DEPARTAMENTO DE BIOLOGIA ANIMAL
Cdx, Wnt signalling and anterior Hox genes in
the regulation of the posterior growth zone
in the mouse embryo.
Ana Rita Soares Monteiro
Dissertação
Mestrado em Biologia Evolutiva e do Desenvolvimento
2012
UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Cdx, Wnt signalling and anterior Hox genes in
the regulation of the posterior growth zone
in the mouse embryo.
Ana Rita Soares Monteiro
Dissertação
Mestrado em Biologia Evolutiva e do Desenvolvimento
Orientadores: Doutora Jacqueline Deschamps e Doutora Sólveig Thorsteinsdóttir
V
Abstract
The Cdx gene family plays a fundamental role in the regulation of the posterior growth zone during mouse development. This region contains populations of long–term neuromesodermal progenitors that contribute to axis elongation [1]. Cdx2+/-‐Cdx4-‐/0 (Cdx2/4)
mutants have a truncation of the axis and defects in the placental labyrinth leading to embryonic lethality. Rescuing experiments showed that Wnt signalling and Hox trunk genes interact with Cdx genes in the regulation of axial extension [2]. In the first part of this work we studied the lethality in mutants that lack one allele of Cdx2 and both alleles of Wnt3a. We show that Wnt3a and Cdx2 interact in the regulation of placental labyrinth precursors and act upstream of Cdx4.
Previous findings revealed that trunk Hox genes and Hox13 differentially regulate posterior axial growth [2]. Here we tested the role of an anterior Hox gene (Hoxb1) in the regulation of axial elongation. We showed that overexpression of Hoxb1 under the Cdx2 promoter in a genetic background of Cdx2/4 mutants aggravates the phenotype instead of rescuing it. Cdx2/4 Cdx2PHoxb1 transgenic embryos present embryonic lethality and a more severe truncation of the axis compared to their Cdx2/4 littermates. Therefore, we propose that anterior Hox genes are epistatic over the trunk Hox genes. To assure the right regulation of axis extension Cdx genes would interact in a positive way with trunk Hox genes. However, the presence of anterior Hox genes would disrupt the balance between anterior and trunk Hox genes.
Keywords: Axial extension, mouse, Cdx , Hox, Wnt
Resumo
Durante o processo de gastrulação as camadas germinativas do embrião são formadas e o plano corporal do organismo é estabelecido. Após a gastrulação o crescimento do eixo em ratinho ocorre por um processo designado de extensão axial. A parte posterior do eixo do ratinho cresce através da adição de tecidos provenientes de populações de progenitores residentes na linha primitiva e tecidos adjacentes, mais tarde no “botão da cauda” [1]. Esta região é por esse motivo denominada de “zona de crescimento posterior”. Nesta região estão presentes progenitores da mesoderme extraembrionária, células germinais primordiais, mesoderme somítica, neuroectoderm, progenitores neuro-‐ mesodérmicos de longo termo e precursores de endoderme. A ordem referida é a ordem da sua localização dos mais posteriores para os a mais anteriores na linha primitiva. A regulação e manutenção destes progenitores é essencial para extensão do eixo, manutenção das células germinais primordiais e desenvolvimento da mesoderme extraembrionária que dará origem ao alantoide. Esta regulação é assegurada pela acção de factores de transcrição, como Cdx (Caudal related homeobox) que actua como regulador dos genes Hox e via de sinalização Wnt/Beta-‐catenina [2]. A família de genes Cdx é constituída por três genes (Cdx1,
Cdx2 e Cdx4). Mutantes de Cdx apresentam o eixo antero-‐posterior truncado, cuja
severidade depende dos genes ou do número de alelos mutados. Alguns destes mutantes apresentam defeitos nos tecidos extraembrionários, ausência de alantoide no caso mais extremo e malformações no labirinto vascular da placenta. Os defeitos nos tecidos extraembrionários provocam letalidade embrionária uma vez que os embriões são incapazes de estabelecer correctamente contacto com o sangue materno e assim prosseguir com a troca de nutrientes. Mutantes de Cdx também apresentam defeitos na padronização do eixo axial com algumas transformações ao nível da identidade vertebral. Um dos mutantes de
Cdx mais estudado é o de Cdx2+/-‐Cdx4-‐/0 (Cdx2/4) [2-‐4]. O fenótipo destes mutantes
apresentam diferentes penetrâncias , o nível de truncamento varia ( no caso mais severo o eixo termina ao nível do sacro) assim como os defeitos que causam letalidade embrionária. Em alguns embriões o alantóide não se funde com o córion, noutros casos os defeitos são ao nível do labirinto placentário. Apenas uma pequena percentagem de embriões nasce [4].
A primeira parte deste trabalho tem como objectivo estudar a interacção de genes
Cdx e a via de sinalização Wnt através do estudo de mutantes Wnt3a-‐/-‐Cdx2+/-‐. Trabalhos
anteriores demonstraram que Wnt actua tanto a jusante como a montante de Cdx no processo de extensão axial, Lef1 (mediador da via Wnt canónica) foi capaz de resgatar o
VII
entre Cdx e a via de sinalização Wnt. Os mutantes gerados, Wnt3a-‐/-‐Cdx2+/-‐ sofrem letalidadeembrionária. Este fenótipo não era espectável uma vez que mutantes Cdx2+/-‐ e mutantes
Wnt3a-‐/-‐ não apresentam qualquer letalidade embrionária. Colocámos a hipótese de que a
causa da letalidade destes mutantes seria a mesma que a observada em mutantes Cdx2/4, e portanto que a mesma via de regulatória estaria a ser afectada. Para testar esta hipótese, analisaram-‐se alantóides de embriões de dia embrionário 8.5 (E8.5) e placentas de embriões de E10.5. Alantóides dos mutantes desenvolvem-‐se correctamente e a maioria funde com o córion. Cortes de placentas mostraram defeitos na ramificação dos vasos sanguíneos embrionários, mas menos severos que os descritos em mutantes Cdx2/4. Devido à semelhança com Cdx2/4 foi testada a hipótese da expressão de Cdx4 estar afectada em mutantes Cdx2+/-‐Wnt3a-‐/-‐ . Os baixos níveis de expressão de Cdx4 observados em mutantes
confirmou esta hipótese. Estes resultados levaram nos a concluir que genes Cdx e a via de sinalização Wnt actuam em conjunto na regulação da população de progenitores dos tecidos que darão origem ao labirinto placentário.
No segundo projecto foi explorada a função de genes Hox anteriores (Hox1-‐3) na regulação da extensão axial. Os genes Hox têm um papel fundamental no estabelecimento da identidade dos segmentos ao longo do eixo anterio-‐posterior. No entanto, os genes Hox do tronco (Hox5-‐9) também estão envolvidos na extensão do eixo. Este papel é desempenhado em paralelo com os genes Cdx. Os genes Hox e genes Cdx têm um gene ancestral em comum e durante o desenvolvimento partilham domínios de expressão na região posterior do embrião [6]. Sobrexpressão de Hoxb8 e Hoxa5 sob o promotor de Cdx2 levou ao resgate do fenótipo de mutantes Cdx2/4, demonstrando assim uma função na regulação da extensão do eixo. Estes embriões transgénicos (Cdx2/4 Cdx2PHoxb8 e Cdx2/4
Cdx2PHoxa5) apresentaram menor letalidade e o eixo axial apresenta truncamento menos
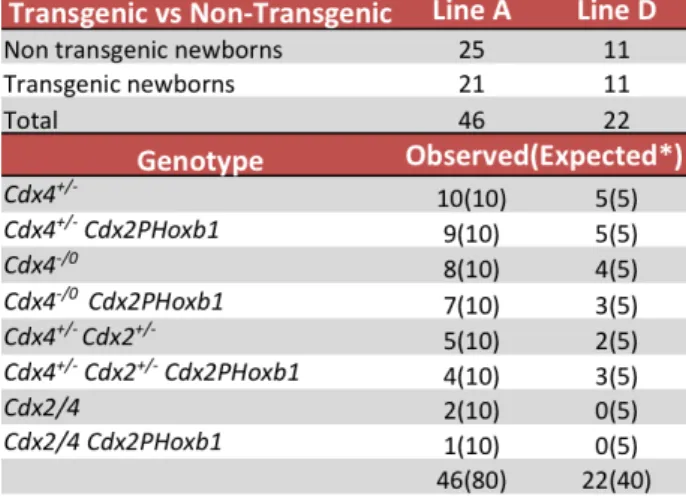
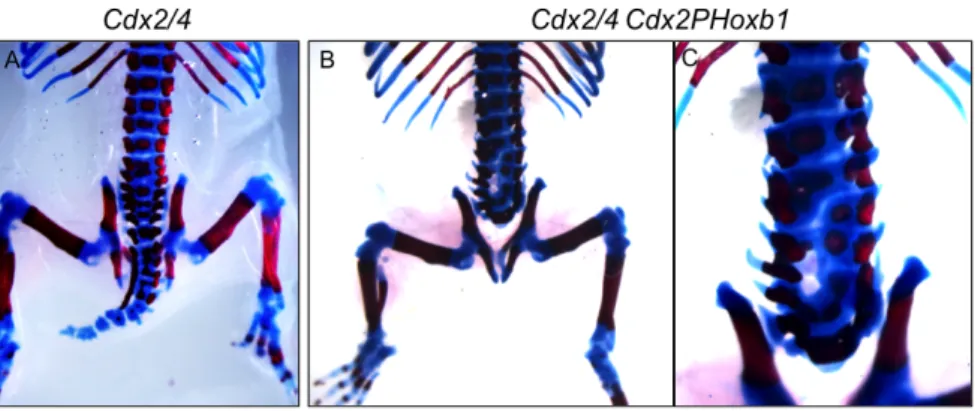
severo, relativamente aos embriões Cdx2/4 [2]. Neste projecto propusemos testar se a sobreexpressão de Hoxb1 (gene Hox anterior) sob o mesmo promotor, resgataria o fenótipo de Cdx2/4. Foram criadas diferentes linhas transgénicas com a construção Cdx2PHoxb1 expressa no fundo genético de mutantes Cdx2/4. A sobrevivência e esqueleto axial destes indivíduos foram analisados. A presença do transgene Hoxb1 não resgatou a letalidade embrionária de mutantes Cdx2/4 e em algumas linhas transgénicas aumentou a letalidade. De todas as linhas obteve-‐se apenas um recém-‐nascido com o genótipo Cdx2/4 Cdx2PHoxb1 o que indica que a presença de Hoxb1 está a agravar o fenótipo de Cdx2/4. A análise do esqueleto axial dos mutantes com e sem o transgene mostrou que em todos os mutantes de
base nestes resultados propomos que Hoxb1 interage com genes Cdx/Hox centrais de forma antagonísitca no processo de extensão axial. Os genes Hox mais afastados de genes centrais do cluster actuam de forma epistática sobre estes, o que explicaria o agravamento do fenótipo de Cdx2/4 Cdx2PHoxb1. Em suma, ao longo do processo de extensão axial é necessário um balanço entre genes Hox anteriores e posteriores, estabelecido através de interacções epistásticas.
Palavras chave: Genes Cdx, extensão axial, sinalização Wnt, genes Hox
IX
Abbreviation List
AP Anterior-‐posterior
C Celsius
ADH Alcohol dehydrogenase
AP Alkaline phosphatase
Cdx2/4 Cdx2+/-‐ Cdx4 null
CHAPS 3[(3-‐Cholamidopropyl)dimethylammonio]-‐propanesulfonic acid
Cyp26a1 Cytochrome P450, family 26, subfamily A, polypeptide 1
DEPC Diethylpyrocarbonate
DIG Digoxigenin
DNA Deoxyribonuclease acid
DNAse Deoxyribonuclease
dNTP Deoxyribonucleotide triphosphate
DTT Dithiothreitol
E Embryonic day
EDTA Ethylene diamine tetraacetic acid
et al. et alii (and others)
EtOH Ethanol
FGF Fibroblast growth factor
FGFR Fibroblast growth factor receptor
H Hour
ICM Inner cell mass
LB Lysogeny broth
Lef1 Lymphoid enhancer-‐binding factor 1
LiCl Lithium Chloride
M Molar MetOH Methanol MgCl2 Magnesium dochloride min Minutes ml Milliliter mM Millimolar
MAB Maleic acid buffer
NaAC Sodium Acetate
ng Nanogram
NTMT Alkaline phosphatase buffer
PBS0 Phosphate buffered saline (without calcium and magnesium)
PCR Polymerase Chain Reaction
PFA Paraformaldehyde
PGC Primordial germ cell
PSM Presomitic mesoderm
RA Retinoic acid
RALDH Retinaldehyde dehydrogenase
Raldh2 Retinaldehyde dehydrogenase type 2
RDH Retinol dehydrogenase
RNA Ribonucleic acid
RNAse Ribonuclease
rpm Revolutions per minute
RT Room temperature
RXR Retinoic X receptor
sec Seconds
SDS Sodium dodecyl sulphate
SRY Sex-‐determining region Y
SSC Saline Sodium Citrate
Taq polymerase Thermicus aquaticus polymerase
TBS Tris buffered Saline
TCF T cell factor
TE Tris EDTA
tRNA Transfer ribonucleic acid
VE Visceral endoderm
VEGF Vascular endothelial growth factor
μg Microgram
μl Microliter
XI
Table of contents
ABSTRACT
V
RESUMO
VI
ABBREVIATION LIST
IX
TABLE OF CONTENTS
XI
GENERAL INTRODUCTION
1
Early mouse development 1
Axis elongation and axial progenitor cells 1
Wnt signalling 2
Retinoic acid signalling 2
FGF signalling 3
Cdx genes 3
Cdx null mutants and the genetic control of axial extension 5
AIM OF THIS THESIS
7
CHAPTER I -‐ INVOLVEMENT OF THE CANONICAL WNT PATHWAY DOWNSTREAM OF
CDX GENES IN THE FORMATION OF THE PLACENTAL LABYRINTH
9
Introduction 9
Placental labyrinth development 9
Cdx genes and Wnt signalling pathway in placenta formation 9
Methods 11
Mice 11
Isolation embryos and processing 11
Genotyping 11
Histological analysis 11
In situ hybridization 12
Results 14
Wnt3a-‐/-‐ Cdx2+/-‐ embryos have defects in placental labyrinth similar to Cdx2/4 mutants 14
Cdx4 is downregulated in Wnt3a-‐/-‐Cdx2+/-‐ mutants 14
Discussion 16
CHAPTER II – ANTERIOR HOX GENES AND AXIAL ELONGATION
17
Introduction 17
The vertebrate axis 17
Hox genes and vertebrate axis 17
Hox genes expression and regulation 18
Generation of transgenic constructs and mice 21
Isolation of embryos 21
Bone and cartilage staining 21
Genotyping 21
RNA isolation 22
DNAse treatment 22
cDNA synthesis 23
Quantitative RT-‐PCR analysis 23
Results 24
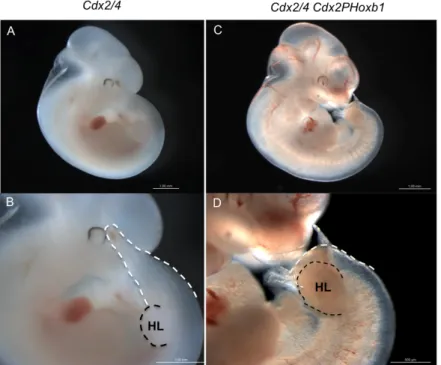
Hoxb1 is overexpressed in the Cdx2PHoxb1 transgenic mice 24
Hoxb1 transgene does not recue defects from the placental labyrinth of Cdx2/4 mutants. 25
Hoxb1 does not rescue the axial defects of Cdx mutants 26
Analysis of the phenotype of Hoxb1 transgenic embryos 31
Discussion 33
CONCLUDING REMARKS
35
REFERENCES
37
ANNEXES
43
Annex I 43Annex II 44
1
General Introduction
Early mouse development
The early patterning of the embryo and the onset of subsequent morphogenesis occurs during what could be considered the most important process in development, gastrulation. Gastrulation is characterized by morphogenetic movements accompanied by cell proliferation and differentiation which will eventually convert the embryo into three germ layers, the ectoderm, mesoderm and endoderm [7]. The mouse gastrulates by the ingression of cells from the epiblast through the primitive streak, a structure that emerges in the posterior region of the embryo at embryonic day (E) 6.2 [8]. During gastrulation, nodal-‐ dependent signals from the VE have a role in the regionalization in the primitive streak, with the node in the most anterior region [9]. Fate maps provided by clonal analysis of single epiblast cells show that the epiblast is regionalized; however individual cells can contribute to multiple germ layers [10]. The node organizes the ingression of epiblast cells through the primitive streak. Once ingressed, mesodermal tissues differentiate in lateral mesoderm (circulatory system, limb bud mesenchyme and wall of the digestive organs), intermediate mesoderm (urogenital system) or paraxial mesoderm (presomitic mesoderm and somites) [8].
Axis elongation and axial progenitor cells
By the end of gastrulation, only the most rostral tissues are formed and the elongation of the anterior-‐posterior (AP) axis continues by the addition of tissues from the primitive streak and adjacent epiblast, and later from the tail bud [1,11]. The source of these axial tissues is a pool of progenitors, some of which have stem cell properties [1]. These axial structures are added in an rostral-‐to-‐caudal sequence as the embryo grows [11]. For this reason both the primitive streak plus the adjacent epiblast, together with tail bud can be called “posterior growth zone”. This region comprises the border region between the node and anterior primitive streak and the epiblast adjacent to the streak [1,12,13]. Cell lineage tracing studies revealed the relative positions of the different progenitor populations, and they indicate a temporal order of cell emergence that corresponds to the building of the AP axis [7]. More recently clonal analysis showed that the stem cell-‐like precursors are neuromesodermal progenitors which persist after the segregation of endodermal and
surface ectoderm layers [14], suggesting that neurectoderm and mesoderm are more closely related than mesoderm and endoderm.
As mentioned above some of these progenitors of the posterior growth zone have stem cell characteristics. Therefore an equilibrium between the generation of differentiated axial tissues and the maintenance of a posterior progenitors is required [1]. The genetic control of the process of axial elongation and maintenance of the posterior growth zone involves a series of highly conserved genes and signalling pathways. Among the known signalling pathways involved are Wnt, Retinoic acid (RA) and Fgf [1,2,15,16] and among the transcription factor-‐encoding genes are Cdx [3,17] and T brachyury [2,3,17,18].
Wnt signalling
Wnt signalling is essential during vertebrate development and is associated with the regulation of many processes. Wnt is the ligand that activates the canonical pathway and the other main components are the transmembrane receptor Frizzled (Fz), and the downstream effectors of the pathway, Dishevelled (Dsh), β-‐catenin and T cell factor/Lymphoid enhancer-‐ binding factor 1 (Tcf/Lef1) [19]. During early development the Wnt pathway controls cell proliferation, stem cell maintenance, cell fate decisions, organized cell movements and establishment of tissue polarity [20]. Wnt3 and Wnt3a have been shown to be essential for axis formation and elongation of vertebrate embryos, respectively. Wnt3a is expressed in the presumptive mesoderm in the posterior region of the developing embryo [5]. Null mutants for Wnt3a have a severe axial truncation, a disrupted notochord and a deficient tailbud [21]. Galceran et al. showed that Wnt3a acts trough Lef1/Tcf1 since Lef1-‐/-‐Tcf1-‐/-‐ mice
have a phenotype similar to that of Wnt3a-‐/-‐ mice [22]. Wnt3a is also involved in the
regulation of somitogenesis acting on the presomitic mesoderm (PSM) [23]. Wnt3-‐/-‐ mice do
not develop a primitive streak and therefore lack mesoderm and node [24], and thus Wnt3 is required in a much earlier developmental stage compared to Wnt3a.
Retinoic acid signalling
Retinoic acid (RA) signalling is involved in a range of developmental processes, for example the control of progenitor cell populations, including the axial precursors [25]. RA is a vitamin A-‐derived compound and its biological action is restricted by the localization of its synthesis regulated by retinol and alcohol dehydrogenase (RDHs and ADHs) and
General Introduction
3
retinaldehyde dehydrogenases (RALDHs) and the presence of enzymes that degrade it, cytochrome P450s (CYP26s) [25].
Raldh2-‐/-‐ embryos die during development from defective heart morphogenesis and
have severe developmental defects like body axis truncation [26]. Cyp26a-‐/-‐ mutant embryos
show several defects among which a truncation of the posterior body region, posterior transformations of cervical vertebrae and abnormal hindbrain patterning [27,28].
FGF signalling
Fibroblast growth factors (FGFs) are a family of ligands that bind tyrosine kinase receptors, the FGF receptors (FGFRs). FGF ligands bind the extracellular domain of the FGFRs to form a complex leading to the transphosphorylation of specific intracellular tyrosine residues [29].
Fgf signalling is required for ingression of epiblast cells through the primitive streak [30,31]. During axial elongation Fgf8 is expressed in the primitive streak and posterior mesoderm [1,4,32-‐34]. A caudal-‐to-‐rostral gradient of Fgf8 is formed from the node region where low Fgf8 levels allow mesoderm to differentiate and high concentrations maintain the stemness of the progenitors in the posterior growth zone [1,16]. Fgf signalling is confined to the posterior region of the embryo as a result of the antagonistic interaction with RA.
Cdx genes
The vertebrate Cdx genes (Cdx1, Cdx2 and Cdx4) are the homologs of the Drosophila
caudal (cad) gene [35] , which is known for playing a role in patterning the AP axis of the
early fly embryo and acts as a posterior homeotic gene [36]. Both Cdx and Hox gene families arose from a common ancestor, the ProtoHox cluster thought to confer anteroposterior identity to axial tissues in all bilatarians [6]. Given their common origin, high similarities between these two families exist. The three Cdx genes are initially expressed in the primitive streak at the late primitive streak stage. Slightly later, Cdx1 has the most anterior expression boundary whilst the expression of Cdx2 and Cdx4 is more posteriorly restricted. This situation is transient and at E9.0 all three Cdx genes are expressed in the posterior growth zone.
Expression of Cdx1 is initiated at E7.2 in the ectodermal and mesodermal cells of the primitive streak [37]. Cdx1-‐/-‐ mutant mice have anterior homeotic transformations of the
cervical region accompanied by a caudal shift in the expression domain of Hox genes [38]. This shows the role of Cdx genes in regulating Hox genes expression.
In addition to its expression in the primitive streak, Cdx2 is already expressed at E3.5 in the trophectoderm. At the blastocyst stage Cdx2 has an essential function in assuring segregation of the inner cell mass (ICM) and trophectoderm and is necessary for the implantation into the uterus wall at E4.5 [39-‐42]. At E7.2 expression of Cdx2 is detected in the embryo in the posterior primitive streak, in the allantois and the chorion. The gene remains expressed at later stages in the posterior neural tube and presomitic mesoderm. The lethality of Cdx2-‐/-‐ mutants at E3.5 can be bypassed by tetraploid rescue, and the
resulting embryos eventually die at E10.5 because of defects in the allantois [40]. In addition, the absence of the allantois leads to agenesis of the placental labyrinth. Around E10.5 the mouse embryo becomes dependent on the correct formation of the placental labyrinth, which will allow exchanges of nutrients and gases between the mother and the embryo.
Cdx2 mutants obtained by tetraploid rescue are severely truncated in all three germ layers
posteriorly to the forelimb bud, and they form a maximum of 17 somites [40]. Heterozygous mutants for Cdx2 get born but they have a subtle shorter axis and occasionally exhibit a short and kinky tail and skeletal analysis showed anterior homeotic transformations of some of the cervical and thoracic vertebrae [41]. Although Cdx2 is not expressed in the somitic mesoderm at cervical levels, Cdx2 mutations do alter egene expression and the identity of vertebrae at this cervical levels which implies that the interactions of Cdx as Hox regulators occur early in the presomitic mesoderm [43]. The phenotypes of Cdx1 and Cdx2 loss of function mutants may result from the fact that Cdx proteins are positive regulators of the
Hox genes in embryonic tissues [44]. Although the possibility that Cdx genes play a role on
their own in the processes of axial extension and patterning should also be consider.
Cdx4 located on the Y chromosome, is first expressed at E7.2 in the allantoic bud and
in the posterior primitive streak. Cdx4 remains expressed in the neuroectoderm, presomitic and lateral plate mesoderm in the posterior embryo, and in the hindgut endoderm until around E10.5 [45,46]. Hemizygous mutants for Cdx4 have a very mild axial defect, an anterior transformation of vertebra 15, with very low penetrance [4].
The Cdx mutant phenotypes discussed so far show that Cdx genes have a crucial role in patterning the anteroposterior axis (together with Hox genes) and in supporting the process of axial elongation of the mouse. Besides the failure of Cdx2 null mutants in generating a functional allantois, the role of Cdx genes in extraembryonic tissues was shown in the compound mutant Cdx2+/-‐/Cdx4-‐/-‐ (Cdx2/4). These embryos also have an axial
General Introduction
5
truncation at the sacral level and only 15% survive until birth. The embryonic lethality is due to defects in placental development, in some cases a failure of chorio-‐allantoic fusion , and most often deficiencies in extension and branching of the allantoic vascular network into the chorionic ectoderm [4].
Cdx null mutants and the genetic control of axial extension
Cdx triple null mutants were generated with mice carrying null alleles for Cdx1, Cdx4
and conditional alleles for Cdx2 [47]. These mutants present the most severe axial truncation of all the Cdx mutants described: only 5 somites are generated. The posterior growth zone of
Cdx null embryos severely lost its activity of generating nascent mesoderm and
neuroectoderm. The complete absence of Cdx alleles in these mutants permits a more clear study of the genetic pathways associated with Cdx and axial elongation. The expression of
Wnt3a is downregulated in these mutants, reinforcing that the Wnt pathway acts
downstream of Cdx genes. Hox gene expression was also affected, Hox anterior genes are well induced but posterior Hox genes show no expression in these mutants. Cdx genes regulate the gene encoding the enzyme that degrades RA, Cyp26a1 directly and positively [2,48]. Cyp26a1 is absent from the posterior region of Cdx null mutants resulting in the deficiency of RA clearance. The persistence of RA in the posterior region is further accounted for the high level of Raldh2 expression in this region of the Cdx null mutants. Due to the
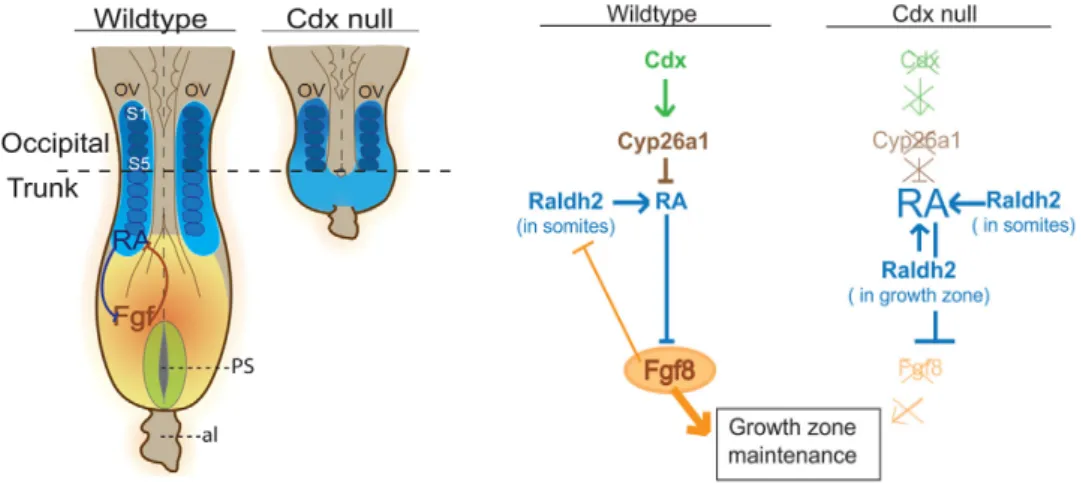
higher levels of RA in the posterior region of Cdx null embryos, Fgf8 expression is completely absent in these mutants. Interestingly, re-‐induction of Fgf signalling was able to partially
Figure 1 -‐ Genetic interactions involved in the maintenance of the posterior growth zone in Wild-‐type and Cdx null embryos. Left: Schematic dorsal view of E8.5 wild-‐type and Cdx null embryos. Right: Schematic
representation of the signalling cascades downstream of Cdx in the growth zone of wild-‐type and Cdx null embryos. Not the absence of Fgf8 in the Cdx null mutants that lead to failure of RA clearance from the posterior region of the embryo. Orange represents the expression domain of Fgf8 and in blue the presence of RA. From: Van Rooijen et al., 2012
rescue the Cdx2 mutant truncation [47]. The rescued embryos regain the expression of
Cyp26a1 also absent in Cdx2 null mutants. The generation of embryos totally deprived of
Cdx activity allowed a better understanding of the mechanisms and genetic interaction involved in axial extension. The model in figure 1 was proposed.
7
Aim of this thesis
This work will focus on the role of Cdx genes and the interaction with other factors in the regulation of the posterior growth zone in the mouse embryo. This thesis has two aims; the first is to study the interaction of Cdx2 and canonical Wnt signalling (Wnt3a) and the second is to test the role of Hoxb1 in the regulation of axis extension and the interaction with Cdx genes. The results of these two projects will be described in two separate chapters in this thesis.
The project described in Chapter I results from previous observations that Wnt3a-‐/-‐
Cdx2+/-‐ mutant embryos were not recovered at E15.5. The aim of this project was to
investigate the origin of early lethality of the Wnt3a-‐/-‐Cdx2+/-‐ mutant embryos. Neither Cdx2
heterozygote mutants nor Wnt3a null mutants are arrested in their development. Cdx2 heterozygotes only present some alterations in vertebrate patterning and a mild defect in the posterior embryonic axis, missing a few caudal vertebrae [41]. Wnt3a homozygous mutants have a severe truncation of the embryonic axis [5], very similar to the posterior body truncations of Cdx2 mutants [49]. Both Wnt signaling and Cdx/Hox genes have important roles during axis elongation and Wnt exerts a positive feedback loop on Cdx that maintains Wnt signalling to sustain progenitor self-‐renewing and tissue elongation [2]. We wanted to test whether the loss of Wnt3a in Cdx2 heterozygotes was causing early lethality of the compound mutant embryos by compromising placental development.
Cdx genes are key regulators of the process of axial extension as they regulate the
niche of the axial progenitors in the posterior growth zone. Previous work showed that trunk
Hox genes collaborate with Cdx genes to stimulate posterior axial growth while posterior Hox genes promote growth termination by interfering with Cdx/trunk Hox genes [2]. The
second question of this work concerns the role of anterior Hox genes in the process of axial elongation and how they interact with Cdx in this regulation; this work is described in Chapter II. In this project we propose to test whether Hoxb1, like Hoxb8 and Hoxa5 [2], is able to rescue the Cdx2/4 mutant phenotype.
9
Chapter I -‐ Involvement of the canonical Wnt pathway
downstream of Cdx genes in the formation of the
placental labyrinth
Introduction
Placental labyrinth development
Mice have a chorioallantoic placenta, which means that it is formed from two extraembryonic components, the chorion and the allantois. The allantois is first visible at E7.0/E7.25 [50,51] as a bud of extraembryonic mesoderm arising from the posterior part of the primitive streak [10,52]. The outer cells of the bud will differentiate into a layer of mesothelium that surrounds an inner core of extraembryonic mesoderm [53] . The next step in the development of the allantois is its growth into the exocoelomic cavity in the direction of the chorion [53]. The allantois vascularizes intrinsically, rather than by angiogenesis. It arises independently from the vessel network of the yolk sac or the fetus and is not accompanied by erythropoiesis [54]. The allantoic vasculature is formed by vasculogenesis, a process characterized by the differentiation of mesodermal cells into endothelial cell precursors or angioblasts. The vascularization starts in the most distal cells of the inner core of the allantois which start to flatten and then coalesce to form the blood vessels [54]. Expression of Flk1, a tyrosine kinase receptor for vascular endothelial growth factor (VEGF) and a marker for endothelial cells, follows the morphological appearance of vascularization, first in the distal part of the allantois and later at the proximal part [54]. The first signs of vascularization occur before the fusion of the allantois with the chorion.
Cdx genes and Wnt signalling pathway in placenta formation
The role of Cdx in the development of extraembryonic tissues was mentioned above.
Cdx2 null mutations impair the generation of embryonic and extra-‐embryonic mesoderm
and Cdx2 null allantois does not fuse with the chorion [40]. This reveals the early Cdx dependence of placental ontogeny, reflected by the fact that one active Cdx2 allele is required for outgrowth of the early allantoic bud. In Cdx2+/-‐ and Cdx2+/-‐Cdx4+/-‐ mutants the
allantois reaches a normal size. Cdx mutants exhibit subsequent defects that compromise the ontogenesis of a proficient chorio-‐allantoic placenta, with a penetrance that increases with the decrease in Cdx dosage [4]. In the case of Cdx2/4 mutants, the majority of mutant
allantoises undergoes chorio-‐allantoic fusion but exhibit a later defect, being impaired in the establishment of a functional endothelial network in the labyrinth. The allantoic vessel branching fails to occur in the placental labyrinth, preventing the necessary proximity of the embryonic and maternal blood [4]. The role of Cdx genes in placentogenesis is an early one acting on the progenitors of endothelial cells in the early allantois since Cdx genes are downregulated in the allantois at E8.5.
Wnt signaling is also involved in the development and differentiation of the placental tissues in the mouse embryo [55]. Several studies showed that Wnt signaling is crucial for extraembryonic development, particularly in chorion-‐allantois fusion, placental vascularization and labyrinth function. Embryos null for both Tcf-‐1 and Lef-‐1 display severe defects in placenta formation due to absence of chorionic-‐allantois fusion [56]. Fzd5 knock-‐ out embryos do not survive beyond E10.0 since their placentae were less vascularized[57]. Labyrinths of Wnt2 null embryos exhibit different defects such as edema and decreased numbers of capillaries [58]. Deletion of Wnt7b results in embryonic death around midgestation due to placental abnormalities [59].
Objective
Previous experiments in the lab showed that Wnt3a-‐/-‐ Cdx2+/-‐ mutant embryos suffer
early lethality during development. The objective of this project is to investigate the cause of this lethality, by analysing mutant embryos at earlier stages. Our hypothesis is that, similarly to Cdx mutants, the early lethality resulted from impairment of the allantoic and/or
placental labyrinth development.
11
Methods
Mice
All mice were in the C57Bl6j/CBA background. Cdx2+/-‐ mice were obtained from F.
Beck (Beck et al., 1995) and Wnt3a mice from S.Takada (Takada et al., 1994). To generate the mutants Wnt3a-‐/-‐ Cdx2+/-‐, Wnt3a+/-‐ females were crossed with Wnt3a+/-‐Cdx2+/-‐ males.
Matings were timed to get embryos from the desired stage. The day of the vaginal plug was designated as E0.5 at noon.
Isolation embryos and processing
Embryos were isolated in PBS0, for E8.5 the allantois was kept intact and for E10.5 the placenta was also isolated. Embryos and placentas (E8.5, E10.5) were fixed in paraformaldehyde (PFA) (4%) at 4°C overnight. Tissue was washed twice (10 minutes (min)) in PBS0 with Tween (1%) (PBT), dehydrated in methanol (10 min) steps of 25%, 50%, 75% and twice 100%) and stored at -‐20°C.
Genotyping
For genotyping of embryos genomic DNA was isolated from the yolk sac and amnion. Tissue was lysed overnight by a lysis solution (100 mM Tris HCl pH 8.5, 5 mM EDTA, 0.2% SDS, 200 mM NaCl, 100 μg/mL proteinase K) at 55°C, precipitated with isopropanol and finally dissolved in TE buffer.
Primer sequences for genotyping Cdx2 are ATATTGCTGAAGAGCTTGGCGGC (forward) and TAAAAGTCAACTGTGTTCGGATCC (reverse). Primer sequences for Wnt3a are ACTACAACCCTCCTCACCTG (forward) and TGGCTACCCGTGATATTGCT (reverse). The PCR reaction conditions are 94°C for 5 min, 94°C for 30 seconds (sec), 61°C for 1 min, 72°C for 1 min for 35 cycles, 72°C for 5min and 12°C until the end of reaction. In 10 μl mixture with 0.5 μM of each primer, 0.2 mM of each dNTP,1.5 mM MgCl2 and 1x PCR buffer (Promega 5x Flexi Green GoTaq Buffer)
Histological analysis
Dehydrated placentas (in 100% methanol at -‐20°C) were put in paraffin (30 min at 60°C) and paraffin was refreshed twice (2 times 30 min at 60°C). Placentas were embedded
in paraffin and sections were cut (6 μm) using a microtome. Sections were afterwards stained with hematoxylin and eosin.
In situ hybridization
i. Probe generation
DNA transformation into competent cells
1 μl of plasmid with Cdx4 cDNA insert was added into 25 μl of DH5α competent cells and incubated for 10 min on ice. Cells were heat shocked for 45 sec at 42°C and after that placed on ice for 2 min. 1ml of prewarmed Lysogeny Broth (LB) medium was added and incubated at 37°C for one hour. 100μl of the transformation mixture was spread on a LB agar plate (with ampicillin). The plates were left overnight incubating at 37°C. Two separate colonies were picked and grown over night in 100 ml LB medium with 200 μl ampicillin.
DNA isolation form DH5α
Overnight bacterial cultures were pelleted by centrifuging 10 min at 3200 rpm. Plasmids were isolated with the Invitrogen PureLinkTM Quick Plasmid Midiprep Kit, following the manufacturer’s protocol. After the isolation the concentration of DNA was determined with NanoDrop.
Linearization and purification
10 μg of DNA was linearised using restriction enzymes for 1 hour. Linearised DNA plasmid was purified by a phenol/chloroform extraction followed by precipitation with NaAC.
Synthesis of digoxygenin-‐labeled (DIG-‐labeled) RNA probe
In a total volume of 20 μl, the following reagents were mixed: 5x Transcription Buffer, 0.1 M DTT, DIG RNA-‐labelling mixture, placental RNAse inhibitor and RNA polymerase (T7) together with 1.5 μg of linearised plasmid DNA. The mixture was incubated for 2 hours at 37°C. The next step was to dilute with 5x transcription buffer followed by the digestion with 2 μl DNAse (RNAse free) for 45 min at 37°C. Next, destilled H20, brewer’s yeast tRNA, LiCl and 100% ethanol (-‐20°C) was added to the mixture and incubated overnight at -‐20°C. The mixture was spinned down (15 min) at 4°C, washed with 70% ethanol and centrifuged again. Finally, the probes were dried under vacuum, redissolved in TE/formamide (1:1) and stored at -‐80°C.
Chapter I – Methods
13
For whole mount in situ hybridization the embryos were rehydrated (75%, 50%, 25% methanol, 2 times PBT; all steps for 10min) and permealized with 10 μg/ml proteinase K for 15 min. Proteinase K was blocked by glycine (2 mg/ml in PBT) for 5 min and was followed by two times wash (5 min) with PBT. Embryos are refixed in 0.2% glutaraldehyde in 4% PFA, followed by two washes (5 min) with PBT. The embryos were washed with 300 μl prehybridization mix (5 min) and subsequently incubated for at least 1 hour at 70°C in 400 μl prehybridization mix. Hybridization takes place over night at 70°C with prehybridization mix with the probes. After hybridization embryos are washed with 800 μl prehybridization mix (10 min at 70°C) and 400 μl 2x SSC (70°C) was added three times (10 min). After 2 times 30 min wash with CHAPS (0.1%)/SSC (2x), the tissue was incubated at 37 °C for at least one hour with 100 μg/ml RNAse-‐A in CHAPS (0.1%)/SSC (2x). Afterwards the samples were washed twice with Maleic acid buffe (MAB) for 10 min at room temperature and twice for 30 min at 70°C. Subsequently a 10 min wash with PBT and two washes (10 min) with TBST with 2mM levamisole. Embryos were preblocked by 10% heat inactivated sheep serum (endogenous alkaline phosphatase activity is inactivated beforehand by 70°C incubation for 30 min) for 2 hours. Beforehand a anti-‐DIG alkaline phosphatase mixture was prepared with 15 mg embryo powder in 2,5 ml TBST, 250 μl 10% inactivated sheep serum and 5 μl of anti-‐DIG conjugated with AP; incubated at 4°C for 4 hours while shaking. Blocking serum was removed and anti-‐DIG mixture was added, tissues were incubated at 4°C overnight with gently shaking. Post antibody washes were done with TBST with 2mM levamisole (3 times 5 min followed by 5 times 60 min wash). Before immunological detection with 1 ml BM Purple (with 1mM levamisole) starts, the samples must be washed 3 times with NTMT (with 2 mM levamisole). To stop the reaction the embryos were washed twice (10 min) with NTMT (with 2 mM levamisole) and 10 min with PBT including 10 mM EDTA. Embryos were postfixed with 0.2% glutaraldehyde in 4% PFA and finally samples were washed (30 min) and stored in PBT/EDTA. Embryos were placed in filtered PBT/EDTA for image acquisition. This protocol is
Results
In order to study the relationship between Cdx genes and Wnt signalling in more detail, Wnt3a-‐/-‐ Cdx2+/-‐, embryos were generated previously to this work by crossing Wnta3+/-‐
females with Wnt3a+/-‐ Cdx2+/-‐ males. At E15.5 these genotypes were not recovered which
indicated early lethality during development. To investigate whether placentation was defective in these mice, embryos were isolated at earlier stages of development and the phenotype was analysed.
Wnt3a
-‐/-‐Cdx2
+/-‐embryos have defects in placental labyrinth similar to
Cdx2/4 mutants
Embryos were isolated at two different embryonic stages. E8.5 embryos were generated to observe whether the allantois was attached or not to the chorion. At E10.5 the umbilical cord and placenta have normally already developed and it is possible to analyse their morphology.
At E8.5 Wnt3a-‐/-‐ and Wnt3a-‐/-‐Cdx2+/-‐ show a narrower posterior region in the
embryo compared to wild type. Only a few Wnt3a-‐/-‐Cdx2+/-‐ embryos had not undergone
chorio-‐allantoic fusion.
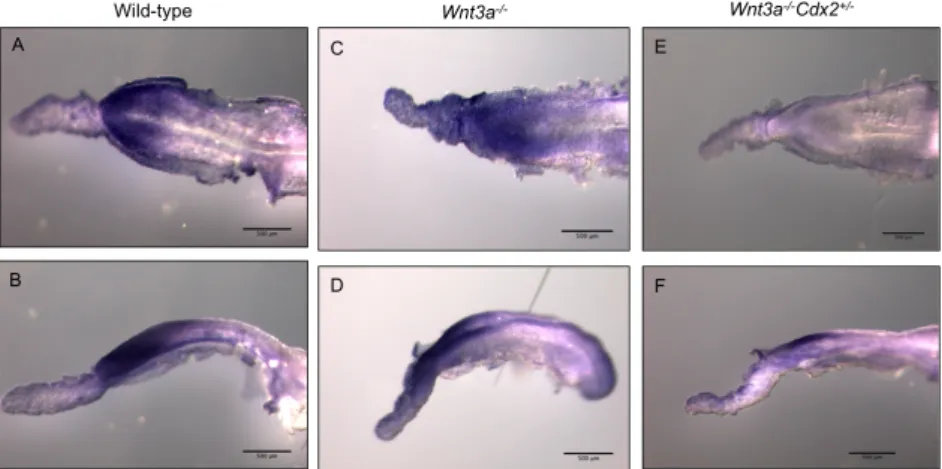
At E10.5 no defects in the morphology of the umbilical cord or aberrant blood was observed. Placentas were isolated and sectioned to analyse the phenotype of the labyrinth. Figure 1.1 shows sections of these placentas, from both wild-‐type and Wnt3a-‐/-‐Cdx2+/-‐
embryos. Fig1.1A and 1.1C show that the labyrinthine area, containing the embryonic and maternal blood, has the same width in the wild-‐type and in the Wnt3a-‐/-‐Cdx2+/-‐ mutants.
However the embryonic vessels are wider in the mutants, and do not penetrate the chorionic plate efficiently (Fig.1.1D). The embryonic blood seems to be held in the base of the placenta and the branching of the vessels is underdeveloped when compared to the wild-‐type (Fig.1.1B and D). As a result, the embryonic vessels and the maternal blood are not in direct contact, impairing the interchange of nutrients, which is a likely cause of the embryonic lethality. The defects in the placental labyrinth resemble that in the Cdx2/4 mutants, although it is less severe.
Cdx4 is downregulated in Wnt3a
-‐/-‐Cdx2
+/-‐mutants
To investigate whether this phenotype is reproducing the Cdx2/4 phenotype we analysed whether Cdx4 was downregulated in these embryos. In mutants isolated at E8.5 in