www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Auditory
evoked
potentials
in
a
newborn
Wistar
rat
model
of
hyperbilirubinemia
夽
,
夽夽
C
¸a˘
gıl
Gökdo˘
gan
a,∗,
Aydan
Genc
¸
b,
Özlem
Gülbahar
c,
Ozan
Gökdo˘
gan
d,
Ays
¸e
Helvacı
e,
Selin
Üstün
Bezgin
f,
Leyla
Memis
¸
eaGaziUniversityHospital,AudiologyDepartment,Ankara,Turkey
bHacettepeUniversityHospital,AudiologyDepartment,Ankara,Turkey
cGaziUniversityHospital,BiochemistryDepartment,Ankara,Turkey
dMemorialHospital,ENTDepartment,Ankara,Turkey
eGaziUniversityHospital,PathologyDepartment,Ankara,Turkey
fGaziUniversityHospital,ENTDepartment,Ankara,Turkey
Received7September2014;accepted6January2015 Availableonline2December2015
KEYWORDS
Hyperbilirubinemia; Wistarrats;
Sensorineuralhearing loss;
Auditoryevoked potentials
Abstract
Introduction:Hyperbilirubinemia isa common health problem innewborns. Its effectscan bedifferentaccordingtothelevelanddurationofthehyperbilirubinemia.Thetoxiceffect ofbilirubinontheauditorysystemcanbeseenasasensoryneuralhearing lossorauditory neuropathyspectrumdisorder(ANSD).
Objective:Thepurposeofourstudywastodeterminetheeffectsoftoxicbilirubinlevelonthe auditorysystembyusingAuditoryBrainstemResponseaudiometry.
Methods:Ratsareusedasanimalmodels duetotheir lowcost andeasyattainability. Audi-toryBrainstemResponsewasusedfor auditoryassessment. Inthisstudy,threegroups were established:experimental,controlandplacebogroups.
Results:
Results:In theexperimental group,which consistsofrats withhyperbilirubinemia, sensory neuralhearinglosswasfoundbilaterallyin4rats(66.67%)andunilaterallyin2rats(16.67%) andauditoryneuropathyspectrumdisorderwas foundunilaterallyin1rat(8.33%).Auditory BrainstemResponsethresholdsweresignificantlyelevatedcomparedtocontrolandplacebo groups(p<0.05).
夽
Pleasecitethisarticleas:Gökdo˘ganC¸,Genc¸A,GülbaharÖ,Gökdo˘ganO,HelvacıA,BezginSÜ,etal.Auditoryevokedpotentialsina newbornWistarratmodelofhyperbilirubinemia.BrazJOtorhinolaryngol.2016;82:144---50.
夽夽Institution:GaziUniversityHospitalDepartmentofAudiology,Ankara,Turkey.
∗Correspondingauthor.
E-mail:cagilgokdogan@gmail.com(C¸.Gökdo˘gan).
http://dx.doi.org/10.1016/j.bjorl.2015.10.008
Conclusion: Hyperbilirubinemiaofnewbornratsmayresultbothinsensoryneuralhearingloss andauditoryneuropathyspectrumdisorder.
© 2015 Associac¸ão Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Published by Elsevier Editora Ltda. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
PALAVRAS-CHAVE
Hiperbilirrubinemia; RatosWistar; Deficiênciaauditiva neurosensorial; Potenciaisevocados auditivos
PotenciaisevocadosauditivosemummodeloderatoWistarneonatocom hiperbilirrubinemia
Resumo
Introduc¸ão: Ahiperbilirrubinemiaéumproblemadesaúdecomumemneonatos.Seusefeitos podemvariar,dependendodoníveledadurac¸ãodahiperbilirrubinemia.Oefeitotóxicoda bilir-rubinanosistemaauditivopodeserobservadonaformadedeficiênciaauditivasensorioneural oudedistúrbiodoespectrodaneuropatiaauditiva.
Objetivo: Afinalidadedenossoestudofoideterminarosefeitosdeníveltóxicodebilirrubina nosistemaauditivo,comousodaaudiometriadarespostaauditivaevocadadetroncocerebral.
Método: Os ratos são empregados como modelos animais grac¸as a seu baixo custo e fácil obtenc¸ão.Utilizamosarespostaauditivaevocadadetroncocerebralparaavaliac¸ãodaaudic¸ão. Noestudo,foramestabelecidostrêsgrupos:experimental,controleeplacebo.
Resultados: Nogrupo experimental, constituído deratoscomhiperbilirrubinemia, disacusia auditivaneurosensorialfoidiagnosticadabilateralmenteem quatroratos(66,67%),e unilat-eralmente em dois (16,67%); e distúrbio do espectro da neuropatia auditiva foi observado unilateralmente em um rato (8,33%). Os limiares da resposta evocada de tronco cerebral estavamsignificantemente elevados,emcomparac¸ãocomosgruposcontroleeplacebo(p< 0,05).
Conclusão:Ahiperbilirrubinemiaderatosneonatospoderesultartantoemdisacusiaauditiva neurosensorialcomoemdistúrbiodoespectrodaneuropatiaauditiva.
© 2015 Associac¸ão Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Publi-cado por Elsevier Editora Ltda. Este é um artigo Open Access sob a licença CC BY (https://creativecommons.org/licenses/by/4.0/deed.pt).
Introduction
Hyperbilirubinemia is a common health problem of new-borns. Newborn hyperbilirubinemia has been accepted as oneofthemainriskfactorsininfants’hearinglosssincethe 1900s.1 Effects of hyperbilirubinemia onhearing function
canbedifferentaccordingtothelevelof hyperbilirubine-miaanddurationofhyperbilirubinemia.2Itisknownthata
highbilirubinlevelisariskfactorforsensoryneural hear-ingloss(SNHL)andauditoryneuropathyspectrumdisorders (ANSD).3
SNHLresultsfrompathologyin thecochlea,8thnerve, brain stem or cortex level. Although 50% of SNHL in children is genetic, it can also develop depending on pathologies in the prenatal, natal, or postnatal periods (infections,metabolicdisordersetc.).4Inpostnatalperiods
hyperbilirubinemia is the most common SNHL cause for newborns,andSNHLprevalencedependingon hyperbiliru-binemia in newborns and infants has been determined as 12.8%.5
ANSD isanissue thathasmany unknownaspectsandit is widely studied. In some cases of ANSD, while only the innerhaircellsintheinnerearareaffected,insomeother casesonlythecentralauditorypathwayisaffected.Among the ANSD risk factors are hyperbilirubinemia, premature birth,andgenetic,perinatalmechanicalventilation.ANSD
relatedtohyperbilirubinemiais seen in2.7% of newborns withhyperbilirubinemia.6
Hyperbilirubinemia may have different pathological effectsontheABRpatternofaffectedindividuals.In new-bornswithhyperbilirubinemia,theABR patterncaneither gobacktonormalorbecomeevenmorepathologicalafter bloodbilirubinlevelsarebroughttonormalwithtreatment.7
Hyperbilirubinemiainthe newbornperiodhasbeen previ-ouslystudiedwithratmodel.8,9
Thepurposeofourstudyistodeterminetheeffectsof toxicbilirubinlevelontheauditorysystembyusingAuditory BrainstemResponseaudiometry.
Method
The study has been conducted in the local Experimental Animals Research and Application Center of the Faculty of Medicine. The approval of the ethical board has been taken(numberofapprovaloftheethicscommittee: G.Ü.ET-12.001).
examinationofallratshasbeenperformedwithendoscopes byanotorhinolaryngologist.
Experimentgroup
Theexperimentgroupconsistedof6newbornWistarAlbino maleratswithhyperbilirubinemia,withweightsbetween18 and20g(average:18.33g).ARatHyperbilirubinemiaModel hasbeencreatedfortheexperimentgroup.
Themethodforestablishinghyperbilirubinemiawas sim-ilartomethods previouslyreported.9 Bilirubin (Sigma, St.
Louis, MO; B4126) was stored in the dark, and the solu-tionwaspreparedjustbeforethe injection.Bilirubin was thawedin0.1% MNaOHjustasin Hansen etal.8
applica-tionandstabilizedwithBSAanddilutedwithKrebs-Ringer buffer(pH:7.4).Thebilirubinconcentrationhasbeen deter-mined as 3mg/mL, and the prepared solution has been preserved at +40◦C, in a shaded place. Each animal in
theexperimental groupreceivedan intraperitoneal injec-tion of bilirubin at 50mg/kg on the postnatal 7th and 10th days. Prior to the 1st injection and 24h after the 1stand2ndbilirubin injections,measurementshave been donethroughTranscutaneousBilirubinometry(TcB)overthe skin,inordertodeterminethebilirubinlevel.Onthe21st day, electrophysiological hearing tests and TcB were per-formedafterintramuscularanesthesia(50mL/kgketamine and 10mL/kg xylazine). Then euthanasia was performed bydrawingintracardiacblood underdeepanesthesia. The serumbilirubin level was determined quantitatively from theintracardiacblooddrawnandwascomparedtoTcB.
Placebogroup
The placebo group consisted of 6 newborn Wistar Albino male rats with weights between 18 and 20g (average: 18.3g).As theplacebosolution,BSAand24mg/mL Krebs-Ringer buffer (pH: 7.5) have been used. The prepared solutionhasbeenpreservedin+40◦C,inashadedplace.In
ordertodeterminewhetherithasaneffectonthehearing systemofrats,thesolutionwhichhasbeenpreparedwithout bilirubinhasbeenappliedinexactlythesamedosagewhich hasbeenappliedtotheexperimentgroup.Theplacebo solu-tionhasbeen giveninthesameprocedure appliedonthe ratsintheexperimentgroupandhasbeensubjectedtothe sameprocesses.
Controlgroup
Thecontrolgroupconsistedof6newbornWistarAlbinomale ratswithweightsbetween18and20g(average:19g). Con-trarytotheexperimentandplacebogroups,theratsinthe controlgrouphavenotbeengivenanyinjections.However, in order to determine their bilirubin levels, coordinated evaluationshavebeenperformedwiththetimeslices deter-minedfortheexperimentandplacebogroupsandtheyhave beensubjectedtothesameprocesses.
AuditoryBrainstemResponse(ABR)test
The ABR evaluations of the rats included in the study have been performed with the Bio-Logic Systems Corp.’s
NavigatorProModel(version2.2.0)device.IntheABR eval-uations, 13.00 rate click stimulus, 10 msn analysis time, 1000sweepinaveraging,100---1500Hzfiltrationhavebeen used. Changes in the intensity have been made in accor-dancewiththeresponsesachievedinthe70dBnHLintensity levelrecordings.InordertodeterminetheCMexistence,the condensation(+)rarefaction(−)polaritychangehasbeen appliedinthesameintensityforbothears.Forthe thresh-oldscan,asitisadvisedin theclickstimulus,ithasbeen continuedwithrarefactionpolarity.
TranscutaneousBilirubin(TcB)measurement
Transcutaneous Bilirubinometry (TcB) has been performed withMinolta/Air---ShieldsJaundice Meter(JM,mode 101, Minolte Corero Co, Osaka, Japan). TcB measurement has been performedonthebackareaoftherats.Priortothe measurement,technicalmaintenanceandcalibrationofthe devicehasbeenperformed.Duringthemeasurements,due tothepressureappliedbytheprobe,anacrylicplaquewas madetopreventtheratsfrombeingdamaged.
SerumTotalBilirubin(STB)measurement
SerumTotalBilirubinlevelshavebeenanalyzedusing ready-made kits (Roche) in auto analyzer (Roche/Integra-800) systems.The totalbilirubinmeasurementprinciple ofthis kitis theDiazomethodbasedonthemeasurementof the amountof azobilirubin,whichis theresultofthereaction ofbilirubinanddiazotizedsulfanilicacid.10
Statisticalevaluation
Statistical analysis hasbeen realized inSPSS for Windows Version 16.0 packaged software. The TcB results used to determine the bilirubin levels in the groups have been comparedwiththeSTBL,ABRfindingsforthehearing eval-uation differences in the groups, hasn’t been normally distributed the Kruskal---Wallis Variance analysis has been applied.Thelevelofsignificancehasbeentakenasp<0.05. The Mann---WhitneyUtesthasbeen performedtotest the significanceofpairwisedifferencesusingBonferroni correc-tiontoadjustformultiplecomparisons(0.05/3=0.016).An overall5%typeIerrorlevelhasbeenusedtoinferstatistical significance.Thecorrelationcoefficientoftherelationships betweenthevariables inthegroups andstatistical signifi-cancehasbeencalculatedbytheSpearmantest.Anoverall 5%typeIerrorlevelhasbeenusedtoinferstatistical signif-icance.
Results
LevelsofTranscutaneousBilirubinometry(TcB)and SerumTotalBilirubin
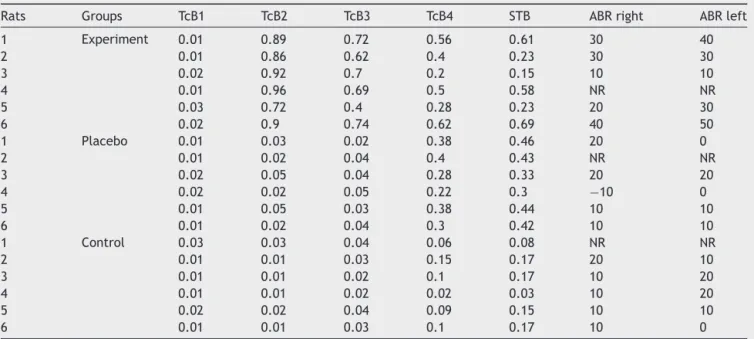
Table1 TcB,STB(mg/dL)valuesandABR(dBnHL)thresholdsofrats.
Rats Groups TcB1 TcB2 TcB3 TcB4 STB ABRright ABRleft
1 Experiment 0.01 0.89 0.72 0.56 0.61 30 40
2 0.01 0.86 0.62 0.4 0.23 30 30
3 0.02 0.92 0.7 0.2 0.15 10 10
4 0.01 0.96 0.69 0.5 0.58 NR NR
5 0.03 0.72 0.4 0.28 0.23 20 30
6 0.02 0.9 0.74 0.62 0.69 40 50
1 Placebo 0.01 0.03 0.02 0.38 0.46 20 0
2 0.01 0.02 0.04 0.4 0.43 NR NR
3 0.02 0.05 0.04 0.28 0.33 20 20
4 0.02 0.02 0.05 0.22 0.3 −10 0
5 0.01 0.05 0.03 0.38 0.44 10 10
6 0.01 0.02 0.04 0.3 0.42 10 10
1 Control 0.03 0.03 0.04 0.06 0.08 NR NR
2 0.01 0.01 0.03 0.15 0.17 20 10
3 0.01 0.01 0.02 0.1 0.17 10 20
4 0.01 0.01 0.02 0.02 0.03 10 20
5 0.02 0.02 0.04 0.09 0.15 10 10
6 0.01 0.01 0.03 0.1 0.17 10 0
TcB1,beforebilirubininjection;NR,noresponse;TcB2and3resultsperformed24hafterthe1stand2ndbilirubininjections;TcB4value priortoeuthanasia.
(p<0.05) in the rats in the experiment groups compared to the rats in the placebo and control groups. When the STBLinbloodiscomparedbetweenthegroups,ithasbeen observed that it is higherin statistically significant terms intheexperimentgroupsincomparisontotheplaceboand controlgroups.Ithasbeenobservedthatthereisa statis-ticallysignificant positiveandstrongrelationshipbetween theTcBvaluepriortoeuthanasiaandtheSTBLvalueinthe experiment,placeboandcontrolgroups.
AuditoryBrainstemResponses(ABR)
Thresholdresults
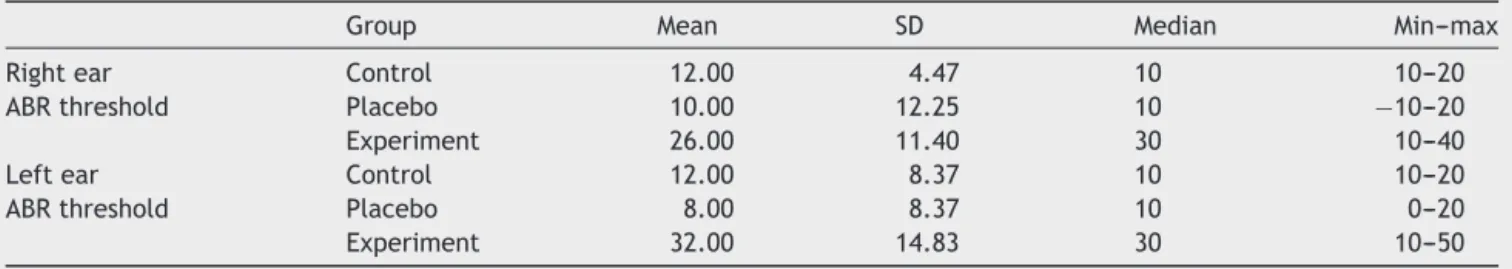
ThecomparisonofABRbetweenthegroups,oneway vari-ance analysis has been used. According to the results of the analysis, while right ear ABR threshold average does not show any significant difference between the groups (p>0.05),leftearABRthresholdshowsastatistically signif-icantdifferencebetweengroups(p<0.05).Thisdifference isaresultofthefactthattheleftearthresholdintensityof theratsintheexperimentgroupsishighercomparedtothe other twogroups (p<0.016)(Tables1and2).Forthetest of the relationship between the STBL valuesin the study groupsandABRthresholds,Spearman’srhocoefficient has been calculated between the variables. According to the results,thereisnosignificantlevelofrelationshipbetween theABRleftandrightthresholdvalueswiththeSTBLvalue intermsofthegroups.
IntheevaluationoftheCMexistenceintheABRpattern achieved from the groups, four aspects have been note-worthy.CMfindingshavebeencategorizedbytakingthese observedaspectsasabasis.InCategory1(Fig.1),whileCM hasnotbeenachievedwiththenormalABRpattern;in Cat-egory2(Fig.2),CMhasbeenobservedwithoutthe2ndwave (theABRthresholdwasconsideredtobethelowestintensity
Figure1 ExampleforCategory1(normalABRpattern).
Table2 MeanthresholdofABRinrightandleftears(dBnHL).
Group Mean SD Median Min---max
Rightear Control 12.00 4.47 10 10---20
ABRthreshold Placebo 10.00 12.25 10 −10---20
Experiment 26.00 11.40 30 10---40
Leftear Control 12.00 8.37 10 10---20
ABRthreshold Placebo 8.00 8.37 10 0---20
Experiment 32.00 14.83 30 10---50
Figure3 ExampleforCategory3(normalABRwithCochlear Microphonic).
Figure4 ExampleforCategory4(thebrokenwave morphol-ogyincludingthe2ndwaveCochlearMicrophonic).
atwhichadefiniterepeatableresponsepeakIIwaspresent). Inaddition,thecategoryachievedinthe2ndwavewiththe CMhasbeenevaluatedasCategory3(Fig.3)andthebroken wavemorphologyincludingthe2ndwavewiththeCMhas beenevaluatedasCategory4(Fig.4).
Discussion
Hyperbilirubinemiaisanimportanthealthproblemin new-borns. The hearing system is also sensitive to bilirubin toxicity. The damages in the central auditory areas have beendefinedinbothanimalsandhumanswith hyperbiliru-binemiainanatomicalandfunctionalterms. However,the physiologicaleffectsandneuromechanismof thedamages caused by hyperbilirubinemia in the peripheral auditory organsarestillamystery.
Ithas been statedin the literature that ANSD may be observed in 1/3---1/2 of the cases with a high level of hyperbilirubinemia.11,12SNHLmayalsobeobservedin
hyper-bilirubinemia(12.8%).5
BilirubintoxicityiscloselyrelatedtoSTBL.Whileahigh correlationisobservedbetweenlevelofbilirubininhumans andSNHL,this correlationdecreases inANSD; although in levelshigherthan20mg/dLthepossibilityofobservingANSD increases.3Therearelimitedpublicationswhichdefinesuch
definite differences and relationships with pathologies in animal models. Due to the difficulties and limitation in forminganimal models,itis difficulttoclearlydetermine bilirubinlevelsandpathologiesresultingfromthis.13
Intheformingof a ratmodelwithhyperbilirubinemia, bilirubin injection is frequently resorted to, due to the easiness ofaccessandapplicationandachievingabsorbed neurotoxicityinthecirculatorysysteminashorttime.13,14
Gaoetal.,14 intheirstudyconductedin2011,havestated
that free bilirubin application is efficientin utilization in bothhyperbilirubinemiaandformationofANSD.Forthis rea-son,intra-abdominalbilirubininjectionhasbeenpreferred intheformationofratmodelwithhyperbilirubinemiainthis study.
Hai Bo Ye et al.13 state that, in cases where a single
andlowdosagebilirubininjectionisdoneinanimalmodels, the damage is not long lasting and, for this reason, high andrepetitivedosagesmaybemoreeffectivein determin-ingthe long termeffects ofhyperbilirubinemia.However, researchersalsounderlinethefactthatthehighest riskin thesemanipulationsis mortality.Inthestudy,afteritwas supportedwithpre-studies,Hansenetal.8modelhasbeen
used,andbilirubindosageappropriatefortheweightofthe newbornratsandwhichdoesnotcausemortalityhasbeen determined.
As Wennbergetal.15 state, due tothe insufficiency of
thestudy,periodicalmeasurementshavebeendonethrough transcutaneousbilirubinometryuntileuthanasia,inorderto determine the bilirubin levels of rats. The bilirubin level determinedthroughthemeasurementspriortoeuthanasia andSTBL’sinbloodtakenthroughintracardiacmethodsafter euthanasiahavebeencomparedandasignificant relation-shiphasbeendeterminedinlinewiththeliterature.
ItisstatedthattheTcBvaluesreachthemaximumlevel 24---48h after the bilirubin injection in Wistar Albino rats andthatthesevaluesdecreasebytime.8,9Accordingtothe
TcBdataachievedinthestudy,thehighlevelofbilirubinin theexperiment group beingsignificantly highercompared toothergroupsisanimportantindicationthatbilirubin tox-icitywasalmostreached.TherelativeincreaseoftheTcB measurementresultspriortoeuthanasiainallgroups may beexplainedwiththevariabilityin thebilirubinvaluesin blooduntiladulthood.
In newborns, ABR is frequentlypreferred inthe deter-minationoftheeffectofbilirubin toxicityontheauditory system.ABR,whichisusedintheevaluationofauditory sen-sitivity,ispreferredinnewbornsduetoitsbeingobjective and non-invasive;its reliabilitybeing higher comparedto othertestmethodsanddeterminationofCMinthe identifi-cationofANSD.17---19
In studies conducted on rats with hyperbilirubinemia, whiledifferences suchaslatencyelongation anddecrease inamplitudeinABRhavebeenevaluated,threshold evalu-ationhasalsobeendone alongwiththesevariablesandit hasbeenobservedthattherearesignificantincreasesinthe thresholdsoftheirABR’s.13,14,19,20 Inthestudy,whilevalues
closetonormaldataandmorphologyhavebeendetermined in the rats of the control group with the exception of 1 rat, an increase in the threshold values of the ABR find-ings and deteriorationin morphology have been observed in theplacebo and experimentgroups. In theexperiment group,whilecompatiblefindingshavebeenachievedinthe CM record and ANSD of 1 rat in one ear,it has not been possibletoreceiveABR responsewithoutCMrecordinthe otherear.Inotherrats,deteriorationsintheABRhavebeen observedindifferentpatterns.
In rats, the anatomical settlement in ABR waves dif-fers from the humans.In humans,while the 1st and 2nd waves originatefromthe8thnerveandthe3rdwave ori-ginates fromthe CN,it is known that in ratsthe 1st and 2nd waves originatefromthe CNand the 3rdwave origi-natesfromSOC.21---23ThedeteriorationsobservedintheCN
inthemorphologicalevaluationconductedintheratsinthe experimentgrouparethoughttocausedeteriorationsinthe morphologyoftheABRresponsesinthisgroupaswell.13,14,19
Shapiroetal.24statethatacutebilirubintoxicityespecially
changestheABR’ssignificantlyinthefirst4hinGunnRats. In the studies conducted on newborns with hyper-bilirubinemia, it has been stated that there has been a spontaneous improvement in about 50% without any interventions in the patterns where ABR has not been achieved and CM has been observed.16 In addition, the
neural damage in the auditory brain stem, which causes ABR change,decreases thesynchronization formedby the activation of the auditory neurons. Thus, the membrane potential of the auditory neurons decrease and neural function in the auditory brain stem is corrupted due to hyperbilirubinemia.13,14,19,20,25Inourstudy,duetobilirubin
injectionsbeingdonepriortotheformationofblood---brain barriersinthenewbornrats,theirneedfor maternalcare untiladulthood andtherisk of mortalitybecominghigher whenthey are takenaway from their mothers, or during anesthesia, ABR evaluations have not been done immedi-atelyaftertheinjections.Forthisreason,theacuteeffect ofbilirubintoxicityanditsfollow-upwerenotevaluatedas well.
Intheliterature,whilethedifficultyoflongterm follow-up in animal studies is underlined, it is also noteworthy thatthe resultsrelated tothisarenot mentioned. Inthe study,afterthe2nddoseinjection,wewaitedfor11days, until the rats reached adulthood. During this time, it is thoughtthatthepotentialeffectsofthehyperbilirubinemia decrease.However,althoughtheeffectof hyperbilirubine-miahasdecreased,asitcanbeseenfromthestudyaswell, duetoachievinghighintensityABRthresholdsinline with thebilirubin level, thisis thoughtto causedeteriorations inthewavemorphologyandinbeingabletoachieveANSD findingsand/orABR.
Podwalletal.26statethat,withoutanyriskfactor,they
havepublishedapatternthathasdeterminedANSDinthe left ear with normal radiological findings, and although bilirubin toxic effect wassystemic, unilateral results can alsobeobserveddependingoninvolvementlocalization.
Duringtheformationofthemodel,duetothefactthat besides therat typeused, the powderbilirubin used was the same in the formation of hyperbilirubinemia, Hansen etal.8modelhasbeenused.Duetothefactthatthesame
solutionhasbeenusedintheplacebogroupintheliterature, apre-studyrelatedtothesolution’seffectsontheauditory systemhasnotbeenconducted.However,intheevaluation offindingsrelatedtotheplacebogroup,similarresultshave beenachievedintheABRevaluationsofsomeratscompared totheratsintheexperimentgroup.
WhenitistakenintoconsiderationthattheSTBLvalues arenot high in the TcB of this group,it gives rise tothe thoughtthatthesolutionis effective ontheauditory sys-temas well. This finding, which may beaccepted asthe weakpointofthestudy,alsoprovidesveryvaluable infor-mationintermsofshowingunderstandingoftherealeffect ofhyperbilirubinemiaontheauditory system, bystudying theeffect of the solutionto beused in the formation of hyperbilirubinemiainanimalmodels.
As in the study hypothesis, the blood bilirubin levels have been allowed toincrease by injecting toxicdosages ofbilirubintonewbornrats,andithasbeenobservedthat hyperbilirubinemiamaycause SNHLand/or ANSD. Besides this,ithasalsobeenfoundnoteworthythattheeffectsof hyperbilirubinemiamayappearindifferentpatternsinABR.
Conclusion
Inourstudy,byformingratmodelfornewbornswith hyper-bilirubinemiathroughatoxicdoseofbilirubin,theeffects oflevelofbilirubinoninfantsandchildrenunderrisk,the resultsoftheelectrophysiological hearingevaluationsand morphologicaldifferencehavebeenanalyzed.
consideration that the deterioration of wave morphology inABR maybeconnectedtothedecreaseof synchroniza-tionformedbytheneuraldamageintheauditorybrainstem andtheactivationofauditoryneurons.Ithasbeenobserved thathyperbilirubinemiamaycauseANSDaswellasSNHLand CMcanbeobservedwithouttheABRresponse,andthatin systemicapplicationsnotonlybilateral butalsounilateral settlementsmayexist.
Funding
All financial support of the study was provided by the authors.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Oysu C¸, Aslan I,Ulubil A, Bas¸erer N. Incidence ofcochlear involvement in hyperbilirubiemic deafness. Ann Otol Rhinol Laryngol.2002;111:1021---5.
2.Ö˘günB, S¸erbetc¸io˘glu B,DumanN,ÖzkanH, KırkımG. Long-termoutcomeofneonatalhyperbilirubinemia:subjectiveand objective audiological measures. ClinOtolaryngol Allied Sci. 2003;28:507---13.
3.NickischA,MassingerC,Ertl-WagnerB,vonVoss H. Pedaudi-ologicfindings aftersevereneonatal hyperbilirubinemia.Eur ArchOtorhinolaryngol.2009;266:207---12.
4.AkyıldızN.KulakHastalıklarıveMikrocerrahisi,vol.1.Ankara: BilimselTıpPress;2002.p.215---332.
5.BooNY,RohaniAJ,AsmaA.Detection ofsensorineural hear-inglossusingautomatedauditorybrainstem-evokedresponse and transient-evokedotoacoustic emissionin term neonates with severe hyperbilirubinaemia. Singapore Med J. 2008;49: 209---14.
6.SharmaA,CardonG, HenionK,RolandP.Corticalmaturation andbehavioraloutcomesinchildrenwithauditoryneuropathy spectrumdisorder.IntJAudiol.2011;50:98---106.
7.Nunez-BatallaF,Carro-FernandezP,Antuna-LeonME, Gonzalez-TrellesT.Incidence ofhypoacusiasecondaryto hyperbilirubi-naemiainauniversalneonatalauditoryscreeningprogramme basedonotoaousticemissionsandevokedauditorypotentials. ActaOtorrinolaringolEsp.2008;59:108---13.
8.Hansen T, Tommarello S, Allen J. Subcellular localization of bilirubin in rat brain after in vivo i.v. administration of [3H]bilirubin.PediatrRes.2001;49:203---7.
9.MesnerO,MillerMJ,IbenSC,PrabhaKC,MayerCA,HaxhiuMA, etal.Hyperbilirubinemiadiminishesrespiratorydriveinarat pupmodel.PediatrRes.2008;64:270---4.
10.BalistreriWF,Shaw LM. Liver function. In:Tietz NW, editor. Fundamentalsofclinicalchemistry. 3rded.Philadelphia:WB Saunders;1987.
11.Madden C, Rutter M, Hilbert L, Greinwaid JH Jr, Choo DI. Clinicalandaudiologicalfeaturesinauditoryneuropathy.Arch OtolaryngolHeadNeckSurg.2002;128:1026---30.
12.RanceG,BriggsRJ.Assessmentofhearingininfantswith mod-eratetoprofoundimpairment:theMelbourneexperiencewith auditorysteady-stateevokedpotentialtesting.AnnOtolRhinol LaryngolSuppl.2002;189:22---8.
13.YeHB,ShiHB,WangJ,DingDL,YuDZ,ChenZN,etal.Bilirubin inducesauditoryneuropathyinneonatalguineapigsviaauditory nervefiberdamage.JNeurosciRes.2012;90:2201---13. 14.GaoX, YangX, Zhang B.Neuroprotection oftaurine against
bilirubin-inducedelevationofapoptosisandintracellularfree calciumioninvivo.ToxicolMechMethods.2011;21:383---7. 15.Wennberg RP, Ahlfors CE, Bhutani VK, Johnson LH, Shapiro
SM. Toward understanding kernicterus: a challenge to improvethemanagementofjaundicednewborns. Pediatrics. 2006;117:474---85.
16.VremanHJ,WongRJ,ChanML,YoungBW,StevensonDK. Tran-scutaneous bilirubinometry: a noninvasive tool for studying newborn jaundiced ratsbefore and after exposure to light. PediatrRes.2006;59:203---9.
17.Ngo RY, Tan HK, BalakrishnanA, Lim SB, Lazaroo DT. Audi-toryneuropathy/auditorydys-synchronydetectedbyuniversal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006;70:1299---306.
18.WilsonWJ,SharpKJ,HansenC,KwongP,KellyA. Especially prominentcochlearmicrophonicactivityintheauditory brain-stemresponse.IntJAudiol.2007;46:362---73.
19.ShapiroSM,NakamuraH.Bilirubinandtheauditorysystem.J Perinatol.2001;21:59---62.
20.ShapiroSM.Bilirubintoxicityinthedevelopingnervoussystem. PediatrNeurol.2003;29:410---21.
21.OverbeckGW,ChurchMW.Effectsoftoneburstfrequencyand intensityontheauditorybrainstemresponse(ABR)fromalbino andpigmentedrats.HearRes.1992;59:129---37.
22.ParhamK,SunXM,KimD.Noninvasiveassessmentofauditory functioninmice: auditorybrainstemresponseand distortion productotoacousticemissions.In:WillottJP,editor.Handbook ofmouseauditoryresearch:frombehaviortomolecularbiology. NewYork:CRCPress;2001.
23.AlvaradoJC,Fuentes-SantamaríaV,Jare˜no-FloresT,BlancoJL, JuizJM.Normalvariationsinthemorphologyofauditory brain-stemresponse(ABR)waveforms:astudyinWistarrats.Neurosci Res.2012;73:302---11.
24.ShapiroSM,TeSelleME.Cochlearmicrophonicsinthejaundiced Gunnrat.AmJOtolaryngol.1994;15:129---37.
25.JiangZD,BrosiDM,WilkinsonAR.ChangesinBAERwave ampli-tudesinrelationtototalserumbilirubinlevelintermneonates. EurJPediatr.2009;168:1243---50.