Braz. J. Pharm. Sci. vol.50 número2
Texto
Imagem

Documentos relacionados
Ousasse apontar algumas hipóteses para a solução desse problema público a partir do exposto dos autores usados como base para fundamentação teórica, da análise dos dados
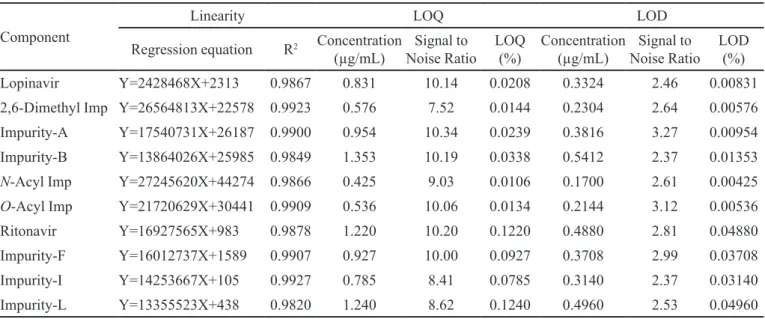
The aim of the present work was the development of a stability- indicating UPLC method for the determination of RSV and its related impurities in the pharmaceutical dosage forms
The investigation includes the determination of organic impurities, inorganic impurities and volatiles, validation of the HPLC-DAD method, stability studies under transport
To assess the role of UVA light on the degradation of MG, the experiments were conducted under optimized conditions and the degradation was followed in the absence of light
and possible degradation products, to evaluate the intrinsic stability of these drugs under stress conditions, and to study the kinetics of degradation of these drugs that are
The probability of attending school four our group of interest in this region increased by 6.5 percentage points after the expansion of the Bolsa Família program in 2007 and
Na hepatite B, as enzimas hepáticas têm valores menores tanto para quem toma quanto para os que não tomam café comparados ao vírus C, porém os dados foram estatisticamente
2,4 In our case an extensive mass involving the nasal septum, maxillary sinus, and orbital, nasal, and oral cavities was ob- served, which was clinically characterized by a