Accepted
Article
This article has been accepted for publication and undergone full peer review but has not
been through the copyediting, typesetting, pagination and proofreading process, which may
lead to differences between this version and the Version of Record. Please cite this article as
Article Type: Original Article
Visceral leishmaniasis-associated nephropathy in hospitalized Brazilian patients: new insights based on kidney injury biomarkers
Gdayllon Cavalcante Meneses1, Elizabeth De Francesco Daher5 , Geraldo Bezerra da Silva Junior2, Gabriela Freire Bezerra1, Thaiany Pereira da Rocha3, Isabella Evelyn Prado de Azevedo3, Alexandre Braga Libório4 and Alice Maria Costa Martins1,3.
1
Pharmacology Post-Graduate Program, School of Medicine, Federal University of Ceará, Fortaleza,
Brazil
2
Collective Health Graduate Program, School of Medicine, Health Sciences Center, University of
Fortaleza, Fortaleza, Brazil
3
Clinical and Toxicological Analysis Department, School of Pharmacy, Federal University of Ceará,
Fortaleza, Brazil
4
Medical Sciences Post-Graduate Program, University of Fortaleza, Fortaleza, Brazil
5
Medical Sciences Post-Graduate Program, School of Medicine, Federal University of Ceará,
Fortaleza, Brazil
Abstract
Objective: To evaluate the usefulness of early acute kidney injury (AKI) biomarkers in clinical management of visceral leishmaniasis.
Accepted
Article
Results: VL patients had hyponatremia, hypoalbuminemia, hypergammaglobulinemia, hematologic and hepatic disorders. AKI was found in 46%, and 1 death (2%) occurred. The AKI group had significant longer hospital stay, lower levels of IFN-y and higher levels of CRP, more clinical renal abnormalities and higher levels of sNGAL, uNGAL, uKIM-1 and uMCP-1. Overall, sNGAL, uKIM-1 and uMCP-1 showed correlations with important clinical renal abnormalities, such as proteinuria, albuminuria, serum creatinine and glomerular filtration rate using adjusted correlations with CRP and IFN-y. Only sNGAL showed an early association with AKI development (OR=2.78, 95% CI=1.429-5.428, per each increase of 50ng/mL), even after adjusting for clinical signals of VL severity and for immune biomarkers. Moreover, sNGAL showed a better performance in predicting AKI development (AUC-ROC=0.81, 95% CI=0.69-0.93; cut-off=154ng/mL, sensitivity=82.6%, specificity=74.1%,
p<0.001).
Conclusions. Visceral leishmaniasis-associated nephropathy showed important proximal tubular injury and glomerular inflammation. Serum NGAL showed an early association with VL-associated nephropathy and may be used to improve clinical management strategies and decrease
morbimortality in VL patients.
Keywords: Visceral leishmaniasis, acute kidney injury, kidney biomarkers, NGAL.
Introduction
Visceral leishmaniasis (VL), also known as kala-azar, a disease caused by parasites of the
Leishmania
genus, is a potentially fatal parasitic disease if left untreated (1). VL severity
depends on the
Leishmania
species and the
host’s immune response
(2). VL patients may
have intense parasitism of the reticuloendothelial system with marked anemia, leukopenia,
thrombocytopenia and an important increase in plasma gamma globulin levels (3). VL
affects approximately 400,000 people around the world and is responsible for up to 40,000
deaths per year (4). In Brazil, VL is endemic with 3298 cases in 2015, being responsible for
272 deaths, with a lethality rate of almost 8% (5,6). VL-associated nephropathy has been
related with higher mortality in these patients (7
–
9).
Acute kidney injury (AKI) is a frequent manifestation in VL (up to 46%), being related with
amphotericin B use, secondary infections, hemodynamic abnormalities, and the parasitic
infection itself
(7). Early AKI diagnosis is crucial to prevent further renal complications, need
Accepted
Article
serum creatinine-based criteria such as RIFLE, AKIN and KDIGO classifications, where the
increased level appears when kidney injury is already advanced (10). Hence, novel kidney
injury biomarkers have been widely studied with a paramount importance in early detection
of kidney injury in different clinical settings (11).
Promising AKI biomarkers for use in clinical practice include kidney injury molecule-1
(KIM-1) and human neutrophil gelatinase
–
associated lipocalin (NGAL). Human NGAL has
been extensively studied as a novel biomarker of kidney injury (12,13). It belongs to the
lipocalin family, extracellular proteins with a molecular mass of 25 kDa (14). NGAL is
produced and secreted by activated neutrophils and poorly expressed in other tissues
during normal conditions, including the kidney (15). Due to its degradation resistance and
small molecular size, NGAL is readily excreted and easily detected in the urine (16). Also,
NGAL expression is induced in the proximal and distal nephron segments during kidney
injury, resulting in elevated serum and urinary levels (17,18). Both urine and serum NGAL
levels have been evaluated in a variety of diseases as an independent predictor of AKI, with
high sensitivity and specificity in the early diagnosis of AKI onset (17).
To the best of our knowledge, there are no studies evaluating the role of NGAL and KIM-1 in VL-associated nephropathy. In a preliminary study of our group with VL patients, elevated levels of urinary MCP-1 (a promising new biomarker of chronic kidney disease) were observed before the start of specific treatment in a cross-sectional design (19). Considering that amphotericin B is the most effective treatment against Leishmania and it is a potentially nephrotoxic drug (1), early diagnosis of kidney injury in VL patients may direct better clinical management strategies. Hence, we hypothesized that NGAL, urinary MCP-1 and urinary KIM-1 levels may provide new insights about VL-associated nephropathy and also predict or identify patients at higher risk for AKI development during hospital stay.
Materials and methods
Study design and VL patient selection
We performed a prospective and single-center study from April 2015 to January 2017, at São José Hospital, a reference hospital for Infectious Diseases in the state of Ceará, Northeast of Brazil.
Accepted
Article
users, and patients with hypertension and diabetes mellitus. Therefore, we aimed to exclude risk factors for kidney disease by evaluating only VL infection and its treatment effect.
All suspected cases of visceral leishmaniasis presenting at hospital had blood and urine samples collected. All samples were collected at admission and before the start of VL treatment. After that, patients with a confirmed diagnosis and those who met the inclusion criteria, including a hospital stay longer than 24 hours, were selected and analyzed in this study. Patients were followed up to hospital discharge (median follow-up time of 11 days). A healthy control group matched for sex and age was also enrolled. The control group comprised participants with confirmed absence of any comorbidities or pathological conditions at the time of sample collection. It consisted of nurses, pharmacists, residents and medical students of the institution who volunteered to participate. The study protocol was reviewed and approved by the Ethics Committee of the Federal University of Ceara (CAAE: 41685514.3.3001.5044), and all participants gave written informed consent prior to study enrollment.
Sample Processing
Urine was centrifuged at 1000 g for 15 min at 4ºC to remove urinary sediments, including whole cells and other debris. The urine supernatant was aliquoted and immediately stored at -80ºC until use. Venous blood was collected in BD Vacutainer® SST with silica clot activator and a polymer gel for efficient serum separation. After 15 minutes the blood samples were centrifuged, and the serum was obtained. Specific serum aliquots were separated for biochemical measurements, while others were used to quantify new biomarkers. Also, serum samples were stored at -80ºC until the analyses were performed.
Biochemical and renal function evaluation
Standard biochemical analyses were performed using a routine automated analyzer provided by the São José Hospital laboratory. Serum sodium and potassium were determined in an ion selective electrolyte analyzer (9180, Electrolyte Analyzer, Roche®) and the results were expressed as mEq/L. Glomerular Filtration Rate (eGFR) was estimated using the CKD-EPI formula (20).
Accepted
Article
Because the Kidney Disease Improving Global Outcome (KDIGO) criteria combine
elements from prior AKI definitions (Acute kidney injury network
–
AKIN and RIFLE criteria),
it was used to classify our patients (21). AKI stage 1 was defined as an increase in serum
creatinine (SCr)
≥0.3 mg/dL and 1.5
-1.9-fold increase in relation to the basal SCr. AKI stage 2
was defined as a 2.0-2.9-fold increase and AKI stage 3 as a 3-fold increase in relation to the
basal SCr. Basal SCr was the lowest SCr level measured during hospital stay.
Enzyme-linked immunosorbent assay (ELISA)
Biomarker levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). In total, urinary and serum NGAL (DY1757-Duoset, R&D Systems), urinary KIM-1 (DY1750B-Duoset, R&D Systems) and urinary MCP-1 (DY279-Duoset, R&D Systems) levels were all evaluated in duplicate. Moreover, IFN-y (DY285-Duoset, R&D Systems) and C-Reactive Protein (CRP: DY1707-Duoset, R&D Systems) levels were quantified in serum samples. To control for urine concentration differences, urinary biomarkers were expressed according to the urinary creatinine content and expressed as “mg-Cr”.
All these assays were sandwich ELISA. Briefly, 96 well plates (UltraCruz® ELISA Plate) were coated for the specific capture of anti-human-biomarker antibodies, followed by human biomarker binding after adding diluted human samples; binding of biotinylated detection antibodies with epitopes of human-biomarker that were fixed by capture antibodies; conjugation of streptavidin-horseradish peroxidase with biotin of the previously immobilized biotinylated antibodies; and quantification of immobilized antibody-biotin-streptavidin-enzyme conjugates by monitoring horseradish peroxidase activity in the presence of the substrate 3,3’,5,5’-tetra-methylbenzidine (TMB ELISA substrate (high sensitivity), Abcam, Cat. No ab171523, Cambridge, MA). Finally, enzyme activity was measured by spectrophotometry at 450nm, with wavelength correction of 570 nm. The increase in absorbance was directly proportional to the concentration of the analyzed biomarker in the sample.
Statistical Analyses
Accepted
Article
distribution of data between the groups. Correlations between clinical and novel kidney biomarkers were evaluated using Pearson’scorrelation or Spearman’s rank correlation, as appropriate.
Moreover, partial (adjusted) correlation was evaluated after controlling for IFN-y and CRP levels. Logistic regression models were used to evaluate the association between kidney biomarkers and AKI development in VL patients after adjusting for age, gender, variables involved in VL
complications (levels of globulins, albumin, aspartate aminotransferase, hemoglobin, white blood cells and platelets), IFN-y and CRP levels. The linearity between continuous variables and the outcome was tested using the Box-Tidwell test (22). Briefly, interactions between the continuous predictors and their logs were included in the model. If such interactions were significant, then the linearity assumption had been violated. Improvement in the maximum log likelihood stepwise method after the kidney biomarker inclusion was used to evaluate the regression model. The ability of renal biomarkers levels in predicting AKI in VL patients at admission was assessed using the area under the receiver-operator characteristic curve (AUC-ROC) as well as the cut-off, sensitivity and specificity values. The statistical analysis was performed using the IBM SPSS Statistics for Windows, version 20.0 (IBM, USA).Vertical scatter plots were created using GraphPad Prism 5 for Windows (version 5.01).
Results
Demographic, clinical and laboratory parameters in VL patients
Serum and urine samples were collected from 82 VL patients, but after applying the exclusion criteria, 50 patients were included. Among the excluded patients (n=32), 10 (31,3%) had hypertension or diabetes, 6 (18,7%) had serum creatinine level > 2mg/dL at admission and 16 patients (50%) had a hospital stay < 48 hours (Figure 1). They were mostly males (86%) and their mean age was 45±19 years. VL patients had important disorders caused by the Leishmania
infestation, such as hematologic disorders (decrease in levels of hemoglobin, hematocrit, platelets and white blood cells counts), hypoalbuminemia, hypergammaglobulinemia and hepatic disorders, with higher levels of enzymes and total bilirubin (Table 1).
Accepted
Article
mean level of serum creatinine, urea and decreased eGFR than the no AKI group and healthy controls. uPCR and uACR were elevated in both VL patient groups (Table 1).
Evaluation of kidney injury biomarkers in VL patients
Regarding the kidney injury biomarkers, serum and urinary NGAL (sNGAL and uNGAL), urinary KIM-1 (uKIM-1) and urinary MCP-1 (uMCP-1) were elevated in the AKI group (Figure 2). The mean level of sNGAL was increased in the AKI group vs. the No AKI group (200.3±59.1 vs. 136.1±56.2 ng/mL, p<0.01)and even in the No AKI group in comparison with the healthy controls (136.1±56.2 vs. 82.8±27 ng/mL, p<0.01). The mean uNGAL (15.8±8.4 ng/mg-Cr) and uKIM-1 (2.21±2.0 ng/mg-Cr) levels and the median and interquartile range of uMCP-1 level (427, 72-659, pg/mg-Cr) were significantly higher (p<0.05) only in the AKI group, when compared with the healthy controls. Regarding the inflammatory biomarkers, the mean level of C-Reactive Protein (CRP) was higher in VL patients when compared with the healthy controls (p<0.001). On the other hand, the systemic levels of IFN-y were lower in VL patients than in the healthy controls (p<0.001) (Table 1).
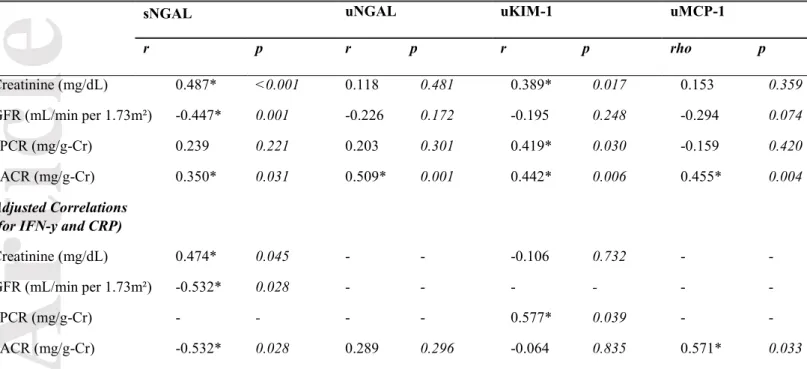
Correlations of kidney injury biomarkers and clinical renal parameters in VL patients
The correlations analysis was performed to evaluate whether the levels of novel kidney biomarkers were associated with clinical renal parameters in VL patients before the start of specific treatment against Leishmania. Moreover, to assess a possible interference of immunological factors in these correlations, adjusted correlations using systemic levels of IFN-y and CRP were applied to previous significant (p<0.05) correlations (Table 2).
Urinary KIM-1 (uKIM-1), a proximal tubule injury biomarker, showed moderate correlations with serum creatinine (r=0.389, p=0.017), serum urea (r=0.397, p<0.05), uPCR (r=0.419, p<0.05), uACR (r=0.442, p<0.01) levels, whereas an inverse correlation was observed with serum sodium (r=-0.490, p<0.05). After adjusting for IFN-y and CRP levels, uKIM-1 remained correlated only with uPCR (r=0.577, p<0.05). Urinary MCP-1 (uMCP-1) and urinary NGAL (uNGAL) were correlated with uACR; however, only uMCP-1 remained associated after adjusting correlation (r=0.571, p<0.05).
Concerning sNGAL levels, there were positive correlations between serum creatinine (r=0.487, p<0.001), urea (r=0.583, p<0.001), and uACR (r=0.350, p<0.05) levels and a negative correlation with eGFR (r= -0.447, p<0.01). In the adjusted correlations, sNGAL remained correlated with creatinine, urea, decreased eGFR and uACR. Hence, sNGAL was associated with most of the important clinical renal parameters, even with the influence of IFN-y mediator and systemic inflammation
Accepted
Article
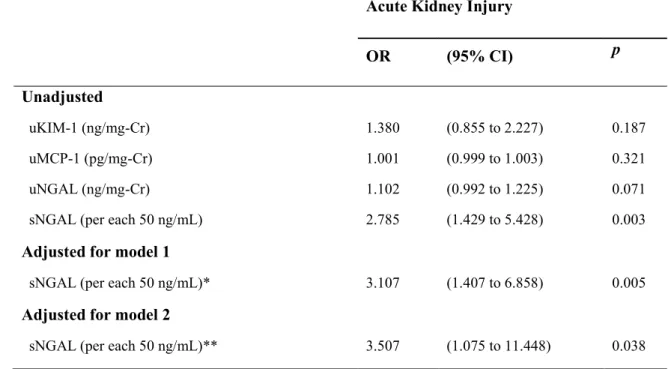
Association of kidney injury biomarkers with AKI development during hospital stay
In a logistic regression model, only sNGAL levels at admission were significantly associated with AKI development in the univariate analysis (OR=2.785 and 95% CI=1.429 - 5.428 per increase of 50ng/mL in sNGAL). In different multivariate models using variables associated with VL severity and
inflammation (age, gender, hemoglobin, white blood cells, platelets, serum globulins and albumin, aspartate aminotransferase, IFN-y and CRP levels), sNGAL maintained an important association with AKI development in VL patients (model 1: OR=3.107 and 95% CI=1.407 - 6.858; model 2: OR=3.507 and 95% CI= 1.075 - 11.448 per each increase of 50ng/mL in sNGAL) (Table 3).
A receiver operating characteristic (ROC) curve was constructed to display the true positive rate (sensitivity) and false positive rate (1 – specificity) of different kidney injury biomarker cut-offs for predicting AKI in VL patients. Also, the performance of each biomarker was assessed by the area under the receiver-operator characteristic curve (AUC-ROC). Only sNGAL and uNGAL could detect AKI with statistical significance. uNGAL had an AUC-ROC=0.68 (95% CI=0.52-0.83), with a cut-off =10.6ng/mg-Cr it had a sensitivity=69.6% and specificity=63% (p=0.029). However, sNGAL had the better performance among biomarkers with the highest AUC-ROC - figure 3. sNGAL levels had an AUC-ROC=0.81 (95% CI=0.69-0.93) and with a cut-off =154ng/mL it had a sensitivity=82.6% and specificity=74.1% (p<0.001).
Sensitivity Analysis
Because only sNGAL remained associated with AKI after multivariate adjustment, we performed a sensitivity analysis using its cut-off point (Table 3). sNGAL levels above of the cut-off (154 ng/mL) remained associated with AKI development in the univariate (OR=13.571 and 95% CI= 3.416 - 53.926; p<0.001) and multivariate models (Model 1: OR=16.506 and 95% CI=3.105 - 87.745; p=0.001 / model 2: O.R= 28.825 and 95% CI=2.094 - 396.734; p=0.012).
Discussion
Accepted
Article
preference. National guidelines have not standardized any treatment preference (23). Although we excluded patients with creatinine > 2mg/dL, patients with reduced renal function at baseline were less frequently treated with amphotericin B.
In VL animal and human studies, VL-associated nephropathy has been attributed to mechanisms that lead to interstitial nephritis and glomerular changes (24). In the present study, the VL patients showed signs of both interstitial and glomerular injury due to the leishmaniasis infection itself, evidenced before the start of the treatment. We observed higher proteinuria and albuminuria levels in VL patients. In VL, glomerular damage has been characterized as a mesangial proliferative lesion or deposition of immune complexes, leading to rapidly progressive glomerulonephritis (8,25). Elevated proteinuria can be a result of hypergammaglobulinemia in VL patients with low-molecular weight protein fractions such as alpha1, alpha2, beta microglobulins and especially gamma globulins, which were freely filtrated in the glomeruli. Elevated proteinuria may accelerate kidney disease progression through chemokine induction and complement activation, which lead to inflammatory cell infiltration in the interstitium (26).
Overall, our best results were found with serum NGAL level evaluation. NGAL levels at admission were significantly higher in VL patients that developed AKI and was independently associated with AKI development in the univariate and multivariate analyses, even after adjustment for clinical markers of VL severity and systemic inflammation. In the present study, we used the KDIGO-based AKI classification. This classification differs from the RIFLE and AKIN classifications, especially regarding the time criterion. KDIGO covers both the AKIN and RIFLE criteria, taking into account changes in creatinine within 48 hours (0.3 md/dL increment in serum creatinine) and for a period of 7 days. sNGAL levels showed good sensitivity and specificity to predict AKI and it is a potential candidate to be included in the clinical care of such patients. Although amphotericin B is emerging as the first-line treatment against VL in some countries, pentavalent antimonial compounds continue to be successfully used. Using NGAL levels as biomarkers to direct the treatment of patients with VL can be an important approach to prevent additional nephrotoxic exposure in high-risk patients.
NGAL is released by activated neutrophils, an important component of antimicrobial innate immunity in the early stages of infection (27). The higher levels of NGAL in the blood acts as a chemoattractant for neutrophils, promoting neutrophil adhesion, extravasation and phagocytosis and also activates CD4+ T regulatory cells (27). Studies with Leishmania major (cutaneous
leishmaniasis) and L. infantum (visceral leishmaniasis) reported that several aspects in the early innate immune reactions were related to neutrophil activation (2). During the early stages of
Accepted
Article
a result, the innate immunity fails to control infection and systemic disease with exacerbated and inefficient neutrophil activation. On the other hand, antigen-presenting cells (APCs) may capture dead parasites and presents to CD8+ and CD4+ cells, which have a huge importance for the control of Leishmania infections through IFN-y production (2), as illustrated in Figure 4. In addition, IFN-y seems to inhibit neutrophil recruitment and activate infected macrophages to kill parasites (2,28).
Thus we suggest that higher levels of systemic NGAL observed in our present VL patients may be associated with exacerbated and inefficient neutrophil activation, which contributes to the infection progression. On the other hand, intrinsic kidney injury resulting from VL infection and inflammation (8) may contribute to increased NGAL expression in nephron segments, leading to a further increase in both urinary and systemic NGAL (29). Furthermore, any GFR impairment due to kidney injury contributes to a decrease in renal clearance of NGAL and, consequently, to an increase in NGAL circulation levels (Figure 4).
In the present study, these systemic levels of NGAL were associated with AKI and showed a better performance than other biomarkers in predicting AKI development, including in comparison to urinary NGAL. Moreover, the levels above the best sNGAL cut-offin our ROC curve analysis showed an increased the risk for AKI development. This association may be affected by systemic immune responses. However, even after adjustment for important inflammatory variables (IFN-y and PCR) sNGAL remained associated with AKI development and clinical renal markers, suggesting an independent association between sNGAL and AKI. NGAL has been extensively studied as a new biomarker for early AKI detection (13). However, the evaluation of NGAL as a kidney injury predictor during tropical infectious diseases has been poorly investigated. Rocha et al. evaluated the
diagnostic performance of urinary NGAL in 22 leishmaniasis patients (5 patients had visceral and 17 were cutaneous leishmaniasis), focusing on amphotericin B-induced AKI. Urinary NGAL was
Accepted
Article
MCP-1 plays a pivotal role in the pathogenesis of renal injury. MCP-1 is produced by intrinsic renal cells, such as podocytes, mesangial and endothelial cells, when stimulated by inflammatory inducers such as immune complexes (32). Moreover, MCP-1 induced a significant reduction in nephrin expression in human podocytes, an important protein of the glomerular filtration barrier, and it is associated with albuminuria (33). In this study, we observed higher levels of urinary MCP-1 (uMCP-1) that were correlated with an increase in albuminuria, even after testing inflammatory interference. Our previous studies with other infectious disease, including VL, hepatosplenic schistosomiasis and leprosy (mostly as the lepromatous form) showed patients also had these clinical complications, including urinary oxidative stress, suggesting an increased risk of kidney disease development in these patients (19,34,35). Despite the important involvement of uMCP-1 in renal interstitium inflammation and glomerulonephritis in VL, the present study showed it was not related with AKI development in the univariate analysis and that it had a poor diagnostic
performance in the ROC curve analysis.
We demonstrated for the first time elevated levels of urinary KIM-1 (uKIM-1) in VL patients before the use of medications against Leishmania, and it was correlated mostly with elevated proteinuria. KIM-1 was significantly expressed early in proximal tubular cells during kidney injury, but not detected in healthy kidneys (36,37). Hence, we suggest that VL patients had an important injury in renal proximal tubules. Proximal tubulopathy was also suggested in a previous study through retinol-binding protein levels in VL patients (25). A possible mechanism is acute
tubulointerstitial nephritis observed in VL-associated nephropathy (38,39), which may be caused by elevated proteinuria, the Leishmania infection itself and inflammatory cell infiltration within the interstitium (40). Evidence has shown that apical exposure to protein overload in proximal tubular cells induces a proinflammatory phenotype, leading to injury (41). Thus, the glomerular
ultrafiltration of excessive amounts of plasma proteins found in VL patients may induce
tubulointerstitial damage, which can stimulate local KIM-1 expression and increase uKIM-1 excretion (41). In fact, in this present study, uKIM-1 levels remained correlated only with proteinuria after adding the immune components’ influence to the partial correlation. However, in the present study, uKIM-1 was not able to predict AKI in VL patients, despite being a promising biomarker for early detection of AKI in various population settings (36).
Accepted
Article
infectious diseases, patients receiving nephrotoxic drugs) can reinforce our findings. Also, although we evaluated several laboratory parameters, clinical complications of VL that are associated with mortality (3,8), such as bacterial infections, dyspnea and diarrhea were not included in the present study. Moreover, the study was performed in a single center, although most VL patients from our endemic areas are referred to this important hospital. Hence, our AKI rates may not be extrapolated to the entire VL population.
In conclusion, we suggest that there is important proximal tubular injury related to protein overload in renal tissue and a glomerular filtration barrier dysfunction associated with glomerular inflammation, but the exact mechanisms remain to be fully elucidated. For the first time, serum NGAL was independently associated with VL-associated nephropathy and it was the most reliable biomarker to predict the risk for AKI development in VL patients. Finally, aiming at reducing patient morbimortality, we believe that serum NGAL can be used in clinical practice to improve clinical management strategies and prevent the immediate exposure to additional nephrotoxic drugs in hospitalized VL patients with a high risk of AKI development.
Acknowledgements
We are very grateful to the patients and the teams of physicians, nurses, pharmacists, residents and medical students from São José Hospital in Fortaleza, who provided great care and follow up to the visceral leishmaniasis patients. We are also grateful to the Renal and Tropical Diseases Research Laboratory (Federal University of Ceara) for the experimental analyses of this research. This study was supported by the Brazilian Research Council – Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (financial support protocol 405963/2016-5) and by Coordination of
Accepted
Article
References
1. McGwire BS, Satoskar a R. Leishmaniasis: clinical syndromes and treatment. QJM. 2014;107:7– 14.
2. Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. Nature Publishing Group; 2011;9(8):604–15.
3. van Griensven J, Diro E. Visceral Leishmaniasis. Infect Dis Clin North Am. 2012;26(2):309–22. 4. Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–54.
5. World Health Organization. Leishmaniasis: epidemiological situation. http://www.who.int/leishmaniasis/burden/en/. 2015.
6. BRASIL. Ministério da Saúde. Situação Epidemiológica, leishmaniose visceral.
http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/leishmaniose-visceral-lv. 2015
7. Oliveira MJC, Silva Júnior GB, Abreu KLS, Rocha NA, Garcia AV V, Franco LFLG, et al. Risk factors for acute kidney injury in visceral leishmaniasis (Kala-Azar). Am J Trop Med Hyg. 2010;82(3):449– 53.
8. da Silva Junior GB, Guardão Barros EJ, De Francesco Daher E. Kidney involvement in leishmaniasis-A review. Brazilian J Infect Dis. 2014;18(4):434–40.
9. Libório AB, Rocha NA, Oliveira MJC, Franco LFLG, Aguiar GBR, Pimentel RS, et al. Acute Kidney Injury in Children With Visceral Leishmaniasis. Vol. 31, The Pediatric Infectious Disease Journal. 2012. p. 451–4.
10. McCullough P a, Bouchard J, Waikar SS, Siew ED, Endre ZH, Goldstein SL, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury:
executive summary from the tenth consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:5–12.
11. Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. Curr Opin Crit Care. 2007 Dec;13(6):638–44.
12. Haase M. NGAL —From discovery to a new era of “ Acute Renal Disease ” diagnosis ? Clin Biochem. 2011;44(7):499–500.
13. Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, et al. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–50.
14. Gelatinase N, Kjeldsens L, Johnsen H, Sengelbv H. Isolation and Primary Structure of NGAL , a Novel Protein Associated. 1993;268(14):10425–32.
Accepted
Article
16. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang JUN, et al. Identification of Neutrophil Gelatinase-Associated Lipocalin as a Novel Early Urinary Biomarker for Ischemic Renal Injury. 2003;(8):2534–43.
17. Davide Bolignano, Valentina Donato, Giuseppe Coppolino, Susanna Campo, Antoine Buemi, Antonio Lacquaniti and MB. Neutrophil Gelatinase–Associated Lipocalin (NGAL) as a Marker of Kidney Damage. Am J Kidney Dis. 2008;52(3):595–605.
18. Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury - Where do we stand today? Nephrol Dial Transplant. 2011;26(3):762–4.
19. Oliveira MJC, Silva Junior GB, Sampaio a. M, Montenegro BL, Alves MP, Henn G a. L, et al. Preliminary Study on Tubuloglomerular Dysfunction and Evidence of Renal Inflammation in Patients with Visceral Leishmaniasis. Am J Trop Med Hyg. 2014;91(5):908–11.
20. Levey AS, Stevens L a, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
21. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. 22. Box, G. E. P. and Tidwell PW. Transformation of the independent variables. Technometrics.
1962;4:531–50.
23. BRASIL. Ministério da Saúde. Manual de vigilância e controle da leishmaniose visceral. 1st ed. Brasília; 2014.
24. Diniz FB, Teoto LO, Oliveira RA De, Seguro C, Jr GBS, Martins A, et al. Renal tubular dysfunction in patients with American cutaneous leishmaniasis. Kidney Int. 2011;1099–106.
25. Elnojomi NAA, Musa AM, Younis BM, Elfaki MEE, El-Hassan AM, Khalil EAG. Surrogate markers of subtle renal injury in patients with visceral leishmaniasis. Saudi J Kidney Dis Transpl. 2010 Sep;21(5):872–5.
26. Gorriz JL, Martinez-Castelao A. Proteinuria: detection and role in native renal disease progression. Transplant Rev (Orlando). 2012 ;26(1):3–13.
27. Nasioudis D, Witkin SS. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol. 2015;204(4):471–9.
28. Xin L, Vargas-Inchaustegui DA, Raimer SS, Kelly BC, Hu J, Zhu L, et al. Type I IFN Receptor Regulates Neutrophil Functions and Innate Immunity to Leishmania Parasites. J Immunol. 2010 Jun 15;184(12):7047–56.
Accepted
Article
30. Rocha PN, Macedo MN, Kobayashi CD, Moreno L, Guimarães LHS, Machado PRL, et al. Role of Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL) in the Early Diagnosis of Amphotericin B-induced Acute Kidney Injury. Antimicrob Agents Chemother. 2015;59(11):6913–21.
31. Liborio a. B, Braz MBM, Seguro a. C, Meneses GC, de Oliveira Neves FM, Pedrosa DC, et al. Endothelial Glycocalyx Damage is Associated with Leptospirosis Acute Kidney Injury. Am J Trop Med Hyg. 2015;92(3):611–6.
32. Kim MJ, Tam FWK. Urinary monocyte chemoattractant protein-1 in renal disease. Clin Chim Acta. 2011;412(23–24):2022–30.
33. Tarabra E, Giunti S, Barutta F, Salvidio G, Burt D, Deferrari G, et al. Effect of the Monocyte Chemoattractant Protein-1 / CC Chemokine Receptor 2 System on Nephrin Expression in. Diabetes. 2009;58(September):2109–18.
34. Meneses GC, Libório AB, Daher EF De, Bezerra G, Felipe M, Araci M, et al. Urinary monocyte chemotactic protein-1 ( MCP-1 ) in leprosy patients : increased risk for kidney damage. BMC Infect Dis. 2014;1(14):1–5.
35. Duarte DB, Vanderlei LA, Bispo RK de A, Pinheiro ME, da Silva GB, Martins AMC, et al. Renal function in hepatosplenic schistosomiasis--an assessment of renal tubular disorders. PLoS One. 2014;9(12):e115197.
36. Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: A meta-analysis. PLoS One. 2014;
37. Ichimura T, Bonventre J V., Bailly V, Wei H, Hession C a., Cate RL, et al. Kidney injury molecule-1 (KIM-molecule-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–42. 38. Clementi A, Battaglia G, Floris M, Castellino P, Ronco C, Cruz DN. Renal involvement in
leishmaniasis: A review of the literature. NDT Plus. 2011;4(3):147–52.
39. Dutra M, Martinelli R, de Carvalho EM, Rodrigues LE, Brito E, Rocha H. Renal involvement in visceral leishmaniasis. Am J Kidney Dis. 1985 Jul;6(1):22–7.
40. Michel DM, Kelly CJ. Acute interstitial nephritis. J Am Soc Nephrol. 1998;9(3):506–15. 41. Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, et al. Protein overload
stimulates RANTES production by proximal tubular cells depending on NF-??B activation. Kidney Int. 1998;53(6):1608–15.
Accepted
Article
FIGURE LEGENDS
Figure 1. Flow chart of visceral leishmaniasis patients that were evaluated in this study, including those that were divided into Acute Kidney Injury (AKI) groups.
Figure 2. Serum NGAL (sNGAL) and urinary biomarker levels (uNGAL, uKIM-1, uMCP-1) at admission in patients with visceral leishmaniasis that had a diagnosis of acute kidney injury (AKI) during hospital stay compared with the No AKI group and healthy controls. Patients with documented AKI were defined by the KDIGO criteria. *p<0.05.
Figure 3. Measurement of serum and urinary NGAL levels (sNGAL and uNGAL, respectively) at hospital admission for acute kidney injury diagnosis in visceral leishmaniasis patients. AUC-ROC: area under the receiver-operator characteristic curve. Significant value: p<0.05.
Figure 4. Hypothesis of pathogenic mechanisms involved in Leishmania infection control and renal injury in visceral leishmaniasis. Glomerulonephritis and interstitial nephritis may be caused by glomerular ultrafiltration of excessive amounts of plasma proteins (protein overload), the
leishmaniasis infection itself, immune complex deposition and renal inflammation. In this context, proteinuria and albuminuria increase and, furthermore, intrinsic MCP-1 (mostly in glomeruli) and KIM-1 (proximal tubular injury) are produced and excreted by renal cells. The innate immunity against Leishmania is associated with neutrophil recruitment and activation that produce and increase systemic NGAL, mostly in exacerbated neutrophil activation during inefficient
Accepted
Article
Table 1. Clinical and laboratory parameters in visceral leishmaniasis patients according to AKI development.
Visceral leishmaniasis
Healthy Control
(n=13)
No AKI patients (n=27)
AKI patients
(n=23) P value
Age 59±8 39±15 48±22 0.001
Male Gender 10 (77)
Hospital Stay (days) - 12 (5-15) 13 (11-18) 0.018
Leishmaniasis-HIV coinfection (%) - 1 (4) 4 (17) 0.108
Amphotericin-B use during hospital
stay - 11 (41) 16 (69) 0.642
SBP (mmHg) 117±5 117±11 120±13 0.368
Hemoglobin (g/dL) 14.2±0.9 8.4±1.9 7.6±2.8 <0.001
Hematocrit (%) 42.7±5.3 26.8±5.3* 26.2±4.9* <0.001
White blood cells (per mm3) 7241±1048 2394±1160* 2327±1379* <0.001
Platelets (10³ /mm³) 222±80 116±74* 91±58* <0.001
Globulin (g/dL) 2.3±0.22 4.14±1.1* 4.8±2.2* <0.001
Albumin (g/dL) 4.2±0.2 3.2±0.6* 2.9±0.7* <0.001
AST (U/L) 30 (20-33) 54 (28-109) * 64 (28-104) * 0.008
ALT (U/L) 33 (19-52) 53 (21-89) 46 (18-68) 0.267
Total Bilirubin (mg/dL) 0.5 (0.4-0.7) 0.5 (0.4-1.2) 0.7 (0.3-1.3) 0.859
Glucose (mg/dL) 102±10 108±18 107±28 0.849
Sodium (mEq/L) 141±4 138±4 136±6 0.483
Potassium (mEq/L) 4.9±0.5 4.1±0.5 4.3±0.6 0.250
Renal parameters
Creatinine (mg/dL) 0.81±0.1 0.89±0.2 1.11±0.36# 0.002
UREA (mg/dL) 30±10 27±9 42±22** 0.004
GFR (mL/min per 1.73m²) 114±12 103±21 76±35# <0.001
uPCR (mg/g-Cr) 16.5±26.5 420±360* 394±294* 0.001
uACR (mg/g-Cr) 2.3±3.2 12.8±10.4* 18.2±11.8* <0.001
Accepted
Article
sNGAL (ng/mL) 82.8±27.2 136.1±56.2* 200.3±59.1# <0.001
uNGAL (ng/mg-Cr) 8.1±7.2 11.5±5.2 15.8±8.4* 0.016
uKIM-1 (ng/mg-Cr) 0.72±0.37 1.63±0.87 2.21±2.0* 0.016
uMCP-1 (pg/mg-Cr) 75 (47-95) 243 (68-451) 427 (72-659)* 0.039
Inflammatory biomarkers
CRP (mg/L) 1.02±0.9 7.9±0.63* 7.5±1.35* <0.001
IFN-y (pg/mL) 38.2±33 9.4±6.6* 7.8±3.2* <0.001
Data are presented as the mean ±standard deviation or as an absolute number with percentage in parenthesis. GFR,
glomerular filtration rate using CKD-EPI formula; uPCR, urinary protein/creatinine ratio; uACR, urinary albumin-creatinine ratio; sNGAL and uNGAL, serum and urinary neutrophil gelatinase-associated lipocalin, respectively; uKIM-1, urinary kidney injury molecule-1; uMCP-1, urinary monocyte chemotactic protein-1; CRP, C-reactive protein; IFN-y, interferon-gamma.
* p<0.05 compared with healthy control. ** p<0.05 compared with “No AKI”.# p<0.05 compared with healthy control and
Accepted
Article
Table 2. Correlations between kidney biomarkers and clinical renal parameters in visceral
leishmaniasis patients.
sNGAL uNGAL uKIM-1 uMCP-1
r p r p r p rho p
Creatinine (mg/dL) 0.487* <0.001 0.118 0.481 0.389* 0.017 0.153 0.359
GFR (mL/min per 1.73m²) -0.447* 0.001 -0.226 0.172 -0.195 0.248 -0.294 0.074
uPCR (mg/g-Cr) 0.239 0.221 0.203 0.301 0.419* 0.030 -0.159 0.420
uACR (mg/g-Cr) 0.350* 0.031 0.509* 0.001 0.442* 0.006 0.455* 0.004
Adjusted Correlations (for IFN-y and CRP)
Creatinine (mg/dL) 0.474* 0.045 - - -0.106 0.732 - -
GFR (mL/min per 1.73m²) -0.532* 0.028 - - - - - -
uPCR (mg/g-Cr) - - - - 0.577* 0.039 -
-uACR (mg/g-Cr) -0.532* 0.028 0.289 0.296 -0.064 0.835 0.571* 0.033
r of Pearson; Spearman’s rho. *Moderate correlation was significant; p<0.05.
GFR, Glomerular filtration rate estimated by CKD-EPI formula.
Accepted
Article
Table 3. Association of admission kidney injury biomarkers levels with acute kidney injury
development during hospital stay in visceral leishmaniasis patients.
Acute Kidney Injury
OR (95% CI) p
Unadjusted
uKIM-1 (ng/mg-Cr) 1.380 (0.855 to 2.227) 0.187
uMCP-1 (pg/mg-Cr) 1.001 (0.999 to 1.003) 0.321
uNGAL (ng/mg-Cr) 1.102 (0.992 to 1.225) 0.071
sNGAL (per each 50 ng/mL) 2.785 (1.429 to 5.428) 0.003
Adjusted for model 1
sNGAL (per each 50 ng/mL)* 3.107 (1.407 to 6.858) 0.005
Adjusted for model 2
sNGAL (per each 50 ng/mL)** 3.507 (1.075 to 11.448) 0.038
CI: confidence interval; OR: Odds ratio.
AKI was defined according to the KDIGO criteria and treated as a dependent variable in the logistic regression analysis.
sNGAL and uNGAL, serum and urinary neutrophil gelatinase-associated lipocalin, respectively; uKIM-1, urinary kidney injury molecule-1; uMCP-1, urinary monocyte chemotactic protein-1.
Model 1: adjusted for age, gender, hemoglobin, white blood cells, platelets, interferon-gamma (IFN-y) and C-reactive protein (CRP)
Model 2: adjusted for age, gender, globulins, albumin, aspartate aminotransferase (AST), IFN-y and CRP.
* Log likelihood reduction after sNGAL addition χ2 14.05, df=1, p<0.01