Stability indicating RP-HPLC method development and validation of cefepime and amikacin in pure and pharmaceutical dosage forms
Texto
Imagem


Documentos relacionados
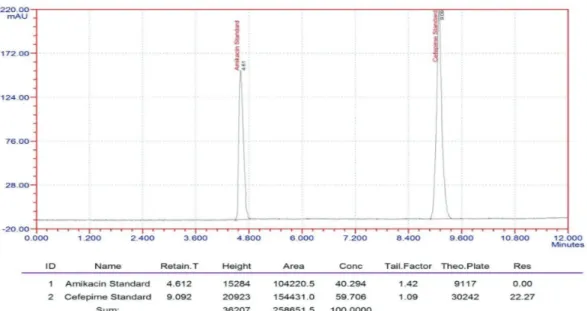
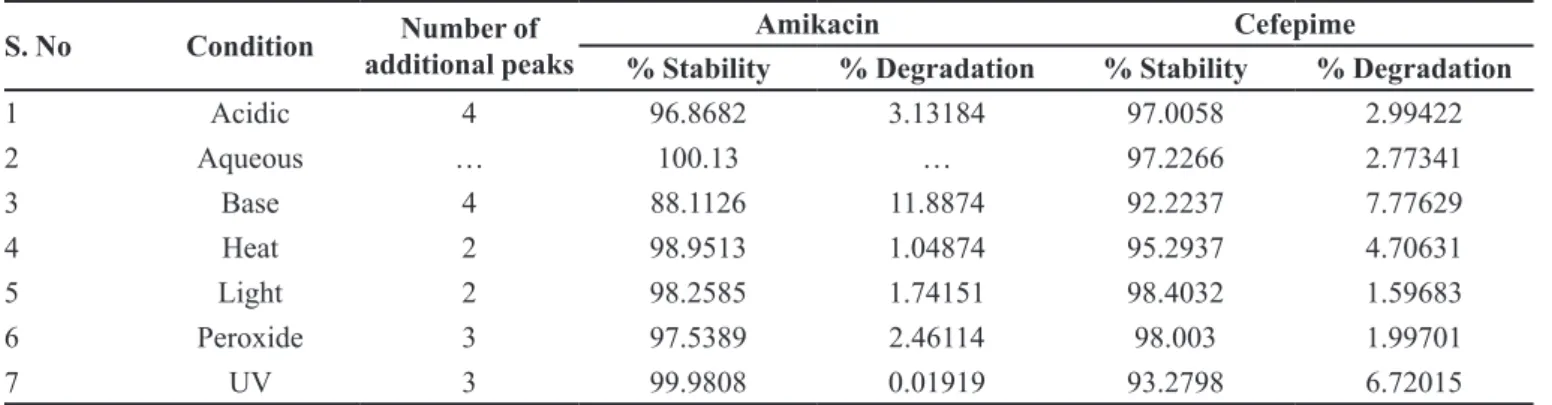
The results show that for solutions, the retention time and peak area of Sexagliptin and pioglitazone remained almost unchanged( %RSD <2) and no
Yadav .Method development and its validation for simultaneous estimation of Atorvastatin and Amlodipine in combination in tablet dosage form by UV spectroscopy,
A simple, sensitive, rapid, robust and reproducible method for the determination of Quetiapine Hemi Fumarate (QHF) in bulk and Pharmaceutical Formulation (Tablets) was developed
Simultaneous estimation and validation of developed method of Nifedipine (NIF) and Atenolol (ATN) in combined dosage form as well as in laboratory mixture
Commercial capsule formulation was successfully analyzed using the developed method and the proposed method is applicable to routine analysis of determination of
Development and validation of a stability-indicating HPTLC method for analysis of Nebivolol hydrochloride and Hydrochlorothiazide in the bulk material and in
For further dilution, 5 ml of sample stock solution was added to a 50 ml volumetric flask and 5 ml of tablet degradation stock was added to a 20 ml flask, and the volumes were
Then pipette out 10ml from the standard stock solution in other 100ml volumetric flask and diluted up to mark with Methanol to give a working standard solution having