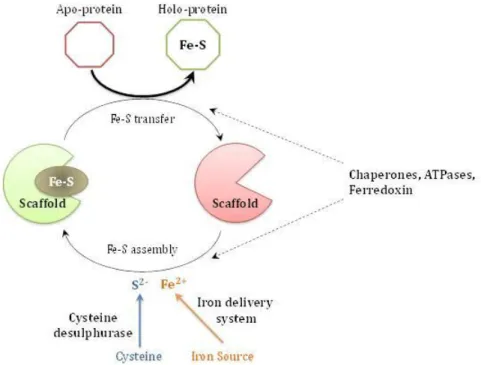
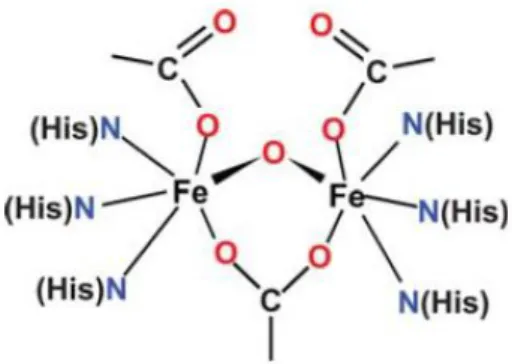
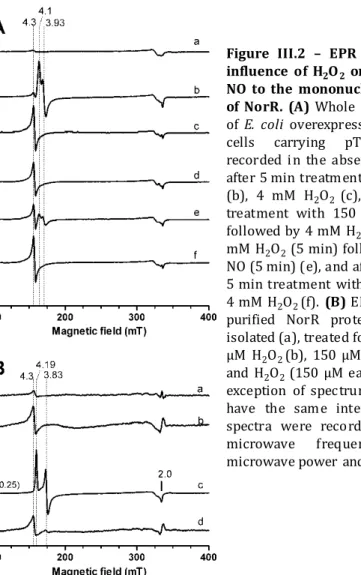
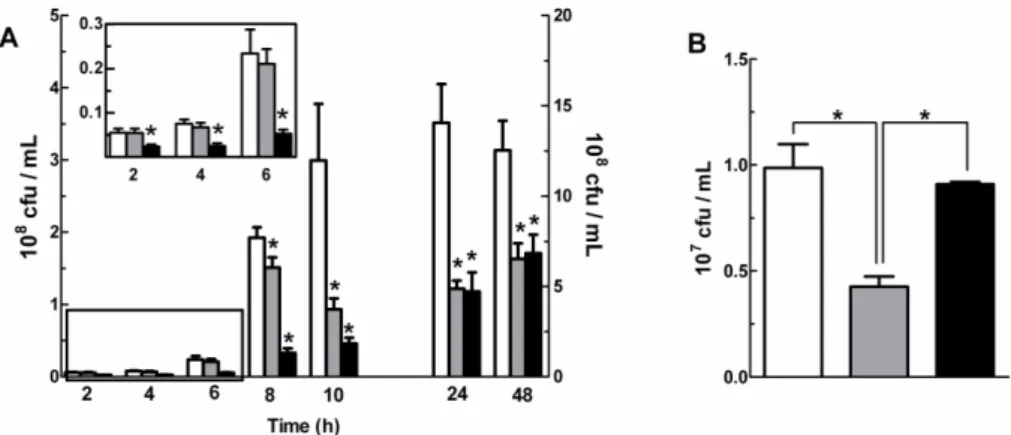
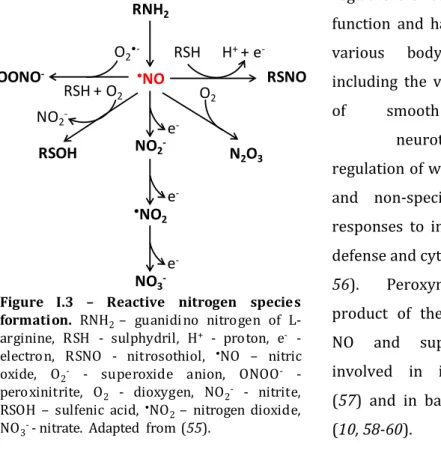
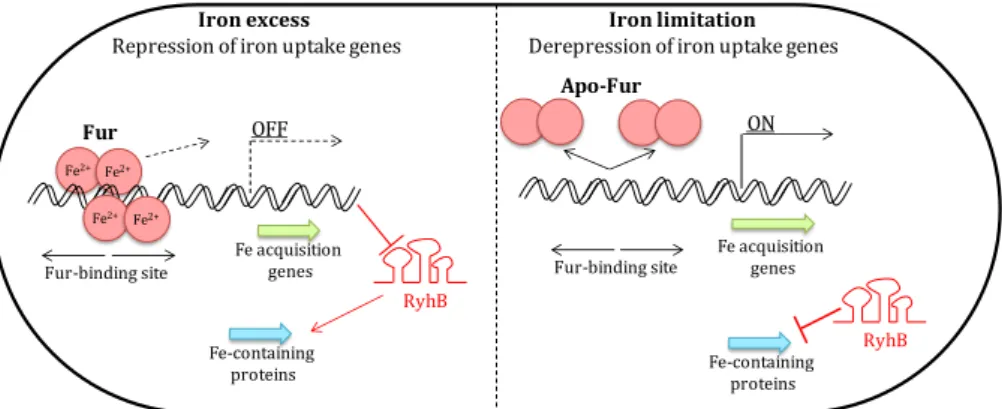
The Role of Di-iron Proteins in Pathogen Resistance
Texto
Imagem


Documentos relacionados
redemocratização no Brasil, expressada pela Constituição de 1988 (BRASIL, 1988), estabeleceu um novo paradigma para as liberdades individuais, diferenciando-se do período de
67 Ora, é perante este conjunto de problemas, que dizem respeito aos domínios da história do Egipto, da ciência, do colonialismo, do Estado, da economia e da política
A teoria Kohlbergiana tem em linha de conta o problema das relações entre o pensamento moral e acção moral, que procura saber quando, como e em que circunstâncias é que
Relativamente à forma como homens e mulheres que passam por um ou mais tratamentos e homens e mulheres que não passaram por nenhum tratamento, caraterizam a relação
De acordo com os normativos vigentes, a avaliação é considerada como parte constitutiva da aprendizagem, um elemento integrante e regulador de toda a prática educativa,
O Estado na prossecução do interesse público e numa articulação entre contenção orçamental e necessidade de realização de grandes empreendimentos, tem optado
mulheres e os homens de forma diferente de acordo com o seu ESE - e um efeito prevalecente de sexo nos participantes do sexo masculino – avaliam mulheres e homens do mesmo ESE de
In a study performed by Barakat, Moustafa and Bikhazi (2012), it was found that 4 weeks of treatment with sodium selenite (5 ppm in drinking water) increased mRNA and protein