and viscosity x
time (c). 126
Figure 5.6 – Chemical stability of AgNP during the stability testing of gel
(1 and 2%) at 25°C (a) and 40°C (b). 130
Figure 5.7– (a) Antibacterial activity against Staphylococcus aureus for 0.5% AgNP gel (marked as 1), 1.0% AgNP gel (marked as 2), 2.0% AgNP gel (marked as 3) and 1.0% silver sulfadiazine (marked as 4). (b) Antibacterial activity against Escherichia coli for gel without AgNPs (marked as 5), 1.0% silver sulfadiazine (marked as 6), 2.0% AgNP gel (marked as 7) and 1.0% AgNP gel (marked as 8). (c) Antibacterial activity against Pseudomonas aeruginosa for 0.5% AgNP gel (marked as 9), 1.0% AgNP gel (marked as 10), 2.0% AgNP gel (marked as 11)
and 1.0% silver sulfadiazine (marked as 12). 132 Chapter 6
Figure 6.1 – UV-Vis spectrum 144
Figure 6.2 – DLS of nanoparticles showing (a) centrifuged AgNPs, (b) AgNPs + Itraconazole 3.2 µg mL-1 and (c) AgNPs + Itraconazole 4.8 µg
mL-1. 146
Figure 6.4 – SEM of (a) centrifuged AgNPs only, (b) AgNPs + ITR 3.2 µg
mL-1, and (c) AgNPs + ITR 4.8 µg mL-1. 149
Figure 6.5 – EDX of (a) centrifuged AgNPs only, (b) AgNPs + ITR 3.2 µg
mL-1, and (c) AgNPs + ITR 4.8 µg mL-1. 150
Figure 6.6 – Histogram of (a) centrifuged AgNPs only, (b) AgNPs + ITR 3.2 µg mL-1, and (c) AgNPs + ITR 4.8 µg mL-1 151 Figure 6.7 – SEM images of samples of centrifuged AgNPs (a) and its compositional map shown at (b). SEM of AgNPs + ITR 3.2 µg mL-1 (c) and its compositional map (d). Finally, SEM of AgNPs + ITR 4.8 µg mL-1
(e) and its compositional map (f). 153
Figure 6.8 – Chromogenic medium to identify C. parapsilosis. 154 Figure 6.9 – Sensibility test of C. parapsilosis against six different combinations. From twelve o’clock and clockwise: ITR 4.8 mg mL-1, centrifuged AgNPs, ITR 3.2 mg mL-1, ITR 80 mg mL-1, AgNPs + ITR 4.8 mg mL-1 and ending, AgNPs + ITR 3.2 mg mL-1. 155 Figure 6.10 – Itraconazole as control drug and its associations with silver
nanoparticles. 156
TABLES LIST
Chapter 2
Table 2.1 – AgNPs samples and temperature of synthesis. 54 Table 2.2 – Particle medium size according FWHM. 63 Table 2.3 – Size distribution by intensity of light scattering. 66
Table 2.4 – Zeta potential values 68
Table 2.5 – Growing of A. niger versus time 79 Table 2.6 – Growing A. terreus versus time. 80 Table 2.7 – Growing of A. fumigatus versus time. 81 Chapter 3
Table 3.1 – Effect of AgNPs produced by green synthesis and Amphotericin B against C. albicans and C. tropicalis. 90 Chapter 5
Table 5.1 – Physicochemical characteristics of 1.0% and 2.0% Gel-AgNP
stored at 25ºC /60%RH. 128
Table 5.2 –Physicochemical characteristics of 1.0% and 2.0% AgNP gel
stored at 40ºC /75%RH. 128
Table 5.3 – Chemical stability of AgNP gel (1.0 and 2.0%) during
SUMMARY
Chapter 1
1 INTRODUCTION 20
1.1 Nanoscience and nanotechnology 20
1.2 Silver nanoparticles 23
1.3 Green synthesis and reducing sugars 24
1.4 Multi-resistant microorganisms 29
2 LITERATURE REVIEW 30
2.1 Silver nanoparticles 30
2.1.1 Synthesis of silver nanoparticles 32
2.1.2 Capping agents used to stabilize silver nanoparticles 33
2.1.3 Plasmon band 37
3 OBJECTIVES 38
3.1 General objective 38
3.2 Specifics objectives 38
3.2.1 Characterization of AgNP trough 38
3.2.2 Obtaining and characterization of composite materials with
AgNPs 38
3.2.3 Perform tests as 38
REFERENCES 39
Chapter 2
1 INTRODUCTION 53
2 EXPERIMENTAL 53
2.1 Materials 53
2.2 Methods 53
2.2.1 Concentrating AgNPs 55
2.2.2 Preliminary antimicrobial assays 55
3 CHARACTERIZATION 56
3.1 UV-Vis spectroscopy 56
3.2 X-ray diffraction (XRD) 56
3.3 Dynamic light scattering (DLS) and zeta potential 56
4 RESULTS AND DISCUSSION 57
4.1 UV-Vis spectroscopy 57
4.2 XRD 64
4.3 DLS and zeta potential 65
4.4 SEM 69
4.5 Antimicrobial activity 75
5 CONCLUSIONS 82
REFERENCES 83
Chapter 3
1 INTRODUCTION 86
2 EXPERIMENTAL 87
3 CHARACTERIZATION 88
4 RESULTS AND DISCUSSION 88
5 CONCLUSION 92
REFERENCES 93
Chapter 4
1 INTRODUCTION 98
2 EXPERIMENTAL 99
2.1 Materials 99
2.2 2.2 AgNPs and AgFilm fabrication 99
3 CHARACTERIZATION 99
3.1 3.1 Ultraviolet-visible (UV-Vis) spectroscopy 99
3.2 Size 99
3.3 Fourier transform infrared (FT-IR) spectroscopy 100
3.4 Powder X-ray diffraction (PXRD) 100
3.5 AgFilm thickness 100
3.6 Microscopic features 100
3.7 Antifungal activity of AgFilm 101
4 RESULTS AND DISCUSSION 102
5 CONCLUSION 111
REFERENCES 112
Chapter 5
2 EXPERIMENTAL 119
2.1 Materials 119
2.2 Methods 120
2.2.1 Synthesis and functionalization of AgNPs 120
2.2.2 AgNPs characterization 121
2.2.3 Formulation of AgNPs based gel. 121
2.2.4 Antibacterial activity 121
2.2.5 Stability evaluation 122
3 RESULTS AND DISCUSSION 123
3.1 AgNPs characterization 123
3.2 AgNPs based gel and properties 126
4 CONCLUSION 133
REFERENCES 134
Chapter 6
1 INTRODUCTION 140
2 EXPERIMENTAL 141
2.1 Materials 141
2.2 Methods 141
2.2.1 Synthesis of AgNPs 141
2.2.2 Association of AgNPs with Itraconazole 141
2.2.3 Antifungal activity of AgNPs + ITR 142
2.2.4 Statistic data 142
2.3 Characterization 142
2.3.1 UV-Vis spectroscopy 142
2.3.2 Infrared spectroscopy 143
2.3.3 X-ray diffraction spectroscopy 143
2.3.4 Dynamic light scattering 143
2.3.5 Scanning electron microscopy 143
3 RESULTS AND DISCUSSION 144
4 CONCLUSIONS 158
CHAPTER 1
CHAPTER 1 – NANOTECHNOLOGY, SILVERNANOPARTICLES AND GREEN SYNTHESIS: AN OVERVIEW
1 INTRODUCTION
1.1 Nanoscience and nanotechnology
Richard Feynman, who is considered the father of nanoscience, made the following question into a lecture at Annual Meeting of American Physical Society (1959): - “Why can't we write the entire 24 volumes of the Encyclopedia Britannica on the head of a pin?” [1]. It is true: it is not possible yet. However, a lot of progress in nanoscience had been obtained, on a way that this science reveals a vast area of researches and applications to be done. Once it is possible to control the characteristics of nanosized particles, the properties of these materials and their functions in different devices may be considerably improved. The term Nanotechnology was first pronounced by the Japanese professor and researcher Norio Taniguchi, in 1974, to describe the technological and scientific progress of these reduced materials [2].
Nanometer (nm = 10-9m) may be realized as the billion part of the meter. A material is considered nanosized when its dimensions area among 0.1 and 100 nm. Figure 1.1 shows a comparison of different materials that exist in various scales.
Figure 1.1 – Different scales of materials and organisms.
To verify the state of the art, a research on the scientific database
ScienceDirect (http://www.sciencedirect.com) reveals the arising of researches envolving the term “nano”, in a projection of the earlier 2000’s to 2015, shown at Figure 1.2
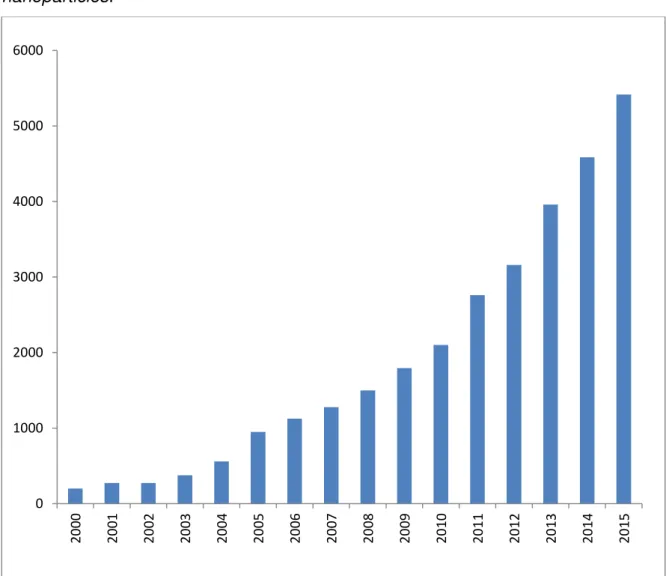
Figure 1.2 –Evolution of “nanoresearch” along 15 years, till 2015.
Source: author
The data shown help us to understand, in a certain way, the importance of nanotechnology along the last years. The performed search reveals that, back at 2000, there were 2,546 published works about the subject. Five years later, this number arises to 10,143 and at the end of 2015, there were 33,055 works on the referred site! This represents a considerable arising of ten times over the beginning of researches.
Then, after searching for the term nanotechnology, it were found 1,043 Brazilian works – at Scopus database (http://www.scopus.com) – from a total of 104,394 published papers. This discrete participation of Brazil represents 0.99%, against 32.16% (USA) and 12.98% (China). Despite having few publications, Brazil leads in the South America. The second country that publishes in a
considerable way is Argentina, with 238 works, which traduces a 0.22%. Brazil occupies the 20th position when the searched term is nanotechnology. Still on this database, when the terms nanoparticles and nanomedicine are searched, it is found 33,387 published works from the first and 8,371 from the last one, which Brazil contributes with 482 and 161 publications, respectively.
These numbers show the running for the technological development on this area of science, and Brazil, despite occupies 20th position among all countries, is working to contribute continuously. Somehow, it is a reasonable overview, when we consider that only in 2004 our country began massive investments on this area [4]. Recently [5] Brazil had firmed a partnership with The US to the Nanotechnology, Energy and Materials Innovation Consortium. This partnership consolidates an investment made by Federal Government, trough Ministry of Science and Technology, of R$ 100 million to the three years 2012-2015 [6].
The research in nanotechnology produced considerable progresses on treatment of several diseases, which originated a new area: nanomedicine. Autoimmune diseases such as rheumatics, conjunctive tissue disorders, neurological, endocrine and dermatologic disorders are now subjects of researches in nanomedicine [7], such as cardiovascular diseases, [8], infections [9] and neoplastic ones, promoting also progress in veterinary medicine.
Zhu and co-workers [10] cataloged several activities and applications of nanoparticles in microbiological infections, especially at diagnoses, treatment, medical devices and vaccines.
The size of nanoparticles or nanodevices applied in nanomedicine is decisive, once they act in cell scale, and many times entering in cells to perform effective activity.
So, today it is understood that nanotechnology is a very promising area of Science, in a way that it expands possibilities to obtain new materials with different properties when nanoscaled [26]. It is very important that researches be performed and constantly encouraged, aiming the production of new materials that supply the demand of industry, academy, health and a lot of areas that may be beneficed.
1.2 Silver nanoparticles
Silver - atomic number 47 and molecular weight 107.86 g mol-1 - is a noble metal found in the earth's crust in the form of minerals such as argentita (Ag2S), chloride (AgCl) or metallic silver [27]. An efficient way to enhance and
improve the biocompatibility of materials is to reduce its size. Nanomaterials once obtained can be modified to improve its efficiency and applicability in different areas such as bioscience, biomedicine and biomaterials.
Among different possibilities of obtaining and using nanomaterials, there are silver nanoparticles. Like other nanostructured particles of other metals, their physico-chemical properties differ from silver metal [28].
Figure 1.3 – Silver nanoparticles solutions: different sizes show different colors.
Source: http://nanocomposix.com/collections/silver (access may 13th 2015)
1.3 Green synthesis and reducing sugars
With the advances in research for obtaining new materials and modernization of industrial processes, there is an increasing demand for raw matter to accompany this development. Furthermore, there is a constant concern for the type of material used in these processes, since it seeks to reduce the toxic and polluting them. In this way, a so-called Green Synthesis technique had been developed over the years to minimize such effects [29-33].
Green Synthesis involves processes that should be in accordance with the Twelve Principles of Green Chemistry [34]. They are the prevention, saving atoms, synthesis of less dangerous products, product design safe, safer solvents and auxiliary, search for energy efficiency, use of renewable raw material, avoiding the formation of derivatives, catalysis, drawing for degradation, real-time analysis for the prevention of intrinsically safe and chemical pollution to prevent accidents . In this way, some methods of green synthesis of silver nanoparticles are cited in the study by Sharma and colleagues [35], and are also highlighted:
cases, also as reducing agents. An example is the use of -D-glucose as reducing sugar and starch as a stabilizing agent, used simultaneously under slight heating [33]. The use of starch prevent the use of toxic organic solvents [36]. These produced nanoparticles can be easily integrated into biological and pharmaceutical use systems.
b) Tollens method: the Tollens reaction basically is to reduce the silver from the diaminesilver complex - [Ag(NH3)2]+ - by an aldehyde. The reaction can be envisaged as:
[Ag(NH3)2]+(aq) + RCHO(aq) → Ag(s) + RCOOH(aq)
It is possible to considerably reduce the concentration in the modified procedure of the Tollens reaction and the silver ions are then reduced by the saccharide in the presence of ammonia, yielding silver nanoparticle films with sizes ranging from 50 to 200 nanometers.
c) Irradiation method: a series of irradiation methods - laser, microwave, radiolysis, photolysis - can be used to reduce silver ions and to produce nanoparticles. It is common, for example, using laser in an aqueous solution containing silver salt and a surfactant. This technique can produce nanoparticles with well-defined shapes and uniform size distribution [37]. This technique does not need reducing agents. The replacement of the laser source with a mercury lamp it is also possible to produce nanoparticles [38], and the visible light radiation employment [39].
e) Polyoxometalates method: this method has the potential to synthesize silver nanoparticles due to the fact that the polyoxometalates (POM) are water soluble and have the processor capacity to polielectronics reduction-oxidation reactions (more electrons en-ware) without a disorder in their [42, 43]. structures. In this synthesis, the POM act as photocatalytic agents, reducing and stabilizing agents, which confers a high efficiency of the method. Zhang and colleagues [44] conducted a study of synthesis and satisfactory stabilization of gold nanoparticles from polioxometalates.
Figure 1.4 – Chemical structure of sugars monomers: gliceraldehyde (a), glycogen (b), starch (c) and cellulose (d).
H
O
H
OH
O
H
(a) O OH OH OH OH (b)O
H
O
H
H
O
H
O
H
O
H
(c) O OH OH
O
H
OH
(d)
Source: author
The monosaccharaides can be further classified as aldoses or ketoses. Both can be classified according to the number of carbons in its chain. Aldoses with four, five or six carbon atoms are termed tetroses, pentoses and hexoses, and successively. The ketoses are classified as the ketotetroses, ketopentoses and ketoexoses for the same number of carbon atoms above-mentioned mind. It is a common occurrence among sugars that are isomers, once asymmetric carbons (stereogenic centers) can be found in these saccharides. Among these possibilities of isomers, we highlight the epimers, which are diastereoisomers: structures that differ in the configuration of the chiral carbon only.
Figure 1.5 – L-arabinose (a), D-ribose (b) and D-xylose (c).
O
H
O
H
O
H
H
H
H
O
H
O
H
(a)O
H
O
H
H
H
H
O
H
O
H
O
H
(b)O
H
H
H
O
H
H
O
H
O
H
O
H
(c) Source: authorIn this context, the use of reducing sugars for the production of the silver nanoparticles is within the expected of green chemistry. These sugars may be aldoses or ketoses. The aldehydes undergo oxidation to form carboxyl groups characteristic of acids. This reaction, also, is the basis for the verification of aldoses. The ketoses, when isomerize as aldoses also assume the character of reducing sugars [45]. In redox reactions, in so far as the sugars are oxidized, another participant of reaction is reduced. Thus, the silver ions present in solution are reduced by these saccharides, and reduction half-reaction may be satisfactory written by Equation 1.1:
1.4 Multi-resistant microorganisms
According to the World Health Organization (WHO), resistance to antimicrobials occurs when a microorganism does not respond to the originally drug developed for the treatment of infections caused by the same. These microorganisms (bacteria, fungi, viruses and parasites) are resistant to the attack of antimicrobial agents, so the standard treatments become ineffective and the infection continues, increasing the risk of spreading to others [46].
Also according to the WHO, the evolution of resistant strains is a natural phenomenon that occurs with microorganisms which erroneously replicate or when resistance traits are exchanged between them. Indiscriminate use of antibiotics accelerates the higher incidence of multidrug-resistant strains. In addition, poor practices of infection control, inadequate and poor sanitary quality conditions in the treatment of foods contribute to the propagation of antimicrobial resistance.
Bacteria are simple, unicellular organisms and prokaryotes, what implicates that their genetic material is spread in the cytoplasm. Since fungi are eukaryotes and more complex bacteria, they may present as uni or multicellular beings. The first are the yeasts: simpler forms of fungi and larger than bacteria. The second are molds: visible and are due to the formation of a mass called mycelium and comprises several filaments called hyphae [47].
The antimicrobial agents used against bacteria and fungi can be classified as antibiotic and antifungal agents, respectively. Both pathogens may develop resistance to antimicrobials. This condition represents a health risk, since the infection by these pathogens can be fatal.
2 LITERATURE REVIEW 2.1 Silver nanoparticles
It is possible to understand that silver nanoparticles – AgNP – had been widely studied along last years, according to data obtained at Science Direct database, showed at Figure 1.6.
Figure 1.6 – Last fifteen years of publishing works involving the term silver nanoparticles.
Source: author
The silver nanoparticles are highlighted for showing uncommon physical-chemical and biological properties. This leads to an extensive application in health care. A lot of products are available, such as materials for surgery and prosthesis [48]. Still according to Heilman, the studies involving silver nanoparticles in Brazil are not significant yet, and need a massive investment to grow according to the demands.
When investigating the diverse possibilities of applications of silver, it can be highlighted its employment on electronic devices, which aims to obtain best responses in conduction [49-51], catalyst [52, 53], increase the efficiency of paints and prototypes in printers [54-56], obtaining of paint with bactericide properties [57], confection of wound healing dressings with anti-inflammatory and antimicrobial properties [58-60], functionalization of tissues on textile industries [40-42], cosmetic industries [44], as sensor to identify, qualitative and quantitative, other molecules [45-48], despite its large use as controlling of microbial growth [49-54].
number of surface atoms will lead to an antimicrobial particle compared with the massive metal [75].
The silver nanoparticles are commonly firstly characterized by UV-Vis spectroscopy, through the appearing of plasmon band, which max will depend on the shape and size of particles [80-82], as well as the composition of system [83, 84].
2.1.1 Synthesis of silver nanoparticles
The nanoparticles may be obtained by physical (top down) or chemical (bottom up) processes. Simplifying, on physical methods, the nanoparticles are obtained through maceration of a macro-material. By the other way, chemical processes involve the manipulation of material at atomic scale, which offers a higher size and properties control [85]. Figure 1.7 shows a short scheme differencing both processes.
Figure 1.7 – Physical and chemical methods of nanoparticles synthesis
Source: adapted from Santos [86].
One way to provide the synthesis is by using plants. In a work performed in 2011, Vidhu and co-workers [87] synthesized AgNPs trough an aqueous extract of Macrotiloma uniflorum. A similar study was made by Rastogi and Arunachalam [88], when they synthesized silver nanoparticles by using garlic –
Allium sativum – aqueous extract and sunlight, and evaluated the antibacterial potential of the obtained nanoparticles. Moldovan and co-workers [89] synthesized AgNPs trough Sambucus nigra and studied its antioxidant activity. Other works had shown a tendency to use aqueous extract of plants to synthesize silver nanoparticles [90-93].
Another way is trough photo induction. El-Sherbiny and co-workers [94] synthesized silver nanoparticles trough a photo-induced process obtaining functionalized silver nanoparticles. Patra [95] worked on a photo-mediated synthesis of silver nanoparticles. Also, antioxidant, antibacterial and anticandidal activities were investigated. A similar work was performed by Verma [96]. In this last work, the system containing AgNPs was synthesized by exposing the mixture to sunlight over 45 min, and the yield increased each minute of synthesis.
It is possible to synthesize silver nanoparticles also trough a micro-wave assisted process, as performed Pal [97]. On his work, he used ethanol as reducing agent. Hydrotermal synthesis is possible as well [98] as sonochemical technique [99, 100] to obtain silver nanoparticles. However, the traditional method to synthesize silver nanoparticles is trough sodium borohydride [101-106]. This method is largely employed, but generates toxic products and does not attend the green synthesis purpose.
2.1.2 Capping agents used to stabilize silver nanoparticles
Figures 1.8a, 1.8b and 1.8c show different types of stabilization of nanoparticles, such as electrostatic, steric and electrosteric.
Figure 1.8 – Stabilization: electrostatic (a), steric (b) and electrosteric (c).
(a) (b) (c)
Source: author, inspired on Santos [86].
It is important to consider the kind of stabilizer, once there are some possibilities of stabilize the nanoparticles. The capping agent must be able to avoid the van der Waals forces that exist between the particles, and this mechanism may be done by three ways: electrostatic, showed at Figure 1.8a, which there are electric forces surrounding the nanoparticle and the repulsion among them produces the stability in solution. The electrostatic stabilization occurs when there are ions adsorbed at the surface of metallic nanoparticles. This adsorption is multilayer, and originates a coulombic repulsive force among the AgNPs. Another way is the steric stabilization, as shown in Figure 1.8b, which the stabilization does not occur because of electrostatic forces, but it is intrinsically about the steric occupation of spaces, and this steric hindrance avoids the contact among nanoparticles, which also confers to the system stability at solution. This kind of stabilization is as better as higher voluminous is the ligand. And as last, the electrosteric stabilization, as shown at Figure 1.8c, which works with both principles: electrostatic and steric. This kind of stabilization may be observed at polioxoanions [109, 110].
Figure 1.9 – Stabilization of AgNP trough polymer (a), surfactant (b) and ligand (c).
(a) (b) (c)
Source: author, inspired on Santos [86].
Therefore, the capping agents play a very important role on the synthesis of nanoparticles as a whole. There are a lot of capping agents. Two of these capping agents are objects of this work: sodium citrate and sodium dodecyl sulfate, illustrated in Figure 1.10.
Figure 1.10 – Sodium citrate (a) and sodium dodecyl sulfate (b).
O
-O
O
H
O
-O
O
O
-Na
+Na
+Na
+ (a)C
H
3O
S
O
O
O
-10
Na
+ (b) Source: authorFigure 1.11 – Silver nanoparticles with capping agent (a) and without it (b).
Source: author (inspired in the work of Shabbeer and co-workers [113])
2.1.3 Plasmon band
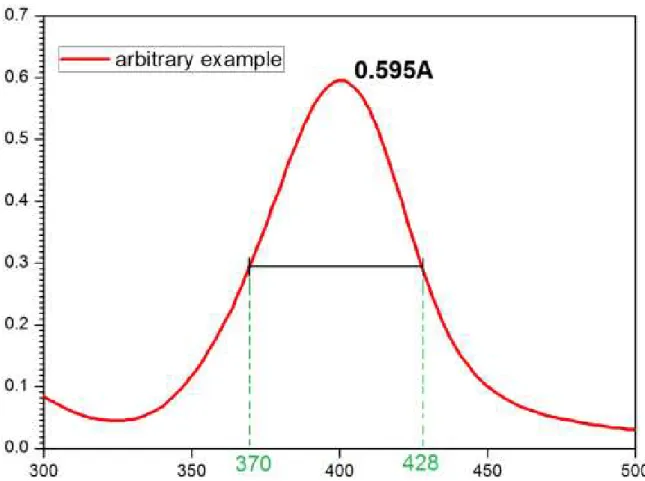
Metallic nanoparticles show different colors when in solution. It is common to observe colors ranging from blue to red of silver nanoparticles, depending on the shape. This changing of colors is due to a phenomenon known as surface plasmon resonance. This occurs because of the collective oscillation of electrons from the surface of a material, which interacts with the light that focuses on it. This collective oscillation leads the electrons, even for a short time, to be cumulated over a certain place on the material. The phenomena is thermodynamically unstable, because reduces the entropy of system, which responds tending to restore its initial condition. This restoring force originates an oscillation frequency inside the particle, then it is possible to observe the plasmon band [118].
Figure 1.12 – Plasmon band
Source: author
3 OBJECTIVES
3.1 General objective
To perform a green synthesis to obtain silver nanoparticles (AgNPs), through reducing sugars and silver nitrate, and promote the stabilization of the system by using sodium citrate and sodium dodecyl sulfate.
3.2 Specifics objectives
Once the AgNPs are obtained, several steps are taken, as described below:
3.2.1 Characterization of AgNP trough:
- UV-vis spectroscopy; - X-ray diffraction;
- Dynamic scattering of light; - Zeta potential;
- Scanning electron microscopy - Atomic force microscopy.
3.2.2 Obtaining and characterization of composite materials with AgNPs:
- Agar films; - Carbomer gel.
3.2.3 Perform tests as:
REFERENCES
1. Feynman, R.P., There's plenty of room at the bottom. J MEMS, 1992. 1: p. 60-66.
2. Taniguchi, N., Bulletin of the Japan Society of Precision Engineering, 1974: p. 18-23.
3. Castro, F.M.B.G.R., Nanotecnología, hacia un nuevo portal científico-tecnológico. QuímicaViva, 2012. 3(11): p. 171-184.
4. Brasil. Relatório referente à gestão do programa "Desenvolvimento da Nanociência e da Nanotecnologia" no exercício de 2005. 2008 [cited 2015 9 de maio].
5. Brasil. Site Portal Brasil. 2015 [cited 2015 9 de maio].
6. Brasil. Investimento em nanotecnologia pode chegar à R$ 110 milhões. 2012 [cited 2015 9 de maio].
7. Gharagozloo, M., S. Majewski, and M. Foldvari, Therapeutic applications of nanomedicine in autoimmune diseases: From immunosuppression to tolerance induction. Nanomedicine: Nanotechnology, Biology and Medicine, 2015. 11(4): p. 1003-1018.
8. Gupta, A.S., Nanomedicine approaches in vascular disease: a review.
Nanomedicine: Nanotechnology, Biology and Medicine, 2011. 7(6): p. 763-779.
9. Bell, I.R., Advances in integrative nanomedicine for improving infectious disease treatment in public health. European Journal of Integrative Medicine, 2013. 5(2): p. 126-140.
10. Zhu, X., Nanomedicine in the management of microbial infection –
Overview and perspectives. Nano Today, 2014. 9(4): p. 478-498. 11. Jr, R.A.F., What is nanomedicine? Nanomedicine, 2005. 1(1): p. 8.
potential bladder cancer biomarkers. Journal of Proteomics, 2012. 75(12): p. 3529-3545.
13. Drake, P.M., A lectin affinity workflow targeting glycosite-specific, cancer-related carbohydrate structures in trypsin-digested human plasma.
Analytical Biochemistry, 2011. 408(1): p. 71-85.
14. Hang Xing, K.H., Ji Li, Seyed-Fakhreddin Torabi, Yi Lu, DNA Aptamer Technology for Personalized Medicine. Curr Opin Chem Eng, 2014. 1: p. 9.
15. Misra, R.V., Developing an integrated proteo-genomic approach for the characterisation of biomarkers for the identification of Bacillus anthracis.
Journal of Microbiological Methods, 2012. 88(2): p. 237-247.
16. Antônio C. H. Barreto, V.R.S., Rafael M. Freire, Selma E. Mazzetto, Juliano C. Denardin, Giuseppe Mele, Igor M. Cavalcante, Maria E. N. P. Ribeiro, Nágila M. P. S. Ricardo, Tamara Gonçalves, Luigi Carbone, Telma L. G. Lemos, Otília D. L. Pessoa, Pierre B. A. Fechine Magnetic Nanosystem for Cancer Therapy Using Oncocalyxone A, an Antitomour Secondary Metabolite Isolated from a Brazilian Plant. Int. J. Mol. Sci., 2013. 14(9): p. 15.
17. Murase, K., Control of the temperature rise in magnetic hyperthermia with use of an external static magnetic field. Physica Medica: European Journal of Medical Physics. 29(6): p. 624-630.
18. Vanessa Zamora-Mora, M.F.-G., Julio San Romána, Gerardo Goya, Rebeca Hernández, Carmen Mijangos, Magnetic core–shell chitosan nanoparticles: Rheological characterization and hyperthermia application.
Carbohydrate Polymers, 2014. 102: p. 8.
20. Manh, D.H., Structural and magnetic study of La0.7Sr0.3MnO3 nanoparticles and AC magnetic heating characteristics for hyperthermia applications. Physica B: Condensed Matter, 2014. 444: p. 94-102.
21. Sekhon, B.S. and S.R. Kamboj, Inorganic nanomedicine—Part 1.
Nanomedicine: Nanotechnology, Biology and Medicine. 6(4): p. 516-522.
22. Fan, Z., Theranostic nanomedicine for cancer detection and treatment.
Journal of Food and Drug Analysis, 2014. 22(1): p. 3-17.
23. Monduzzi, M., From self-assembly fundamental knowledge to nanomedicine developments. Advances in Colloid and Interface Science, 2014. 205: p. 48-67.
24. Loeve, S., B.B. Vincent, and F. Gazeau, Nanomedicine metaphors: From war to care. Emergence of an oecological approach. Nano Today, 2013. 8(6): p. 560-565.
25. Jee, J.P., Cancer targeting strategies in nanomedicine: Design and application of chitosan nanoparticles. Current Opinion in Solid State and Materials Science, 2012. 16(6): p. 333-342.
26. Zarbin, A.J.G., Química de (nano)materiais. Química Nova, 2007. 30: p. 1469-1479.
27. Lee, J.D., Química inorgânica não tão concisa. 1999, Edgard Blücher São Paulo.
28. V. J. Schacht, L.V.N., S. K. Sandhi, L. Chen, T. Henni, P. J. Klar, K. Theophel, S. Schnell, M. Bunge, Effects of silver nanoparticles on microbial growthdynamics. Journal of Applied Microbiology 2012. 114: p. 11.
29. Anstas PT, W.J., Green Chemistry: Theory and Practice. 1998, New York: Oxford University Press, Inc.
31. Gross, R.A. and B. Kalra, Biodegradable Polymers for the Environment.
Science, 2002. 297(5582): p. 803-807.
32. Poliakoff, M. and P. Anastas, A principled stance. Nature, 2001. 413(6853): p. 257-257.
33. Raveendran, P., J. Fu, and S.L. Wallen, Completely “Green” Synthesis
and Stabilization of Metal Nanoparticles. Journal of the American Chemical Society, 2003. 125(46): p. 13940-13941.
34. Lenardão, E.J., "Green chemistry": os 12 princípios da química verde e sua inserção nas atividades de ensino e pesquisa. Química Nova, 2003. 26: p. 123-129.
35. Sharma, V.K., R.A. Yngard, and Y. Lin, Silver nanoparticles: Green synthesis and their antimicrobial activities. Advances in Colloid and Interface Science, 2009. 145(1–2): p. 83-96.
36. Amanullah, M. and L. Yu, Environment friendly fluid loss additives to protect the marine environment from the detrimental effect of mud additives. Journal of Petroleum Science and Engineering, 2005. 48(3–4): p. 199-208.
37. Yu, D. and V.W.W. Yam, Hydrothermal-Induced Assembly of Colloidal Silver Spheres into Various Nanoparticles on the Basis of HTAB-Modified Silver Mirror Reaction. The Journal of Physical Chemistry B, 2005. 109(12): p. 5497-5503.
38. Eustis, S., Growth and fragmentation of silver nanoparticles in their synthesis with a fs laser and CW light by photo-sensitization with benzophenone. Photochemical & Photobiological Sciences, 2005. 4(1): p. 154-159.
39. Sudeep, P.K. and P.V. Kamat, Photosensitized Growth of Silver
40. Collera-Zúñiga, O., F. Garcı́a Jiménez, and R. Meléndez Gordillo,
Comparative study of carotenoid composition in three mexican varieties of Capsicum annuum L. Food Chemistry, 2005. 90(1–2): p. 109-114.
41. Jagadeesh, B.H., T.N. Prabha, and K. Srinivasan, Activities of β
-hexosaminidase and α-mannosidase during development and ripening of
bell capsicum (Capsicum annuum var. variata). Plant Science, 2004. 167(6): p. 1263-1271.
42. Weinstock, I.A., Homogeneous-Phase Electron-Transfer Reactions of
Polyoxometalates. [(Chem. Rev. 1998, 98, 113 (this issue). Published on
the Web January 22, 1998.]. Chemical Reviews, 1998. 98(1): p. 389-390. 43. Hill, C.L., Introduction: PolyoxometalatesMulticomponent Molecular
Vehicles To Probe Fundamental Issues and Practical Problems. Chemical Reviews, 1998. 98(1): p. 1-2.
44. Zhang, G., Synthesis of various crystalline gold nanostructures in water: The polyoxometalate [small beta]-[H4PMo12O40]3- as the reducing and stabilizing agent. Journal of Materials Chemistry, 2009. 19(45): p. 8639-8644.
45. Campbell, M.K., Bioquímica. Vol. 3. 2007, São Paulo: Thomson Learning. 46. Organization, W.H. Antimicrobial resistance. 2015 [cited 2015
Jun-7-2015]; Available from:
http://www.who.int/mediacentre/factsheets/fs194/en/.
47. Tortorra, G.J., B.R. Funke, and C.L. Case, Microbiologia. 8 ed. 2005, Porto Alegre: Artmed.
48. Heilman, S., Efeito da radiação ionizante nos revestimentos de cateteres de poliuretano com nanopartículas de prata. 2015, IPEN - USP: São Paulo. p. 90.
50. Fukuda, K., Organic integrated circuits using room-temperature sintered silver nanoparticles as printed electrodes. Organic Electronics, 2012. 13(12): p. 3296-3301.
51. Natsuki, J. and T. Abe, Synthesis of pure colloidal silver nanoparticles with high electroconductivity for printed electronic circuits: The effect of amines on their formation in aqueous media. Journal of Colloid and Interface Science, 2011. 359(1): p. 19-23.
52. Hsieh, C.T., C. Pan, and W.Y. Chen, Synthesis of silver nanoparticles on carbon papers for electrochemical catalysts. Journal of Power Sources, 2011. 196(15): p. 6055-6061.
53. Seo, J.H., Cytotoxicity of serum protein-adsorbed visible-light photocatalytic Ag/AgBr/TiO2 nanoparticles. Journal of Hazardous Materials, 2011. 198: p. 347-355.
54. Huang, Q., W. Shen, and W. Song, Synthesis of colourless silver precursor ink for printing conductive patterns on silicon nitride substrates. Applied Surface Science, 2012. 258(19): p. 7384-7388.
55. Zhou, X., Enhanced dispersibility and dispersion stability of dodecylamine-protected silver nanoparticles by dodecanethiol for ink-jet conductive inks. Applied Surface Science, 2014. 292: p. 537-543.
56. Kosmala, A., Synthesis of silver nano particles and fabrication of aqueous Ag inks for inkjet printing. Materials Chemistry and Physics, 2011. 129(3): p. 1075-1080.
57. Ataeefard, M. and S. Sharifi, Antibacterial flexographic ink containing silver nanoparticles. Progress in Organic Coatings, 2014. 77(1): p. 118-123. 58. Said, J., An in vitro test of the efficacy of silver-containing wound
59. Gaisford, S., An in vitro method for the quantitative determination of the antimicrobial efficacy of silver-containing wound dressings. International Journal of Pharmaceutics, 2009. 366(1–2): p. 111-116.
60. Hebeish, A., Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. International Journal of Biological Macromolecules, 2014. 65: p. 509-515.
61. Silver, S. and L.T. Phung, Bacterial heavy metal resistance: New Surprises. Annual Review of Microbiology, 1996. 50(1): p. 753-789. 62. M, C., Antibacterial and bioactive silver-containing Na2OCaO2SiO2 glass
prepared by sol-gel method. Journal of Materials Science and Materials for Medicine, 2004. 15: p. 7.
63. Crabtree, J., The efficacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Peritoneal Dialysis International, 2003. 23(4): p. 368-374.
64. Zhao, G. and J.S. Stevens, Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. .
Biomaterials, 1998. 11: p. 6.
65. Aymonier, C., Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties.
Chemical Communications, 2002(24): p. 3018-3019.
66. Ansari, M., Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biology and Medicine, 2011.
67. Libor, K. and S. Jana, Comment on 'Preparation and antibacterial activity of Fe 3 O 4 @Ag nanoparticles'. Nanotechnology, 2009. 20(2): p. 028001. 68. Rai, M., A. Yadav, and A. Gade, Silver nanoparticles as a new generation
of antimicrobials. Biotechnology Advances, 2009. 27(1): p. 76-83.
70. Kim, J.S., Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2007. 3(1): p. 95-101.
71. Magaña, S.M., Antibacterial activity of montmorillonites modified with silver. Journal of Molecular Catalysis A: Chemical, 2008. 281(1–2): p. 192-199.
72. Shin, S.H., The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. International Immunopharmacology, 2007. 7(13): p. 1813-1818.
73. Arjunan, N.K., Green Synthesis of Silver Nanoparticles for the Control of Mosquito Vectors of Malaria, Filariasis, and Dengue. Vector-Borne and Zoonotic Diseases, 2011. 12(3): p. 262-268.
74. Kim, T.N., Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. Journal of Materials Science: Materials in Medicine, 1998. 9(3): p. 129-134.
75. Cho, K.H., The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochimica Acta, 2005. 51(5): p. 956-960. 76. Meenal, K., Extracellular synthesis of silver nanoparticles by a
silver-tolerant yeast strain MKY3. Nanotechnology, 2003. 14(1): p. 95.
77. Souza, G.I.H., Utilization of Fusarium oxysporum in the biosynthesis of silver nanoparticles and its antibacterial activities. , in Xth National Meeting of Environmental Microbiology. 2004: Curitiba, PR (Brazil)
78. Durán, N., Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. Journal of Nanobiotechnology, 2005. 3: p. 8-8.
79. Wright, G.D., Resisting resistance: new chemical strategies for battling superbugs. Chemistry & Biology, 2000. 7(6): p. R127-R132.
Monitoring the Onset of Surface Screening Effects. The Journal of Physical Chemistry C, 2014. 118(48): p. 28075-28083.
81. Mock, J.J., Shape effects in plasmon resonance of individual colloidal silver nanoparticles. The Journal of Chemical Physics, 2002. 116(15): p. 6755-6759.
82. Malinsky, M.D., Chain Length Dependence and Sensing Capabilities of the Localized Surface Plasmon Resonance of Silver Nanoparticles Chemically Modified with Alkanethiol Self-Assembled Monolayers. Journal of the American Chemical Society, 2001. 123(7): p. 1471-1482.
83. Sonnichsen, C., A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat Biotech, 2005. 23(6): p. 741-745. 84. Duval Malinsky, M., Nanosphere Lithography: Effect of Substrate on the
Localized Surface Plasmon Resonance Spectrum of Silver Nanoparticles.
The Journal of Physical Chemistry B, 2001. 105(12): p. 2343-2350.
85. Melo, A.D.Q., Estudo da utilização de coacervatos de polifosfato de sódio na obtenção de materiais com nanopartículas metálicas e magnéticas, in
Departamento de Química Orgânica e Inorgânica. 2011, Universidade Federal do Ceará: Brasil. p. 89.
86. Santos, K.O., Nanopartículas de prata e prata-paládio estabilizadas pela polietilenoimina linear funcionalizada: formação, caracterização e aplicações catalíticas, in Departamento de Química. 2012, Universidade Federal de Santa Catarina: Brasil. p. 153.
87. Vidhu, V.K., S.A. Aromal, and D. Philip, Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2011. 83(1): p. 392-397.
89. Moldovan, B., A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L. fruits extract and their antioxidant activity. Journal of Molecular Liquids, 2016. 221: p. 271-278.
90. Espenti, C.S., K.S.V.K. Rao, and K.M. Rao, Bio-synthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential. Materials Letters, 2016. 174: p. 129-133.
91. Kumar, V.A., Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from needles of Pinus densiflora. Materials Letters, 2016. 176: p. 169-172.
92. Ramanibai, R. and K. Velayutham, Synthesis of silver nanoparticles using 3,5-di-t-butyl-4-hydroxyanisole from Cynodon dactylon against Aedes aegypti and Culex quinquefasciatus. Journal of Asia-Pacific Entomology. 93. Ravichandran, V., Green synthesis of silver nanoparticles using
Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Materials Letters, 2016. 180: p. 264-267.
94. El-Sherbiny, I.M., A. El-Shibiny, and E. Salih, Photo-induced green synthesis and antimicrobial efficacy of poly (ɛ -caprolactone)/curcumin/grape leaf extract-silver hybrid nanoparticles.
Journal of Photochemistry and Photobiology B: Biology, 2016. 160: p. 355-363.
95. El-Shamy, A.G., W. Attia, and K.M. Abd El-Kader, The optical and mechanical properties of PVA-Ag nanocomposite films. Journal of Alloys and Compounds, 2014. 590: p. 309-312.
97. Pal, A., S. Shah, and S. Devi, Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent. Materials Chemistry and Physics, 2009. 114(2–3): p. 530-532.
98. Yang, J. and J. Pan, Hydrothermal synthesis of silver nanoparticles by sodium alginate and their applications in surface-enhanced Raman scattering and catalysis. Acta Materialia, 2012. 60(12): p. 4753-4758. 99. Darroudi, M., Green synthesis of colloidal silver nanoparticles by
sonochemical method. Materials Letters, 2012. 66(1): p. 117-120.
100. Zhanjiang, Z. and L. Jinpei, Synthesis and Characterization of Silver Nanoparticles by a Sonochemical Method. Rare Metal Materials and Engineering, 2012. 41(10): p. 1700-1705.
101. Mandal, A., Synthesis, characterization and comparison of antimicrobial activity of PEG/TritonX-100 capped silver nanoparticles on collagen scaffold. Colloids and Surfaces B: Biointerfaces, 2012. 90: p. 191-196. 102. Wojtysiak, S. and A. Kudelski, Influence of oxygen on the process of
formation of silver nanoparticles during citrate/borohydride synthesis of silver sols. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012. 410: p. 45-51.
103. Yan, J., Antibacterial activity of silver nanoparticles synthesized In-situ by solution spraying onto cellulose. Carbohydrate Polymers, 2016. 147: p. 500-508.
104. Xu, L., Droplet synthesis of silver nanoparticles by a microfluidic device.
Chemical Engineering and Processing: Process Intensification, 2016. 102: p. 186-193.
106. Yang, C., S. Jung, and H. Yi, A biofabrication approach for controlled synthesis of silver nanoparticles with high catalytic and antibacterial activities. Biochemical Engineering Journal, 2014. 89: p. 10-20.
107. Jia, C.J. and F. Schuth, Colloidal metal nanoparticles as a component of designed catalyst. Physical Chemistry Chemical Physics, 2011. 13(7): p. 2457-2487.
108. Signori, A.M., Formation of Catalytic Silver Nanoparticles Supported on Branched Polyethyleneimine Derivatives. Langmuir, 2010. 26(22): p. 17772-17779.
109. Gentry, S.T., S.J. Fredericks, and R. Krchnavek, Controlled Particle Growth of Silver Sols through the Use of Hydroquinone as a Selective Reducing Agent. Langmuir, 2009. 25(5): p. 2613-2621.
110. Aiken Iii, J.D. and R.G. Finke, A review of modern transition-metal nanoclusters: their synthesis, characterization, and applications in catalysis. Journal of Molecular Catalysis A: Chemical, 1999. 145(1–2): p. 1-44.
111. Patakfalvi, R., S. Papp, and I. Dékány, The kinetics of homogeneous nucleation of silver nanoparticles stabilized by polymers. Journal of Nanoparticle Research, 2007. 9(3): p. 353-364.
112. Bönnemann, H., Nanoscale colloidal metals and alloys stabilized by solvents and surfactants Preparation and use as catalyst precursors.
Journal of Organometallic Chemistry, 1996. 520(1): p. 143-162.
113. SHABBEER, H.S., Effect of Acidic and Basic Conditions on the Plasmon Band of Colloidal Silver. 2012, 2012. 9(3): p. 9.
115. Lah, N.A.C. and M.R. Johan, Facile shape control synthesis and optical properties of silver nanoparticles stabilized by Daxad 19 surfactant.
Applied Surface Science, 2011. 257(17): p. 7494-7500.
116. Hebeish, A., T.I. Shaheen, and M.E. El-Naggar, Solid state synthesis of starch-capped silver nanoparticles. International Journal of Biological Macromolecules, 2016. 87: p. 70-76.
117. Vanamudan, A. and P.P. Sudhakar, Biopolymer capped silver nanoparticles with potential for multifaceted applications. International Journal of Biological Macromolecules, 2016. 86: p. 262-268.
118. Barnes, W.L., A. Dereux, and T.W. Ebbesen, Surface plasmon subwavelength optics. Nature, 2003. 424(6950): p. 824-830.