REPOSITORIO INSTITUCIONAL DA UFOP: Stereochemistry of 16α-hydroxyfriedelin and 3-oxo-16-methylfriedel-16-ene established by 2D NMR spectroscopy.
Texto
Imagem

Documentos relacionados
The NMR data of this new compound 136 (Figure 33 ) was assigned by 1D and 2D experiments and an R configuration at C-11 was confirmed by 1 H NMR analysis of the Mosher’s
Structure identification of isolated compounds involved analysis of spectral data of 1D and 2D-NMR.. The isolated compounds are here reported for the first time
Based on different spectroscopic data including HRESIMS, 1D ( 1 H NMR and 13 C NMR) and 2D NMR (COSY, HSQC, and HMBC), the structures of the compounds were established as
The structural characterization of the compounds isolated was established based on infrared spectroscopy, mass spectrometry, one- and two-dimensional nuclear magnetic resonance,
The structures of all compounds were determined by spectrometric methods such as 1D and 2D NMR (COSY, HSQC and HMBC) and HREISMS beside comparison with spectral data for
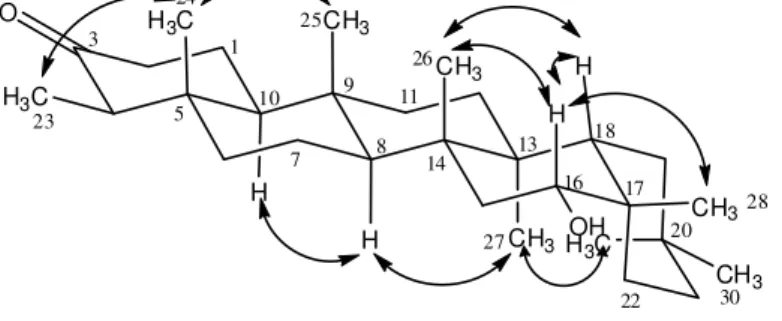
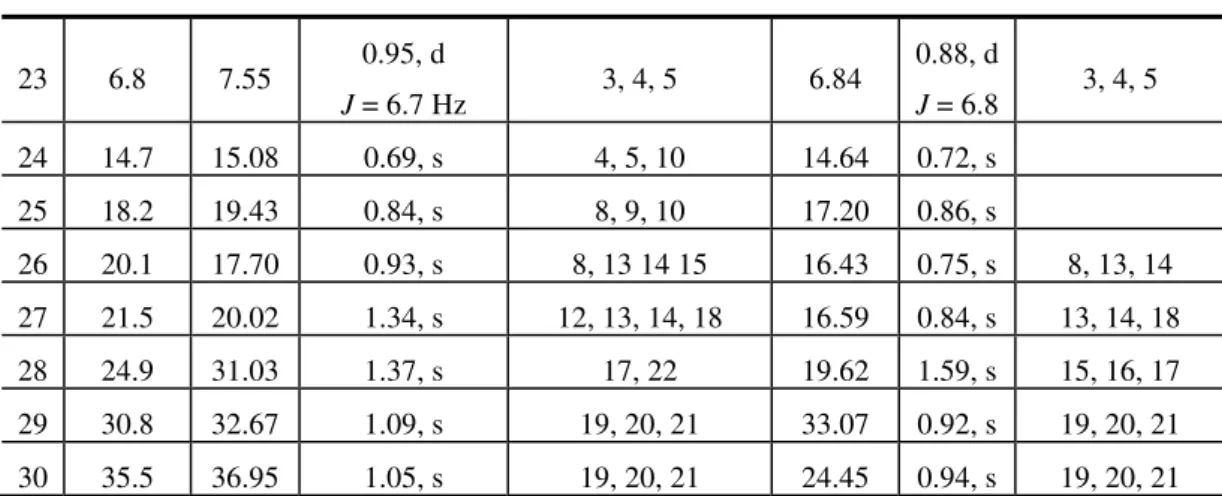
The relative stereochemistry of the hydroxyl group at C-16 was established by the NOESY experiment,. which showed cross-peaks between the hydrogens CH
The structures of the new compounds were elucidated with the help of 1D and 2D nuclear magnetic resonance (NMR) and infrared spectroscopy together with high resolution
The analysis of exopolysaccharides produced by the Leuconostoc pseudomesenteroides R2 strain by infrared, 1 H, 13 C and DEPT-135 NMR spectra indicated that it is a