ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Ozone
decreases
sperm
quality
in
systemic
lupus
erythematosus
patients
Juliana
Farhat
a,
Sylvia
Costa
Lima
Farhat
a,
Alfésio
Luís
Ferreira
Braga
a,b,
Marcello
Cocuzza
c,
Eduardo
Ferreira
Borba
d,
Eloisa
Bonfá
d,
Clovis
Artur
Silva
d,e,∗ aGroupofEnvironmentalEpidemiologyStudies,LaboratoryofExperimentalAirPollution,FacultyofMedicine,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
bGroupofEnvironmentalExposureandRiskAssessmentStudies,Post-GraduatePrograminPublicHealth,UniversidadeCatólicade
Santos,Santos,SP,Brazil
cDivisionofUrology,FacultyofMedicine,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil dDivisionofRheumatology,FacultyofMedicine,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
eUnitofPediatricRheumatology,InstitutodaCrianc¸a,FacultyofMedicine,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received3November2014
Accepted6July2015
Availableonline23November2015
Keywords:
Systemiclupuserythematosus
Airpollution
Spermquality
Fertility
Cyclophosphamide
a
b
s
t
r
a
c
t
Objective:ToinvestigatethedeleteriouseffectsofairpollutantsexposureintheSaoPaulo
metropolitanregiononsemenqualityinsystemiclupuserythematosus(SLE).
Methods:Aseven-yearslongitudinalrepeated-measurespanelstudywasperformedatthe
LaboratoryofExperimentalAirPollutionandRheumatologyDivision.Twosemensamples
from28post-pubertalSLEpatientswereanalyzed.Dailyconcentrationsofairpollutants
exposure:PM10,SO2,NO2,ozone,CO,andmeteorologicalvariableswereevaluatedon90
daysbeforeeachsemencollectiondatesusinggeneralizedestimatingequationmodels.
Results:Intravenous cyclophosphamide (IVCYC) and ozone had an association with a
decreaseinspermqualityofSLEpatients.IVCYCwasassociatedwithdecreasesof64.3
millionofspermatozoa/mL(95%CI39.01–89.65;p=0.0001)and149.14millionof
spermato-zoa/ejaculate(95%CI81.93–216.38;p=0.017).Withregardtoozone,themostrelevantadverse
effectswereobservedfromlags80–88,whentheexposuretoaninterquartilerangeincrease
inozone9-daymovingaverageconcentrationledtodecreasesof22.9millionof
spermato-zoa/mL (95% CI 5.8–40.0; p=0.009) and 70.5 million of spermatozoa/ejaculate (95% CI
12.3–128.7;p=0.016).Furtheranalysisof17patientsthatneverusedIVCYCshowed
associa-tionbetweenexposuretoozone(80–88days)anddecreaseof30.0millionofspermatozoa/mL
(95%CI 7.0–53.0;p=0.011)and79.0 millionofspermatozoa/ejaculate (95% CI 2.1–155.9;
p=0.044).
Conclusion:Ozoneand IVCYChad a consistent adverse effecton semenqualityof SLE
patientsduringspermatogenesis.Minimizingexposuretoairpollutionshouldbetakeninto
account,especiallyforpatientswithchronicsystemicinflammatorydiseaseslivinginlarge
cities.
©2015ElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthor.
E-mail:clovis.silva@hc.fm.usp.br(C.A.Silva).
http://dx.doi.org/10.1016/j.rbre.2015.08.005
O
ozônio
diminui
a
qualidade
do
sêmen
em
pacientes
com
lúpus
eritematoso
sistêmico
Palavras-chave:
Lúpuseritematososistêmico
Poluic¸ãodoar
Qualidadedosêmen
Fertilidade Ciclofosfamida
r
e
s
u
m
o
Objetivo: Investigar os efeitosdeletérios da exposic¸ão aospoluentes do ar na Região
MetropolitanadeSãoPaulosobreaqualidadedosêmendepacientescomlúpuseritematoso
sistêmico(LES).
Métodos: Foifeitoumestudolongitudinaldepainelcommedidasrepetidasdeseteanosno
LaboratóriodePoluic¸ãoAtmosféricaExperimentaleReumatologia.Foramanalisadasduas
amostrasdesêmende28pacientescomLESpós-púberes.Foramavaliadasasconcentrac¸ões
diáriasdeexposic¸ãoaospoluentesdoarPM10,SO2,NO2,ozônioeCOevariáveis
meteo-rológicas90diasantesdecadadatadecoletadesêmencomousodométododeequac¸ões
deestimativasgeneralizadas.
Resultados: Aciclofosfamidaintravenosa(CICIV)eoozônioestiveramassociadosauma
diminuic¸ãonaqualidadedosêmendospacientescomLES.ACICIVesteveassociadaaum
decréscimode64,3milhõesdeespermatozoides/mL(IC95%39,01-89,65;p=0,0001)e149,14
milhõesdeespermatozoides/ejaculado(IC95%81,93-216,38;p=0,017).Emrelac¸ãoaoozônio,
osefeitosadversosmaisrelevantesforamobservadosentreoslags(intervalodetempo)80
e88,quandoaexposic¸ãoaumaconcentrac¸ãomédiadeozônioumintervalointerquartil
maioremnovediasmóveislevouaumdecréscimode22,9milhõesdeespermatozoides/mL
(IC95%5,8-40;p=0,009)e70,5milhõesdeespermatozoides/ejaculado(IC95%12,3-128,7;
p=0,016).Umaanálisemaisaprofundadados17pacientesquenuncausaramCICIVmostrou
associac¸ãoentreaexposic¸ãoaoozônio(80-88dias)eodecréscimode30milhõesde
esper-matozoides/mL(IC95%7-53;p=0,011)e79milhõesdeespermatozoides/ejaculado(IC95%
2,1-155,9;p=0,044).
Conclusão: OozônioeaCICIVtiveramumefeitoadversoconsistentesobreaqualidadedo
sêmendepacientescomLESduranteaespermatogênese.Deve-seconsideraraminimizac¸ão
daexposic¸ãoàpoluic¸ãodoar,especialmenteparapacientescomdoenc¸asinflamatórias
sistêmicascrônicasquevivemnasgrandescidades.
©2015ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Gonad function is severely affected in male SLE patients.
Recently,severespermabnormalitieswithtesticularatrophy,
high follicle-stimulating hormone (FSH) levels and
testi-cular Sertoli cell dysfunction associated with intravenous
cyclophosphamide(IVCYC)treatmentwere reported byour
groupinsystemiclupuserythematosus(SLE).1–3
Exposuretoairpollutantshasalsobeencorrelated with
male reproductive outcomes, especially sperm quality,4–9
howeverthisenvironmentalfactorwasnotstudiedinmale
SLEpopulationwithgonadalfunctionassessment.
Infact,airpollutioniscomposedofaheterogeneous
mix-tureofgasesandparticlesthatincludeozone(O3),particulate
matter(PM10), nitrates (NO), sulfur dioxide (SO2), toxic
by-product oftobacco smoke and carbon monoxide (CO) and
may trigger systemic inflammation and autoimmunity in
SLE.10,11
Therefore, theobjective ofthis study wastoinvestigate
prospectivelythecorrelationofairpollutantsexposure
con-centrations and semen quality in Sao Paulo metropolitan
regioninSLEpatients.
Material
and
methods
Alongitudinalrepeated-measurespanelstudywasperformed
with35nonsmokerspost-pubertalmaleSLEpatientsregularly
followedatthePediatricRheumatologyUnitandtheLupus
Clinics ofthe Rheumatology Division. All patients fulfilled
the American CollegeofRheumatology classification
crite-riaforSLE.12 None ofthemhad cryptorchidism, hydrocele,
hypospadia,testicular infection (e.g.,mumps),orchitis,
tes-ticularvasculitis,testicularcancer,ureteralimpairmentand
previoushistoryofanyscrotaloringuinalsurgery(e.g.,
varic-ocelectomy,vasectomyandherniarepair).NineSLEpatients
wereexcludedsincetheydidnotresidewithinthe
metropoli-tanregionofthecityofSaoPaulo,presentedazoospermiaor
hadonlyonesampleofspermcollected.Therefore,from
Jan-uary2000toJanuary2006,26SLEpatientsresidentofSaoPaulo
metropolitanregionperformedaglobalreproductivehealth
evaluationincludingtwospermsamplesofeachpatientwith
amedianintervalof1month(range0.7–8months).
TheEthicsCommitteeofourUniversityHospitalapproved
this study and aninformed consentwas obtainedfrom all
Globalreproductivehealthevaluation
Demographicdataandlifestylehabits:Currentage,disease
duration,yearsofeducation,smokingandalcohol
consump-tionwererecorded.
Urological evaluationandtesticular doppler ultrasound:
A clinical examination of the genitalia included
evalua-tion of testicles, epididymis, vas deferens, scrotum and
penis was performed blinded to the semen analysis. The
patientswere examined inawarm room(temperature not
inferior to 22◦C), with and without Valsalva manoeuver
and in both the standing and supine positions to assess
clinical varicocele.13,14 Testicular volumes were measured
using the Prader orchidometer. Testicular ultrasound was
performed in all SLE patients by an expert sonographer
blindedtothesemenanalysistoassessradiographic
varic-ocele and testicular volumes. Thelargest measurement in
each dimension was recorded and used to calculate the
testicular volume according to the formula to an ellipsoid
(length×width×thickness×0.52).Thenormalmeanvaluein
malepost-pubertaladolescentsandadultsis15±8mL.15Low
testicularvolumewasdefinedifaSLEpatienthadareduced
testicularvolumebyPrader’sorchidometerand/orultrasound.
VaricocelewasdefinedifaSLEpatienthadpresented
clini-calorradiographicenlargementofthepampiniformvenous
plexusinthescrotum.13
Semen analysis and anti-sperm antibodies: Fifty-two
spermanalysiswereperformedbytwoexpertmedical
tech-nologistswhowereblindedtotheotherparameters.Sperm
volume,concentration,totalspermcount(totalspermatozoa
per ejaculate), progressivemotility were carried out based
onguidelinesoftheWorldHealthOrganization(WHO).16All
patientscollectedatleasttwosemensamples(median
inter-valof1month,range0.7–8months)after48–72hofsexual
abstinence.Thespermatozoawereanalyzedbymanualhand
countaswellasbyacomputer-assistedsemenanalysis
sys-temunder400×magnification,usinganHTM-2030.Eachslide
wasscannedtoestimatethenumberofspermatozoaperfield
equivalentto1mL,toobtainanapproximatesperm
concen-tration in millions of spermatozoa per mL of semen. The
motilityofeachspermatozoawasgraded‘a’(rapidprogressive
motility),‘b’(sloworsluggishprogressivemotility),‘c’
(non-progressivemotility)and‘d’(nomotility).17Thepresenceof
antispermantibodieswasdeterminedbydirectImmunobead
test using ImmunobeadR rabbit anti-human Ig (IgA, IgG,
and IgM) kits (Irvine Scientific, Santa Ana, CA, USA)in all
patients.
Hormonal status: Hormone determinations were
per-formed atstudy entry blinded to the other parameters of
gonadalfunction:FSH(normalvalue:1–10.5IU/L)andmorning
totaltestosterone(271–965ng/dL)weredetectedby
fluoroim-munoassayusingDELFIAtime-resolvedfluoroimmunoassay
kits(Wallac,Turku,Finland).Intra-andinter-assaycoefficients
of variation were limited to 3.5% and 2.1%, respectively.
Inhibin B levels [normal value: 74–470pg/mL (12–17 years
old)and 60–300pg/mL(18–50 yearsold)] weremeasured by
enzymatically amplified, two-site, two-step, sandwich-type
immunoassay(DiagnosticSystemsLaboratories,Inc.,Webster,
TX,USA).Intra-andinter-assaycoefficientsofvariationwere
limited to 3.5–5.6% and 6.2–7.6%, respectively. Gonadal
hormonal abnormality was considered if the testosterone
and/orinhibinBserumlevelswerereducedortheFSHserum
levelswereincreased.
Clinicalevaluationandtreatment
SLEdisease activity andcumulativedamageatthetimeof
study entry were measured in all patients, using the SLE
Disease Activity Index (SLEDAI)18 and the Systemic Lupus
InternationalCollaboratingClinics/ACR(SLICC/ACR)Damage
Index.19Dataconcerningthetherapyweredetermined.
Airqualityandmeteorologicaldata
Dailyrecordsofstudiedpollutants,includingO3(thehighest
hourlyaverage),SO2(24-haverage),NO2(thehighesthourly
average),PM10 (24-haverage)andCO(thehighest8-h
mov-ingaverage),wereobtainedfortheentirestudyperiodfrom
the Sao Paulo StateEnvironmental Agency (CETESB) by 13
automatic monitoringstation spread aroundthe city.PM10
concentration (betaradiation – FH62lNGraseby Andersen)
was measured in12 ofthese stations. The highest hourly
averageofO3(ultraviolet–ThermoE.I.I-Model49)and NO2
(chemiluminescence–ThermoE.I.I-Model42)wasmeasured
atfour stations.Thehighest8hmovingaveragewas
mea-suredatfivestationsforCO(non-dispersiveinfrared–Thermo
E.I.I-Model48)andat13stationsforSO2(pulse
fluorescence-ultraviolet – Thermo E.I.I-Model 43).20 All pollutants were
measured from 00:01 AM to 12:00 PM. The average of all
stations that measured each pollutant was adopted as an
exposurestatusthroughoutthecity,sinceairpollutantslevels
recordedineachstationwerehighlycorrelatedwiththe
oth-ers.Thedailyminimumtemperatureandmean relativeair
humiditywereobtainedfromtheInstituteofAstronomyand
GeophysicsattheUniversityofSãoPaulo.Pollutants’
concen-trationandmeteorologicalvariableswereevaluateddailyon
90daysbeforesemencollectiondates.
Statisticalanalysis
We usedthe generalized estimatingequation model (GEE),
consideringfixedeffectsforrepeatedmeasurementsto
esti-mate the association and effect of pollutants on sperm
concentration, sperm count and sperm progressive
motil-ity. S-Plus 2000 Professional Release 3 software (MathSoft
Inc.,Seattle, WA, USA)wasemployed,adjustingthe model
for the independent variables by way of an exchangeable
correlation asaworkingmatrix,whichassumes equal
cor-relation formeasurementsineach subject. Thedependent
variablesweredefinedbasedonthefifthpercentileofthe
ref-erencelimitsofWHOguidelines forsperm analysis:sperm
concentration (minimum value 15 million per mL), total
sperm count(minimumvalue39 millionperejaculate)and
spermprogressivemotility(sumofpercentageof“a”,“b”of
spermatozoamotility–minimumvalue32%).Theregressions
were adjustedforindependentvariables:current age,years
ofeducation,smoking,alcoholconsumption,timeofsexual
abstinence, reduced testicular volume, presence of
Table1–Demographicdata,diseaseactivity,hormoneevaluation,urologicalevaluation,spermanalysisandtreatmentof malesystemiclupuserythematosus(SLE)patients.
Characteristics Referencevalues Patientvalues WithoutIVCYCuse WithIVCYCuse p
(n=26) (n=17) (n=9)
Demographicdata
Ageatstudyentry,years 29.8(8.9) 29.5(2.1) 30.4(3.2) 0.8
AgeatSLEonset,years;mean(SD) 20.4(9.6) 20.3(10.4) 19.7(8.5) 0.8
Ageatspermarche,years;mean(SD) 12.9(1.0) 12.9(1.2) 12.78(0.6) 0.7
Diseaseduration,years;mean(SD) 9.3(6.5) 8.7(6.9) 10.6(5.8) 0.5
Educationallevel,years;mean(SD) 10.0(3.3) 10.1(2.9) 9.8(3.0) 0.8
Smoking,n(%) 4(15) 4(28.6) 0(0) 0.3
Alcoholconsumption,n(%) 8(31) 5(29.4) 3(33.3) 0.5
SLEDAIscores,n(%)
>4 5(19.2) 3(17.6) 2(22.2) 0.46
>8 3(11.5) 1(5.9) 2(22.2)
Hormoneevaluation
Folliclestimulatinghormone,IU/liter;mean(SD) 7.6(5.8) 6.3(5.9) 10.0(5.2) 0.16
Elevatedlevels;n(%) 7(26.7) 3(17.6) 4(44.4) 0.34
InhibinB,pg/mL;mean(SD) 125.5(78.0) 138.8(73.6)
3(17.6)
97.3(84.5) 0.22
Decreasedlevels;n(%) 7(26.7) 500.5(264.8)
2(11.8)
4(44.4) 0.34
Totaltestosterone,ng/dl;mean(SD) 526.3(243.3) 576.8(201.2) 0.5
Decreasedlevels;n(%) 2(7.7) 0(0) 0.5
Intravenouscyclophosphamide(IVCYC)use 0
Currentuse;n(%) 9(34.6)
Previoususe;n(%) 8.5(12.01)
Cumulativedose(g);(mean/SD) 9(34.6)
Numberofpulsetherapy;n(%) 1.69(1.47)
Durationoftreatment;(meanyears/SD)
Othersdrugsthatalterspermqualityn(%) 10(38.5) 5(29.4) 5(55.6) 0.2
Urologicalevaluationtesticular
Reducedvolume(clinicalandUS);n(%) 7(26.9) 5(29.4) 2(22.2) 0.4
Clinicalorradiographicvaricocele;n(%) 11(42.3) 7(41.2) 4(44.4) 1.0
Spermanalysis WHO2010
Sexualabstinence,days;(median/IQR) ≥2 3(2.5)
Spermvolume,mL;(mean/SD) ≥1.5 2.3(1.2) 2.1(0.9) 2.9(1.5) 0.1
SpermpH;(mean/SD) ≥7.2 7.6(0.3) 7.6(0.3) 7.6(0.3) 1.0
Spermconcentration,×106/mL;(mean/SD) >15 63.2(72.0) 86.3(77.8) 19.6(28.7) 0.02 Totalspermcount,×106perejaculate(mean/SD) >39 147.3(223.2) 209.9(302.4) 46.1(55.6) 0.1
Progressivemotility,%;(mean/SD) >32 53.0(23.0) 57.6(17.7) 47.0(26.4) 0.2
Antispermantibodies,%;(mean/SD) <20 29.0(14.4) 29.3(14.8) 28.8(14.7) 0.9
Resultsarepresentedinn(%),mean±standarddeviationormedian(interquartilerange).
antibodies,SLEdiseaseactivityandcumulativedamage, pred-nisone use, immunosuppressive use (IVCYC, azathioprine, mycophenolate mofetil and methotrexate), use of others medicationsthatalterspermquality (angiotensin-converting-enzyme inhibitor, spironolactone, cimetidine, haloperidol, carbamazepineand thalidomide),and factors regarding air pollutants: temperature, relative humidity and daily con-centrationofthe pollutants. Thelag structurebetween air pollutantexposureandthedependentvariableswasassessed usinglagsof0–90daysandmovingaveragesof2,8,and 9 daysforpollutantsthatwasstatisticallysignificant on sin-gle models. For instance, a 2 day moving average is the mean of the pollutants levels in the concurrent and pre-vious day assigned for the concurrent day. Changes were analyzed,mainly,inrespecttospecificperiodsof spermato-zoa development,which correspondtoepididymalstorage,
development ofsperm motility and total duration of sper-matogenesis (0–9, 10–14 and 70–90 days before collection, respectively).21,22 Single-pollutantmodelswereusedforthe
analysis.However,ifmorethanonepollutanthada
signifi-canteffectontheoutcomethentwo-pollutantmodelswere
adopted.Pearsoncorrelationcoefficientswereestimatedfor
airpollutantvariables.Effectswerereportedasdecreasesin
theoutcomes[withtherespective95%confidenceinterval(CI)]
foraninterquartilerange(IQR)increaseineachpollutant.
Results
Demographicdata,diseaseactivity,hormoneevaluation,
uro-logicalevaluation,spermanalysisandtreatmentofmaleSLE
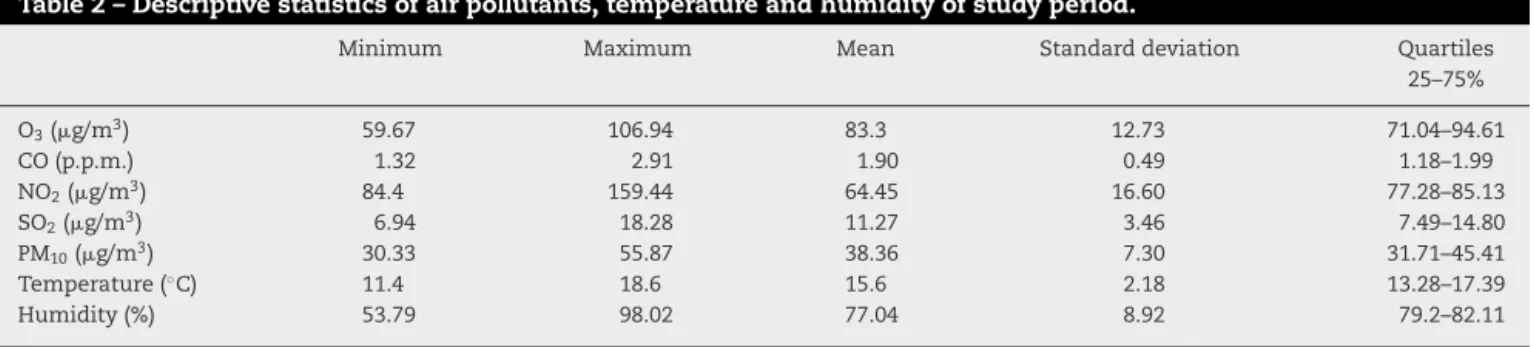
Table2–Descriptivestatisticsofairpollutants,temperatureandhumidityofstudyperiod.
Minimum Maximum Mean Standarddeviation Quartiles
25–75%
O3(g/m3) 59.67 106.94 83.3 12.73 71.04–94.61
CO(p.p.m.) 1.32 2.91 1.90 0.49 1.18–1.99
NO2(g/m3) 84.4 159.44 64.45 16.60 77.28–85.13
SO2(g/m3) 6.94 18.28 11.27 3.46 7.49–14.80
PM10(g/m3) 30.33 55.87 38.36 7.30 31.71–45.41
Temperature(◦C) 11.4 18.6 15.6 2.18 13.28–17.39
Humidity(%) 53.79 98.02 77.04 8.92 79.2–82.11
O3,ozone;CO,carbonmonoxide;NO2,nitrogendioxide;SO2,sulfurdioxide;PM10,particulatematter.
corticosteroids and chloroquine diphosphate treatment beforepuberty.Themeantimeintervalbetweenthelastdose ofIVCYCandspermcollectionwas5.4±3.7years.
SpermevaluationsofSLEpatientsshowedthatthemedian ofsexualabstinence,meansofspermvolume,pH,sperm con-centration,totalspermcountandspermprogressivemotility valueswereattheparametersregardedaspercentile5%by WHOguidelines(Table1).
Thehumidityandthetemperatureandtherangeof
vari-ation in pollutant concentrations and weather conditions
duringtheassessedperiodarepresentedinTable2.Noneof
thepollutantsurpassedtheBraziliannationalstandardsfor
airqualitylimitsinthestudiedperiod.
Primary airpollutants were highlycorrelated witheach
other,withPearson’scoefficientsvaryingfrom0.48(SO2and
NO2)to0.77(PM10andCO)p<0.001andp<0.001respectively.
Ozone’slowest Pearson’scoefficient wasobserved withCO
(0.28), and ozone’s highest coefficient was with NO2 (0.60)
p=0.04and p<0.001 respectively.SinceNO2measurements
wereperformedonadailybasis,onthosedayswithhighNO2
concentrations,therewasahighformationofthesecondary
pollutantasozone.
Intheregressionmodels,onlyIVCYCuseandozonehad
anassociationwithdecreaseinspermquality.
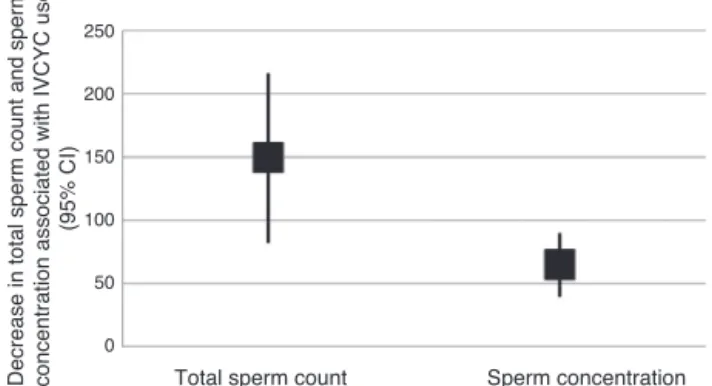
IVCYCusewasassociatedwithdecreasesinsperm
con-centration (64.3million/mL; 95% CI,39.01–89.65; p=0.0001),
total sperm count (149.14 million per ejaculate; 95%
CI, 81.93–216.38; p=0.017) and progressive sperm motility
(20.94%;95%CI,4.75–37.05;p=0.001)intheevaluatedperiods
(Table3).
Withregard toozone,anincrease ofinterquartile range
(IQR)of23.57g/m3inthispollutantaveragedoverthe0–90
dayperiodwasassociatedwithadecreaseof30.6millionof
spermatozoa/mL (95%CI, 2.0–59.3; p=0.040)in sperm.The
specific analysis of daily exposure to ozone revealed that
the critical periodwas 80–88days beforethe dateof
sam-plecollectionwithacumulativedecrease of22.9 millionof
spermatozoa/mL(95%CI,5.8–40.0;p=0.009)and70.5million
ofspermatozoa/ejaculate(95%CI,12.3–128.7;p=0.016)
asso-ciated withozone cumulativeeffectexposure toanIQRof
23.57g/m3(Fig.1).Noeffectswereobservedwiththeothers
pollutantsonspermconcentrationortotalcount.
Nopollutants’effectsonprogressivespermmotilitywere
observedinthetwoweeksafterexposure(periodofmotility
Table3–Decreaseintotalspermcountandspermconcentrationassociatedwithexposuretoozone80–90daysbefore thedateofspermsamplecollect.
Decrease(95%CIa)O
3(IQRb=23.57g/m3)
Totalspermcount(millionperejaculate) Spermconcentration(millionpermL)
Decrease (95%CI)a Decrease (95%CI)a
Lag80 21.3 (4.8;37.8)c 5.1 (0.2;10.0)e
Lag81 21 (7.8;34.1)d 2.8 (−1.4;7.1)
Lag82 3 (−18.7;24.8) 1.6 (−10.3;7.1)
Lag83 8.9 (−10.9;28.8) 2 (−4.0;7.9)
Lag84 5.2 (−30.2;19.8) 2.9 (−10.7;4.9)
Lag85 13.8 (−16.3;43.9) 3.4 (−6.7;13.6)
Lag86 26.8 (4.3;49.4)c 6.1 (−1.24;13.4)
Lag87 12.6 (−3.6;28.7) 4.1 (−0.8;9.0)
Lag88 9.9 (−7.72;27.5) 5.1 (0.2;9.9)
9daymovingaverage(80–88) 70.5 (12.3;128.7)c 22.9 (5.8;40.0)e
Lagn=ndaysafterexposure. a Confidenceinterval. b Interquartilerange. c p=0.01.
Total sperm count Sperm concentration
Decrease in total sperm count and sperm concentration associated with IVCYC use
(95% CI)
250
200
150
100
50
0
Fig.1–Effectsofintravenouscyclophosphamideonsemen quality.
development),but wefound anegativeeffectin weekssix
andsevenafterexposuretoozone(fromlag31tolag38).The
eight-daycumulativeeffectofexposuretoanIQRincreaseof
23.57g/m3inozonewasassociatedwith5.7%decrease(95%
CI,0.6–10.7;p=0.023)intheprogressivespermmotility.
Further analysis of 17 patients that never used IVCYC
showednine-daycumulativeeffect(80–88days)ofexposureto
anIQRincreaseinozonewasassociatedwithadecreaseof30.0
millionofspermatozoa/mL(95%CI,7.0–53.0;p=0.011),79.0
millionofspermatozoa/ejaculate(95%CI,2.1–155.9;p=0.044).
Theeight-daycumulativeeffect(31–38days)wasassociated
withadecreaseof6.6%(95%CI,2.9–10.3;p=0.001)inthe
pro-gressivespermmotility.
No statistically significant associations were found
betweenotherpollutants andother dependentvariables at
thespecificperiodsofspermatozoadevelopment.
Discussion
Toourknowledge,thiswasthefirststudytoidentifythelag
structureeffectsofexposuretotroposphericpollutiononthe
spermqualityinSLEpatients.TheozoneandIVCYChad
sig-nificantdeleteriouseffectonspermatogenesis.
Themainstrength ofthe presentstudy wasacomplete
assessment of gonadal parameters in post-pubertal lupus
patientsandanevaluationofairpollutantsinametropolitan
areaofalargecity.Inaddition,thestudydesignof
repeated-measures of sperm analysis had advantages to require a
smallernumberofindividualsthanacompletelyrandomized
studyanditprovidesmoresuitableconditionsofco-variables
thatmayinfluencethespermquality,withouttheneedofa
healthycontrolgroupevaluation.23Moreover,theanalysisofa
subgrouppatientnotexposedtoIVCYC,aknowngonadotoxic
treatment,allowedamoreaccuratedefinitionofozonesperm
harmful effect. The main limitations of the present study
werethesmallsamplesize,particularlyinthoseSLEpatients
thatwerepreviouslyexposedtocyclophosphamide,andthe
factthat fixedmonitoring stationsdid notfully reflectthe
individualvariationinexposuretopollutants.Inaddition,the
cityofSãoPaulohasapopulationof10,886,518peoplewith
morethan 6million vehicles. This automotive fleetis the
mainsourceofairpollutionanditisobservedthattheozone
concentration has progressively increased.20 Airpollutants
levels recorded in the stations in São Paulo were highly
correlatedwitheachotherandevenozonepresentedpositive
statistically significant correlations to primary pollutants
asobservedinthisstudy.Thelackofeffectofothers
pollu-tantsonspermqualitymayresultfromthehighcorrelation
amongairpollutantsobservedherein.Infact,eachanalyzed
pollutantcanbeconsideredagoodindependentmarkerfor
complexmixtureofairpollution.Inthisway,itispossibleto
assumethattheeffectsonthesemenqualitywereduetothe
actionofallcriteriaairpollutants.
Airpollutionconsistsofaheterogeneousmixtureofgases
and particles that includeO3. Oxidativestress and
inflam-mation induced bythis pollutantmay result inrespiratory
disorders,24–26 as well as contribute to a stateof systemic
inflammation27,28andautoimmunity.10
Additionally,ozonecaninducetesticularoxidativestress
and excessive generation of freeradicals thatcan provoke
damageon maturespermatozoa and germ cells apoptosis,
resultinginreducedspermconcentrationandmotility,29as
observedherein.Inthisregard,Sokoletal.8observedan
asso-ciation between spermquality and exposureto ozone and
Hansenetal.9reportedthesameassociationwithincreaseof
PM2.5.Airpollutionmayalsoinfluencemalefertility7–9dueto
endocrinedisruption,spermDNAdamageandtoxicity
medi-atedbythearylhydrocarbonreceptor.30,31
Interestingly,exposuretoairpollutantsmayaffectgerm
celldevelopmentincludingtheentireperiod(90days)or
spe-cificperiodsofspermdevelopmentbeforesamplecollection
(epididymal storageor developmentof sperm motility).4,8,9
Hammoudetal.4foundthatairpollutionleadtoadecreasedin
progressivemotilityaftermorethan4weeksoftheexposure.
Indeed,thespermmotilityisalateeventin
spermatogene-sisandduringepididymistransit,thespermatozoaundergo
changes in morphology, chemistry and motility. Ourstudy
suggestedthatexposuretopollutantcouldbedeleteriousfor
progressivemotility inSLEpatients, evenbeforethe sperm
reachtheepididymis(0–14daysbeforesemencollecting).
Moreover,weconfirmedandextendedthroughacomplex
statisticalmodelthatIVCYCwasanimportantcauseofinjury
tospermatogenesis1–3,13,14,32–37emphasizingtherelevanceof
cryopreservationofsemenforpost-pubertalmalesinorderto
guaranteethepossibilityofreproductionafterthis
immuno-suppressivetherapy.38,39
Inconclusion,ozoneandtheuseofIVCYChadaconsistent
adverseeffectonsemenqualityofSLEpatients.Wefurther
identifiedthatthisabnormalityisnotrestrictedtoearly
sper-matogenesis but alsooccurs in later stages. Consequently,
minimizing exposureto airpollution should be takeninto
account,especiallyforpatientsresidinginlargecities.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
This study was supported by grants from Conselho
NacionaldeDesenvolvimentoCientíficoeTecnológico(CNPq
de Amparo à Pesquisa do Estado de São Paulo (FAPESP
2004/07832-2toCAS,2005/56482-7toCASand 2008/58238-4
toCAS),FedericoFoundation(toCASandEB)andbyNúcleo
deApoioàPesquisa“SaúdedaCrianc¸aedoAdolescente”da
USP(NAP-CriAd)toCAS.
r
e
f
e
r
e
n
c
e
s
1. SoaresPM,BorbaEF,BonfaE,HallakJ,CorreaAL,SilvaCA. Gonadevaluationinmalesystemiclupuserythematosus. ArthritisRheum.2007;56:2352–61.
2. SuehiroRM,BorbaEF,BonfaE,OkayTS,CocuzzaM,Soares PM,etal.TesticularSertolicellfunctioninmalesystemic lupuserythematosus.Rheumatology.2008;47:1692–7. 3. VecchiAP,BorbaEF,BonfáE,CocuzzaM,PieriP,KimCA,etal.
Penileanthropometryinsystemiclupuserythematosus patients.Lupus.2011;20:512–8.
4. HammoudA,CarrellDT,GibsonM,SandersonM,Parker-Jones K,PetersonCM.Decreasedspermmotilityisassociatedwith airpollutioninSaltLakeCity.FertilSteril.2010;93:1875–9. 5. SelevanSG,BorkovecL,SlottVL,ZudováZ,RubesJ,Evenson
DP,etal.Semenqualityandreproductivehealthofyoung Czechmenexposedtoseasonalairpollution.EnvironHealth Perspect.2000;108:887–94.
6. GuvenA,KayikciA,CamK,ArbakP,BalbayO,CamM. Alterationsinsemenparametersoftollcollectorsworkingat motorways:doesdieselexposureinducedetrimentaleffects onsemen?Andrologia.2008;40:346–51.
7. RubesJ,SelevanSG,EvensonDP,ZudovaD,VozdovaM, ZudovaZ,etal.Episodicairpollutionisassociatedwith increasedDNAfragmentationinhumanspermwithoutother changesinsemenquality.HumReprod.2005;20:2776–83. 8. SokolRZ,KraftP,FowlerIM,MametR,KimE,BerhaneKT.
Exposuretoenvironmentalozonealterssemenquality. EnvironHealthPerspect.2006;114:360–5.
9. HansenC,LubenTJ,SacksJD,OlshanA,JeffayS,StraderL, etal.Theeffectofambientairpollutiononspermquality. EnvironHealthPerspect.2010;118:203–9.
10.FarhatSC,SilvaCA,OrioneMA,CamposLM,SallumAM, BragaAL.Airpollutioninautoimmunerheumaticdiseases:a review.AutoimmunRev.2011;11:14–21.
11.VidottoJP,PereiraLA,BragaAL,SilvaCA,SallumAM,Campos LM,etal.Atmosphericpollution:influenceonhospital admissionsinpaediatricrheumaticdiseases.Lupus. 2012;21:526–33.
12.HochbergMC.UpdatingtheAmericanCollegeof Rheumatologyrevisedcriteriafortheclassificationof systemiclupuserythematosus.ArthritisRheum. 1997;40:1725.
13.NukumizuLA,Gonc¸alvesSaadC,OstensenM,AlmeidaBP, CocuzzaM,Gonc¸alvesC,etal.Gonadalfunctioninmale patientswithankylosingspondylitis.ScandJRheumatol. 2012;41:476–81.
14.AlmeidaBP,SaadCG,SouzaFH,MoraesJC,NukumizuLA, VianaVS,etal.TesticularSertolicellfunctioninankylosing spondylitis.ClinRheumatol.2013;32:1075–9.
15.ColliAS,BerquioES,MarquesRM.Crescimentoe desenvolvimentopubertárioemcrianc¸aseadolescentes brasileiros.In:ColliAS,BerquioES,MarquesRM,editors. Volumetesticular.1sted.SãoPaulo:BrasileiradeCiências Ltda;1984.p.1–34.
16.CooperTG,NoonanE,vonEckardsteinS,AugerJ,BakerHW, BehreHM,etal.WorldHealthOrganizationreferencevalues forhumansemencharacteristics.HumReprodUpdate. 2010;16:231–45.
17.RowePJ,ComhaireFH,HargreaveTB,MahmoudAM.World HealthOrganization(WHO)forthestandardized
investigation,diagnosisandmanagementoftheinfertile men.Cambridge:CambridgeUniversityPress;2000. 18.BombardierC,GladmanD,UrowitzMB,KaronD,ChangCH.
TheCommitteeonPrognosisStudiesinSLE.Derivationofthe SLEDAI:adiseaseactivityindexforlupuspatients.Arthritis Rheum.1992;35:630–40.
19.GladmanD,GinzlerE,GoldsmithC,FortinP,LiangM,Urowitz M,etal.Thedevelopmentandinitialvalidationofthe systemiclupusinternationalcollaboratingclinics/American CollegeofRheumatologydamageindexforsystemiclupus erythematosus.ArthritisRheum.1996;39:363–9.
20.CETESB.Availableat:http://www.cetesb.sp.gov.br/Ar/
arautomatica.asp[accessed10.06.13].
21.JohnsonL,WelschTHJr,WilkerCE.Anatomyandphysiology ofthemalereproductivesystemandpotentialtargetsof toxicants.In:SipesIG,McQueenCA,GandolfiAJ,editors. Comprehensivetoxicology.NewYork:Pergamon;1997.p. 5–98.
22.RobaireB,HermoL.Efferentducts,epididymisandvas deferens.In:KnobilE,NiellJ,editors.Physiologyof reproduction.3rded.NewYork:RavenPress;1994.p. 999–1000.
23.AndreßHJ,GolschK,SchmidtAW.Appliedpaneldata analysisforeconomicandsocialsurveys.1sted.Berlin: Springer-Verlag;2013.
24.KosmiderB,LoaderJE,MurphyRC,MasonRJ.Apoptosis inducedbyozoneandoxysterolsinhumanalveolarepithelial cells.FreeRadicBiolMed.2010;48:1513–24.
25.FragaJ,BotelhoA,SáA,CostaM,QuaresmaM.Thelag structureandthegeneraleffectofozoneexposureon pediatricrespiratorymorbidity.IntJEnvironResPublic Health.2011;8:4013–24.
26.FarhatSC,AlmeidaMB,Silva-FilhoLV,FarhatJ,RodriguesJC, BragaAL.Ozoneisassociatedwithanincreasedriskof respiratoryexacerbationsincysticfibrosispatients.Chest. 2013;144:1186–92.
27.GryparisA,ForsbergB,KatsouyanniK,AnalitisA,TouloumiG, SchwartzJ,etal.Acuteeffectsofozoneonmortalityfromthe airpollutionandhealth:aEuropeanapproachproject.AmJ RespirCritCareMed.2004;15(170):1080–7.
28.WatkinsonWP,CampenMJ,NolanJP,CostaDL.
Cardiovascularandsystemicresponsestoinhaledpollutants inrodents:effectsofozoneandparticulatematter.Environ HealthPerspect.2001;109:539–46.
29.AgarwalA,SalehRA,BedaiwyMA.Roleofreactiveoxygen speciesinthepathophysiologyofhumanreproduction.Fertil Steril.2003;79:829–43.
30.KnucklesTL,DreherKL.Fineoilcombustionparticle bioavailableconstituentsinducemolecularprofilesof oxidativestress,alteredfunction,andcellularinjuryin cardiomyocytes.JToxicolEnvironHealth.2007;70:1824–37. 31.VerasMM,CaldiniEG,DolhnikoffM,SaldivaPH.Airpollution
andeffectsonreproductive-systemfunctionsgloballywith particularemphasisontheBrazilianpopulation.JToxicol EnvironHealthBCritRev.2010;13:1–15.
32.SilvaCA,HallakJ,PasqualottoFF,BarbaMF,SaitoMI,KissMH. Gonadalfunctioninmaleadolescentsandyoungmaleswith juvenileonsetsystemiclupuserythematosus.JRheumatol. 2002;29:2000–5.
33.MoraesAJ,PereiraRM,CocuzzaM,CasemiroR,SaitoO,Silva CA.Minorspermabnormalitiesinyoungmalepost-pubertal patientswithjuveniledermatomyositis.BrazJMedBiolRes. 2008;41:1142–7.
35.SilvaCA,CocuzzaM,BorbaEF,BonfáE.Cutting-edgeissuesin autoimmuneorchitis.ClinRevAllergyImmunol.
2012;42:256–63.
36.Rabelo-JúniorCN,FreiredeCarvalhoJ,LopesGallinaroA, BonfáE,CocuzzaM,SaitoO,etal.Primaryantiphospholipid syndrome:morphofunctionalpenileabnormalitieswith normalspermanalysis.Lupus.2012;21:
251–6.
37.Rabelo-JúniorCN,BonfáE,CarvalhoJF,BonfáE,CocuzzaM, SaitoO,etal.Penilealterationswithseveresperm
abnormalitiesinantiphospholipidsyndromeassociatedwith systemiclupuserythematosus.ClinRheumatol.
2013;32:109–13.
38.SilvaCA,BrunnerHI.Gonadalfunctioningandpreservation ofreproductivefitnesswithjuvenilesystemiclupus erythematosus.Lupus.2007;16:593–9.