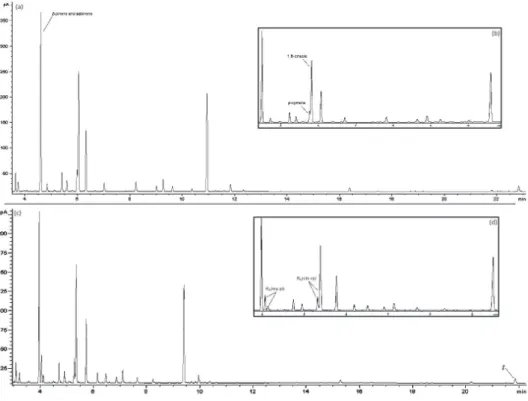
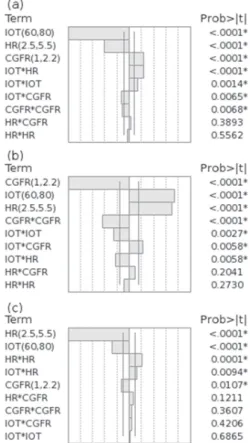
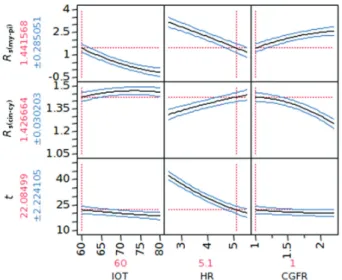
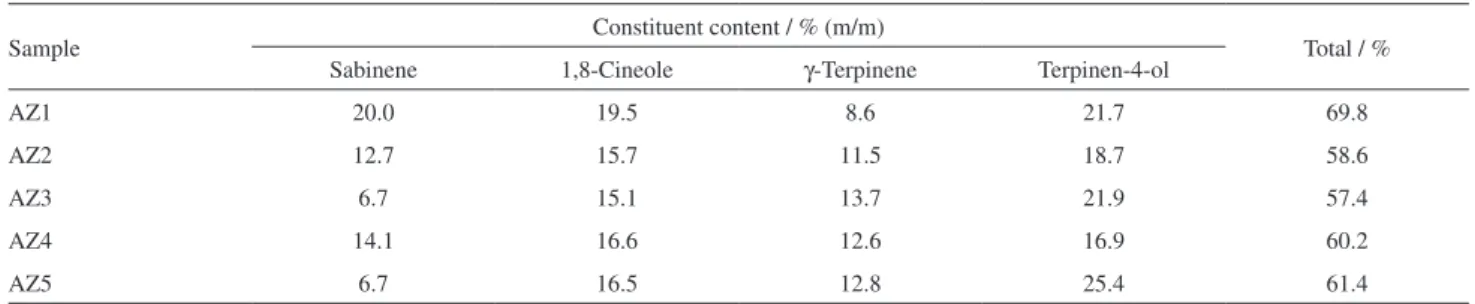
Development, Optimization and Validation of a GC Method by Polarity Phase Constants and Statistical Design of Experiments for the Determination of Monoterpenes in Alpinia zerumbet Essential Oil
Texto
Imagem


Documentos relacionados
The multivariate experimental design technique allowed the optimization of the analytical conditions of HS extraction followed by GC–PID analysis for BTEX determination in dif-
Dealing successfully with the "disturbers" cannot be left to a general health service, Their control requires special efforts, for which special sub-services of
The probability of attending school four our group of interest in this region increased by 6.5 percentage points after the expansion of the Bolsa Família program in 2007 and
Thus, the objec- tive of this study was the development of an analytical method for the determination of Ni and Cd concentrations in biodiesel samples by GFAAS, using microemulsions
2 — As áreas referidas no número anterior são constituídas por uma faixa de protecção non -aedificandi de 25 metros para cada lado do eixo indicado na Planta de
objective of the present work was the optimization and validation of a simplified method employing SLE and capillary GC-MS for the determination of hexachlorobenzene,
Due to the existence of few methods for the determination of pesticide residues in grapes and the occurrence of these compounds, this paper describes the optimization and
The present study describes the development and subsequent validation of simple and accurate stability indicating RP-HPLC method for the determination of sparloxacin and