UNIVERSIDADE ESTADUAL JULIO DE MESQUITA FILHO INSTITUTO DE BIOCIÊNCIAS
CÂMPUS DE BOTUCATU
EFFECTS OF LITHIUM ON THE PROLIFERATION AND MIGRATION OF NEUROBLASTS IN THE ROSTRAL MIGRATORY STREAM IN ADULT MICE
GIANCARLO DE MATTOS CARDILLO
Monografia apresentada ao Instituto de Biociências, Câmpus de Botucatu, para obtenção do título de Bacharel em Ciências Biomédicas
UNIVERSIDADE ESTADUAL JULIO DE MESQUITA FILHO INSTITUTO DE BIOCIÊNCIAS
CÂMPUS DE BOTUCATU
EFFECTS OF LITHIUM ON THE PROLIFERATION AND MIGRATION OF NEUROBLASTS IN THE ROSTRAL MIGRATORY STREAM IN ADULT MICE
GIANCARLO DE MATTOS CARDILLO
Orientadora: Dra. Evelin Lisete Schaeffer
Supervisor: Prof. Dr. José de Anchieta de Castro e Horta Júnior
Monografia apresentada ao Instituto de Biociências, Câmpus de Botucatu, para obtenção do título de Bacharel em Ciências Biomédicas
Effects of lithium on the proliferation and migration of neuroblasts in the rostral
migratory stream in adult mice
Giancarlo M. Cardillo 1, Sergio Catanozi 2, Wagner F. Gattaz 1, Evelin L. Schaeffer 1*
1 Laboratory of Neuroscience (LIM-27), Department and Institute of Psychiatry, Faculty of
Medicine, University of Sao Paulo, Rua Dr. Ovídio Pires de Campos 785, 05403-010, Sao
Paulo, SP, Brazil
2 Lipids Laboratory (LIM-10), Endocrinology and Metabolism Division of Clinical Hospital,
Faculty of Medicine, University of Sao Paulo, Av. Dr. Arnaldo 455, 01246-000, Sao Paulo,
SP, Brazil
* Corresponding author: Tel.: +55 11 2661 7283; Fax: +55 11 2661 7535; E-mail:
schaffer@usp.br (E.L. Schaeffer)
Abbreviations
AD, Alzheimer’s disease; BrdU, 5-bromo-2-deoxyuridine; DG, dentate gyrus; OB, olfactory
bulb; RMS, rostral migratory stream; SGZ, subgranular zone; SVZ, subventricular zone; RT,
Abstract
The discovery of neurogenesis in adult brains opened the possibility of cellular therapy
strategies for the treatment of neurodegenerative diseases, such as Alzheimer’s disease.
Neurogenesis in the adult brain occurs in two areas: subgranular zone of the hippocampus and
subventricular zone (SVZ) of the lateral ventricles. Neurons that originate from the SVZ
migrate to the olfactory bulb (OB) through the rostral migratory stream (RMS). In
Alzheimer’s disease, there is a progressive neuronal dysfunction and degeneration, resulting
in brain atrophy and cognitive impairments including olfactory dysfunction. Several studies
have demonstrated that pharmacological treatment with lithium exerts positive effects on
adult neurogenesis, and one pathway seems to be the modulation of factors that regulate the
migration of neuroblasts. The objective of this study was to investigate whether treatment
with lithium promotes the increase of migratory neuroblasts using as parameter the RMS.
Adult male C57BL/6 mice were divided into control and lithium-treated groups. The animals
were treated for 6 weeks and, at four different time points, i.e., 10 days, 7 days, 3 days and 1
day before the end of treatments, they received an injection of BrdU (cell proliferation
marker). The animals were sacrificed by perfusion fixation and the brains were
immunohistochemically labeled for BrdU for analysis of migrating neuroblasts in the RMS.
The results showed that the number of BrdU+ cells in the RMS was not significantly different
between the two groups, suggesting that lithium, alone, is not capable of increasing the
number of neuroblasts migrating from the SVZ to the OB.
Keywords: lithium; neurogenesis; cell migration; rostral migratory stream; Alzheimer’s
disease
1 Introduction
Neurogenesis, the birth of new neurons, constitutively occurs in two privileged areas of the
adult brain: the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus and the
subventricular zone (SVZ) of the lateral ventricles (Yamashima et al., 2007). Adult
neurogenesis is a multi-step process that encompasses the slow proliferation of early stem or
progenitor cells, the subsequent faster proliferation of more restricted progenitors, migration,
the selection for survival or elimination of young cells, the integration of the surviving cells
into the pre-existing neuronal network, and lastly the later phases of postmitotic development
that include gradually increasing neuronal connectivity and changes in physiological neuronal
properties (Ehninger and Kempermann, 2008). Newborn neurons leaving the SGZ migrate to
the adjacent granule cell layer of the DG (Altman and Bayer, 1990; Kuhn et al., 1996).
Neurons that originate from the SVZ migrate to the olfactory bulb (OB) through a well
defined pathway, the rostral migratory stream (RMS) (Pencea et al., 2001; Bédard and Parent,
2004; Shapiro et al., 2006). Newborn neuroblasts of the SVZ enter the RMS and take 2 to 7
days to reach the OB and 5 to 9 days to start the radial migration to the granular cell layer of
the OB (Whitman and Greer, 2009).
In Alzheimer’s disease (AD), the most common cause of dementia, there is a progressive
neuronal dysfunction and degeneration, resulting in severe brain atrophy and cognitive
impairments (Selkoe, 2003). It is well established that, in AD, neurodegeneration occurs in
the CA1 region and DG of the hippocampus, entorhinal cortex (Gómez-Isla et al., 1996;
Scheff and Price, 1998; Csernansky et al., 2000, 2005; Kordower et al., 2001; Price et al.,
2001; Kril et al., 2004; von Gunten et al., 2006), and association neocortex (parieto-temporal,
inferolateral temporal and prefrontal) (Schuff et al., 1997; Kantarci et al., 2000; Block et al.,
Additionally, there is evidence that olfactory dysfunction occurs in AD. For instance, a study
in postmortem OB of AD patients showed reduced volume of the bulb and decreased number
of neurons in the anterior olfactory nucleus, being limited to younger and very severe patients,
when compared to healthy subjects (ter Laak et al., 1994). Furthermore, in vivo studies
showed reduced olfactory bulb and tract in patients with early AD when compared to healthy
subjects, and this atrophy was associated to an impairment of global cognitive performance
(Thomann et al., 2009). Interestingly, studies of neurogenesis on postmortem brains from AD
patients have shown that endogenous neurogenesis can be triggered in response to the disease.
For example, in patients with AD it was found a significant reduction of progenitor cells in
the SVZ associated with the cholinergic deficit of AD, but an increase in astrocyte-like cells
with progenitor characteristics, indicating the activity of endogenous neurogenesis (Ziabreva
et al., 2006). Moreover, a study in the SVZ, SGZ and granule cell layer of postmortem brain
tissue of AD patients demonstrated that while hippocampal stem cells decrease, proliferation
increases and differentiation/migration phase as well as axonal/dendritic targeting remains
virtually unchanged in all areas studied, suggesting an attenuation of stem cells together with
compensatory increased proliferation that, however, does not result in an increased number of
migratory neuroblasts and differentiated neurons in AD (Perry et al., 2012).
Studies in experimental animals have shown that the pharmacological treatment with lithium
can stimulate the adult neurogenesis. In this matter, lithium treatment for 1 month was
reported as being able to restore cell proliferation in the SVZ of the Ts65Dn mouse model of
Down syndrome (Bianchi et al., 2010). Short-term lithium pre-treatment (24 h) was also
demonstrated as being capable of promoting neuronal proliferation and survival in the
striatum (which seems to be a target of newborn cells derived from the SVZ; Yamashita et al.,
linked lithium with factors that regulate the start and speed of the migration of neuroblasts.
For instance, a study showed that the levels of vascular endothelial growth factor (which
affects the speed of migration; Wittko et al., 2009) are decreased in the hippocampus of rats
that suffer induced stress, however, when the animals are treated with lithium, this deficit is
lower, suggesting that lithium prevents the reduction of vascular endothelial growth factor
induced by stress (Silva et al., 2007). Another study demonstrated that the concentration of
insulin-like growth factor-binding protein (which controls the bioactivity of insulin-like
growth factor, important to the start of cell migration; Hurtado-Chong et al., 2009) is
decreased in postmortem prefrontal cortex of patients with bipolar disorder when compared to
healthy subjects, specially in patients not treated with lithium, suggesting that lithium
preserves the levels of insulin-like growth factor-binding protein (Bezchlibnyk et al., 2007).
Since lithium has been reported as having neurogenic properties, we were interested to
examine whether it could increase the number of migrating neuroblasts in the RMS.
2 Materials and methods
2.1 Animals and treatment protocols
Twenty (20) male C57BL/6 mice, 5-month-old at the beginning of the experiments, were used
in this study. The animals were obtained from the Animal Facility of the Faculty of Medicine,
University of Sao Paulo and kept in conventional facility in standard cage sized 41 34 16
cm (length width height) under constant temperature (22 ± 1°C) and relative humidity
(50-60%) conditions, on a 12-h light/dark cycle with ad libitum access to food and fresh
water. The animals were submitted to one of two treatment groups for 6 weeks: (a) Control:
treatment with standard chow (n = 11); and (b) Lithium: treatment with chow containing 2.0 g
-supplemented chow was prepared by mixing Li2CO3 (Merck, Germany) with a commercial
powdered chow (AIN-93G; Nutri Experimental®, Brazil), then the standard chow was
powdered chow free of Li2CO3. The powdered diets were pelleted fresh every week in our
laboratory. Mice receiving lithium had access to a drinking bottle of water and another one of
0.9% NaCl (saline) solution to prevent dehydration and hyponatremia. The lithium dose used
in this study was determined in a pilot experiment conducted in our laboratory which showed
that 2.0 g Li2CO3/kg chow was the minimum dose needed to achieve therapeutic serum
lithium levels (which vary between 0.5 and 1.2 mmol/L; Solomon et al., 1996; Perlis et al.,
2002) and to significantly increase the proliferation of newborn cells in the hippocampus of
4-month-old adult C57BL/6 mice after a 28-day treatment period (data not shown). The weight
of all animals was monitored at the beginning and at the end of treatments. Chow
consumption was measured per cage weekly and divided by the number of animals per cage
and then per day to determine feed consumption per animal per day in each group.
2.2 Bromodeoxyuridine (BrdU) administration
After 31 days of treatment and at 4 different time points, i.e., 10 days, 7 days, 3 days and 1
day before their sacrifice, the animals received a single intraperitoneal injection of the cell
proliferation marker 5-bromo-2-deoxyuridine (BrdU; Sigma, Germany; 50 mg/mL dissolved
in sterile, warm 0.9% NaCl pH 7.4) at a dose of 100 mg/kg bw. BrdU, a thymidine analogue,
is incorporated into the DNA of dividing cells during the S phase (DNA replication phase),
and can subsequently be detected by immunohistochemistry using a specific, monoclonal
antibody (Gratzner, 1982). The animals were sacrificed after 6 weeks (42 days) of treatment
for analysis of migrating neuroblasts in the RMS.
After 28 days of treatment, thus 4 days before the beginning of BrdU injections, lithium
concentration or lithemia (mmol/L) was determined in serum of all mice. The animals were
put on a heating stage, blood was collected from the tail and the serum separated by
centrifugation. Analyses were performed using a digital ion analyzer (Electrolyte Analyzer
9180; Roche, Germany). The blood of the animals was collected before BrdU administration
to avoid any effect of animal restraint-induced stress on cell proliferation (Kirby et al., 2013).
2.4 Brain tissue collection and cutting
At the end of treatments and, therefore, 10 days, 7 days, 3 days and 1 day after BrdU
injections, the animals were anesthetized with an intraperitoneal injection of ketamine (100
mg/kg bw)/xylazine (10 mg/kg bw) and sacrificed through intracardiac perfusion with
Tyrode’s solution (37ºC) followed by fixation with 4% paraformaldehyde in 0.2 M Sörensen
buffer (NaH2PO4 and Na2HPO4) pH 7.6 (4ºC) using a peristaltic pump (120S;
Watson-Marlow, Wilmington, MA). The fixed brains were removed, post-fixed in 4%
paraformaldehyde for 2 h, immersed in 15% sucrose in phosphate buffered saline pH 7.4
(4ºC) overnight, and then stored in freezer at -70°C. Afterwards, the fixed frozen brains were
sectioned sagittally throughout the whole RMS and OB of both brain hemispheres on a
cryostat (CM3050S; Leica, Germany) into 60-µm-thick adjacent serial sections, and the
sections were collected in 24-well plates containing cryoprotectant solution (30% ethylen
glycol, 30% glycerol 85% w/w, and 40% phosphate buffered saline pH 7.4) and stored in
freezer at -20ºC.
2.5 BrdU immunohistochemistry
All free-floating sections throughout the whole RMS of one brain hemisphere were processed
min) in Tris buffered saline (TBS) pH 7.5 at room temperature (RT). Next, the sections were
incubated with 0.6% H2O2 in TBS for 30 min at RT to eliminate endogenous peroxidase
activity. After washing (3 5 min) in TBS, DNA was denaturated with 50% formamide/2
saline sodium citrate pH 7.0 for 2 h at 65°C followed by acid. After washing (2 5 min) in
TBS, the sections were then incubated with 2 N HCl for 30 min at 37ºC. The sections were
washed in 100 mM borate buffer pH 8.5 for 10 min at RT to neutralise the acid. After
washing (3 10 min) in TBS, the sections were incubated for 60 min at RT with blocking
solution A (2% bovine serum albumine and 5% normal goat serum in 0.25% Triton
X-100-TBS). They were then incubated overnight at 4ºC with 1:500 mouse monoclonal anti-BrdU
(Roche, Germany) diluted in blocking solution A. After washing (3 10 min) in TBS, the
sections were incubated for 2 h at RT with 1:400 biotinylated anti-mouse (Vector,
Burlingame, CA) diluted in blocking solution B (2% bovine serum albumine and 2% normal
goat serum in 0.25% Triton X-100-TBS). The sections were washed (3 10 min) in TBS and
then incubated with avidin-biotin-peroxidase complex (Vectastain ABC Kit; Vector) for 90
min at RT. After washing (3 10 min) in TBS, the sections were treated with
diaminobenzidine (Vector) for 10 min at RT and then washed (2 5 min) in TBS. Finally, the
sections were mounted on slides, air dried overnight and coverslipped using VectaMount
(Vector).
2.6 Stereological analysis of BrdU-labeled cells
The immunopositive cells for BrdU (BrdU+) were counted in the RMS of one brain
hemisphere using a light microscope (Eclipse 80i; Nikon, Japan) and a stereology software
(StereoInvestigator, version 9.14.5; MicroBrightField, Colchester, VT). Contours of the
region of interest (RMS) were drawn in digital images displayed on the computer screen using
objective lens. BrdU+ cells were quantified using all sections containing the region of interest
(i.e., a section sampling fraction ssf of 1), a counting frame (disector) size of 10 10 m, a
disector height of 10 m, an upper guard zone of 2 m, and a grid size (distance between
counting frames) of 125 125 m. The estimated total number (N) of BrdU+ cells per RMS
was determined as the number of cells counted (Q-) multiplied by the inverse of the ssf (1/1),
by the inverse of the area sampling fraction asf (area of the counting frame/area of the grid),
and by the inverse of the thickness sampling fraction tsf (height of the disector/section
thickness), according to the formula N = Q- 1/(ssfasftsf). Cell counts were performed
on blind-coded slides. The average coefficient of error (CE) calculated via the
Gundersen-Jensen method (Gundersen and Gundersen-Jensen, 1987) was 0.098. A ssf of 1 was used because a
reliable CE value was achieved only with an interval of 1 (but not of 2 or 3) between the
sections, perhaps due to the irregular shape of the RMS.
2.7 Statistical analysis of data
Comparisons of BrdU+ cell number and body weight data between groups were made using
Student’s t-test. Data are presented as mean ± standard deviation (SD). Differences were
considered statistically significant if the p value was less than 0.05.
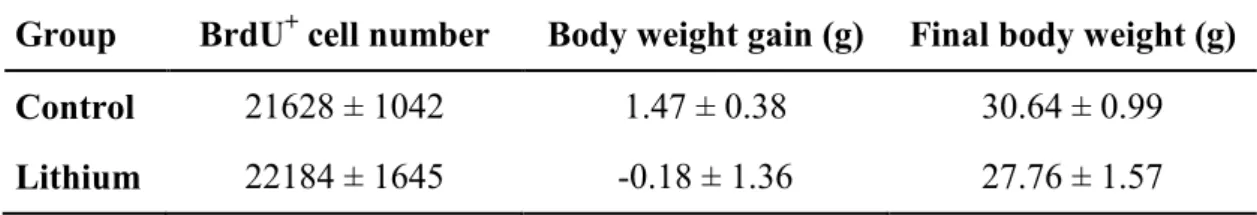
3 Results
The average chow consumption per animal per day in the Control group was 5.81 g whereas
in the Lithium group was 4.83 g. The mean serum lithemia in lithium-treated animals was
0.78 ± 0.20 mmol/L. No significant differences were observed in body weight at the end of
treatments (t-test: t = 1.61, df = 18, p = 0.125) as well as in body weight gain since the
beginning of treatments (t-test: t = 1.28; df = 18; p = 0.218) between the Control and Lithium
between the two groups (t-test: t = 0.30; df = 18; p = 0.771). The data are shown in Table 1
and Figure 2.
4 Discussion
The idea of a substance capable of affecting positively the main stages of adult neurogenesis,
namely cell proliferation, migration, differentiation and survival (Ehninger and Kempermann,
2008), brings the possibility of a cell therapy to treat neurodegenerative diseases such as AD
(Abdel-Salam, 2011). Lithium is the major drug used to treat bipolar disorder (Geddes and
Miklowitz, 2013), and it has been shown as a robust neuroprotective agent in preventing
apoptosis of neurons (Quiroz et al., 2010). This neuroprotective property opened the
possibility to study its effects on the neurogenic process (Chiu et al., 2013), and it has been
proved that lithium can indeed stimulate the adult neurogenesis (Chen et al., 2000; Fiorentini
et al., 2010; Hanson et al., 2011; O’Leary et al., 2012). Based on these findings and
hypotheses, the present study investigated whether lithium would have a positive impact on
the migration stage of adult neurogenesis by analyzing a possible increase in the number of
migrating neuroblasts in the RMS. The results, though, pointed that lithium, alone, has no
effect on the RMS, therefore, impacting neither positively nor negatively on migrating
neuroblasts. Because in our study there was no significant difference between the weights of
lithium-treated mice and controls, and serum lithemia was within the human therapeutic range
(0.5-1.2 mmol/L; Solomon et al., 1996; Perlis et al., 2002) in all lithium-treated mice, the
present results may be due to a true lack of effect of lithium on migrating neuroblasts.
To our knowledge, this is the first study to examine the effect of lithium on the migration of
newborn neuroblasts in the RMS of adult brain. Previous studies reported that, at least under
with lithium for 3-4 weeks showed increased number of proliferative cells in the DG (Chen et
al., 2000). This result was later corroborated by Son et al. (2003) in adult rats, by Hanson et
al. (2011) in 8-week-old rats, and by Schaeffer et al. (in press) in 4-month-old adult mice
treated with lithium for 28 days. Hanson et al. (2011) and Schaeffer et al. (in press) also
demonstrated that lithium has no impact on the survival of newborn cells in the DG, and
O’Leary et al. (2012) observed a decrease in the survival of newborn cells in the whole DG
and SGZ of 8-week-old mice treated with lithium for 21 days. It should be noted that, in our
study, the BrdU injections were administrated 1, 3, 7 and 10 days before sacrifice. By
delivering BrdU to animals at these time points, lithium treatment (lasting from 31 days
before the first BrdU injection until 1 day after the last BrdU injection) could affect both
mitotic (proliferating; 1- to 3-day-old) and postmitotic (surviving; 7- to 10-day-old)
neuroblasts, once both proliferation and survival stages of neurogenesis can be found in the
migration pathway from the SVZ to the OB (Whitman and Greer, 2009). If considering that
lithium treatment might have had opposite effects on proliferating and surviving cells, as has
been reported by others (Chen et al., 2000; Son et al., 2003; Hanson et al., 2011; O'Leary et
al., 2012; Schaeffer et al., in press), then it might be possible that the lack of effect of lithium
on the number of migrating neuroblasts in our study was due to a counterbalance between a
possible increase in cell proliferation and a possible decrease in cell survival. Future studies
could investigate whether the null finding in our study represented a true lack of effect or was
due to a counterbalancing effect, by injecting BrdU at each of those time points (1, 3, 7 and 10
days before sacrifice) in different animals to avoid possible overlapping effects of lithium.
It is noteworthy that the study by Schaeffer et al. (in press) demonstrated that chronic lithium
treatment, when combined with long-term exposure to an enriched environment with physical
generated cells in the hippocampal DG of 4-month-old adult mice. Interestingly,
Martončíková et al. (2011) demonstrated that rats submitted to daily olfactory stimulation
from the day of their birth up to 1, 2 or 3 weeks with different odorants showed an increased
number of proliferating cells and also down-regulation of the number of dying cells in the
RMS. Additionally, Rochefort and Lledo (2005) observed that the number of surviving cells
in the OB of 2-month-old mice was elevated right after the withdraw of the animals from their
odor-enriched housing, where they stayed for 40 days, and this number returned to control
level 1 month later. Similarly, it was observed an improvement of olfactory short-term
memory, also induced by enriched odor exposure, and it lasted for 1 month, then returning to
control level, indicating that the survival of newborn cells is closely associated to the degree
of environment complexity and that the olfactory memory is connected with the neurogenesis.
Therefore, future studies could combine the potentiating effect of lithium with the olfactory
stimulation.
5 Conclusion
In summary, the present study showed that chronic lithium treatment of adult mice had no
effect on the total number of 1- to 10-day-old BrdU+ cells in the RMS. This result suggests
that lithium is not capable of increasing the number of proliferating/surviving neuroblasts
migrating from the SVZ to the OB.
6 Acknowledgments
This study was funded by the State of Sao Paulo Research Support Foundation (FAPESP;
Projects 2009/52825-8, 2009/53008-3, 2013/00100-5). The Laboratory of Neuroscience
(LIM-27) receives financial support from the Alzira Denise Hertzog Silva Benevolent
7 References
Abdel-Salam OM (2011) Stem cell therapy for Alzheimer's disease. CNS Neurol Disord Drug
Targets 10:459-485.
Altman J, Bayer SA (1990) Development of layer I and the subplate in the rat neocortex. Exp
Neurol 107:48-62.
Bédard A, Parent A (2004) Evidence of newly generated neurons in the human olfactory bulb.
Brain Res Dev Brain Res 151:159-68.
Bezchlibnyk YB, Xu L, Wang JF, Young LT (2007) Decreased expression of insulin-like
growth factor binding protein 2 in the prefrontal cortex of subjects with bipolar disorder and
its regulation by lithium treatment. Brain Res 1147:213-217.
Bianchi P, Ciani E, Guidi S, Trazzi S, Felice D, Grossi G, Fernandez M, Giuliani A, Calzà L,
Bartesaghi R (2010) Early pharmacotherapy restores neurogenesis and cognitive
performance in the Ts65Dn mouse model for Down syndrome. J Neurosci 30:8769-8779.
Block W, Jessen F, Träber F, Flacke S, Manka C, Lamerichs R, Keller E, Heun R, Schild H
(2002) Regional N-acetylaspartate reduction in the hippocampus detected with fast proton
magnetic resonance spectroscopic imaging in patients with Alzheimer disease. Arch Neurol
59:828-834.
Chantal S, Labelle M, Bouchard RW, Braun CM, Boulanger Y (2002) Correlation of regional
proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild
Alzheimer disease. Arch Neurol 59:955-962.
Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK (2000) Enhancement of
Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller
MI (2000) Early DAT is distinguished from aging by high-dimensional mapping of the
hippocampus. Dementia of the Alzheimer type. Neurology 55:1636-1643.
Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, Miller MI, Morris JC
(2005) Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict
dementia onset in the elderly. Neuroimage 25:783-792.
Chiu CT, Wang Z, Hunsberger JG, Chuang DM (2013) Therapeutic potential of mood
stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105-142.
Ehninger D, Kempermann G (2008) Neurogenesis in the adult hippocampus. Cell Tissue Res
331:243-250.
Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F (2010) Lithium improves
hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice.
PLoS One 5:e14382.
Geddes JR, Miklowitz DJ (2013) Treatment of bipolar disorder. Lancet 381:1672-1682.
Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT (1996)
Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease.
J Neurosci 16:4491-4500.
Gratzner HG (1982) Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new
reagent for detection of DNA replication. Science 218:474-475.
Gundersen HJ, Jensen EB (1987). The efficiency of systematic sampling in stereology and its
prediction. J Microsc 147:229-263.
Hanson ND, Nemeroff CB, Owens MJ (2011) Lithium, but not fluoxetine or the
corticotropin-releasing factor receptor 1 receptor antagonist R121919, increases cell
Hurtado-Chong A, Yusta-Boyo MJ, Vergaño-Vera E, Bulfone A, de Pablo F, Vicario-Abejón
C (2009) IGF-I promotes neuronal migration and positioning in the olfactory bulb and the
exit of neuroblasts from the subventricular zone. Eur J Neurosci 30:742–755.
Kantarci K, Jack CR Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF,
Kokmen E, Tangalos EG, Petersen RC (2000) Regional metabolic patterns in mild cognitive
impairment and Alzheimer's disease: A 1H MRS study. Neurology 55:210-217
Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D (2013)
Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn
neurons via secreted astrocytic FGF2. Elife 2:e00362.
Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ (2001)
Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive
impairment. Ann Neurol 49:202-213.
Kril JJ, Hodges J, Halliday G (2004) Relationship between hippocampal volume and CA1
neuron loss in brains of humans with and without Alzheimer's disease. Neurosci Lett
361:9-12.
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the
adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci
16:2027-2033.
Martončíková M, Lievajová K, Orendáčová J, Blaško J, Račeková E (2011) Odor enrichment
influences neurogenesis in the rostral migratory stream of young rats. Acta Histochem
113:326-332.
O'Leary OF, O'Connor RM, Cryan JF (2012) Lithium-induced effects on adult hippocampal
neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice.
Pencea V, Bingaman KD, Freedman LJ, Luskin MB (2001) Neurogenesis in the
subventricular zone and rostral migratory stream of the neonatal and adult primate
forebrain. Exp Neurol 172:1-16.
Perlis RH, Sachs GS, Lafer B, Otto MW, Faraone SV, Kane JM, Rosenbaum JF (2002) Effect
of abrupt change from standard to low serum levels of lithium: a reanalysis of double-blind
lithiummaintenance data. Am J Psychiatry 159:1155-1159.
Perry EK, Johnson M, Ekonomou A, Perry RH, Ballard C, Attems J (2012) Neurogenic
abnormalities in Alzheimer's disease differ between stages of neurogenesis and are partly
related to cholinergic pathology. Neurobiol Dis 47:155-162
Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC (2001) Neuron number in the
entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 58:1395-1402.
Quiroz JA, Machado-Vieira R, Zarate CA Jr, Manji HK (2010) Novel insights into lithium's
mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology
62:50-60.
Rochefort C, Lledo PM (2005). Short-term survival of newborn neurons in the adult olfactory
bulb after exposure to a complex odor environment. Eur J Neurosci 22:2863-2870.
Scheff SW, Price DA (1998) Synaptic density in the inner molecular layer of the hippocampal
dentate gyrus in Alzheimer disease. J Neuropathol Exp Neurol 57:1146-1153.
Selkoe DJ, Schenk D (2003) Alzheimer's disease: molecular understanding predicts amyloid
based therapeutics. Annu Rev Pharmacol Toxicol 43:545-584.
Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM (2004) Short-term lithium treatment
promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an
Shapiro EM, Gonzalez-Perez O, Manuel García-Verdugo J, Alvarez-Buylla A, Koretsky AP
(2006) Magnetic resonance imaging of the migration of neuronal precursors generated in the
adult rodent brain. Neuroimage 32:1150-1157.
Schuff N, Amend D, Ezekiel F, Steinman SK, Tanabe J, Norman D, Jagust W, Kramer JH,
Mastrianni JA, Fein G, Weiner MW (1997) Changes of hippocampal N-acetyl aspartate and
volume in Alzheimer's disease. A proton MR spectroscopic imaging and MRI study.
Neurology 49:1513-1521.
Silva R, Martins L, Longatto-Filho A, Almeida OF, Sousa N (2007) Lithium prevents
stress-induced reduction of vascular endothelium growth factor levels. Neurosci Lett 429:33-38.
Solomon DA, Ristow WR, Keller MB, Kane JM, Gelenberg AJ, Rosenbaum JF, Warshaw
MG (1996) Serum lithium levels and psychosocial function in patients with bipolar I
disorder. Am J Psychiatry 153:1301-1307.
Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK (2003) Lithium enhances
long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus.
J Neurochem 85:872-881.
ter Laak HJ, Renkawek K, van Workum FP (1994) The olfactory bulb in Alzheimer disease: a
morphologic study of neuron loss, tangles, and senile plaques in relation to olfaction.
Alzheimer Dis Assoc Disord 8:38-48.
Thomann PA, Dos Santos V, Toro P, Schönknecht P, Essig M, Schröder J (2009) Reduced
olfactory bulb and tract volume in early Alzheimer's disease--a MRI study. Neurobiol Aging
30:838-841.
von Gunten A, Kövari E, Bussière T, Rivara CB, Gold G, Bouras C, Hof PR, Giannakopoulos
P (2006) Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in
Whitman MC, Greer CA (2009) Adult neurogenesis and the olfactory system. Prog Neurobiol
89:162-175.
Wittko IM, Schänzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH (2009)
VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors
in the rostral migratory stream in vivo. J Neurosci 29:8704–8714.
Yamashima T, Tonchev AB, Yukie M (2007) Adult hippocampal neurogenesis in rodents and
primates: endogenous, enhanced, and engrafted. Rev Neurosci 18:67-82.
Yamashita T, Ninomiya M, Hernández Acosta P, García-Verdugo JM, Sunabori T, Sakaguchi
M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K
(2006) Subventricular zone-derived neuroblasts migrate and differentiate into mature
neurons in the post-stroke adult striatum. J Neurosci 26:6627-6636.
Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S, Ballard C (2006)
Altered neurogenesis in Alzheimer's disease. J Psychosom Res 61:311-316.
Figure legends
Figure 1. Representative photomicrograph (400 magnification) of a saggital section of adult
mouse brain immunostained for BrdU, showing the rostral migratory stream (RMS) and part
of the lateral ventricle (LV) and the olfactory bulb (OB). BrdU, 5-bromo-2-deoxyuridine.
Figure 2. Bar graph that depicts the stereologically estimated total number of BrdU+ cells in
the rostral migratory stream of control (n = 11) and lithium-treated mice (n = 9). Data are
presented as mean ± SD. No significant differences were observed between the two groups (t
Figure 2
C o n t r o l L i t h i u m
0 6 0 0 0 1 2 0 0 0 1 8 0 0 0 2 4 0 0 0 3 0 0 0 0
6 - w e e k t r e a t m e n t
N
u
m
b
e
r
o
f
B
r
d
U
+
c
e
ll
Table 1. Estimated total number of BrdU+ cells and body weight data
Group BrdU+ cell number Body weight gain (g) Final body weight (g)
Control 21628 ± 1042 1.47 ± 0.38 30.64 ± 0.99
Lithium 22184 ± 1645 -0.18 ± 1.36 27.76 ± 1.57