“There is grandeur in this view of life, with its several powers, having been originally breathed into a few forms or into one; and that, whilst this planet has gone cycling on according to the fixed law of gravity, from so simple a beginning endless forms most beautiful and most wonderful have been, and are being, evolved.”
Charles Darwin, 1859
I. A
CKNOWLEDGMENTSNo effort of this nature is feasible without assistance and supervision of others, directly or not related to the work itself. I feel I couldn´t have been more or better supported than I was, and that is reflected in the smile I wore along the journey herein reported.
It started with a lucky strike that made me arrive in Feijó´s lab at the IGC by the hand (a big one) of Alessandro, the myco-ruela-post-doc. For being the one that first explained me how to do science, for the endless happiness and inspiration, pure friendship and generosity, and for teaching me SO much, obrigado mané, meu irmão! Most of all, thanks for showing me how to aim the probe! Fungi will never be the same…cada um no seu quadrado!
Pollen tubes arrived in my life with the departure of Alessandro to Brazil. Monsieur Michard introduced me in a more than electrifying project, teaching me everything he could and guiding me throughout a fantastic work. These times were great…Merci pour tout mon ami Erwan!
For all the past and present Plant Dev phyto-urologists, and especially to my friends Catarina, Filipe, Eng. Doutor Moreno, Pedro, Kai, Barbie, Filipa and Herr Becker, who paciently gave me the opportunity to learn so much from them, thank you! “Assim se vê a força do DV”!
Dear JF, peer of the science Gods realm: thanks for receiving me in the lab, asking nothing in exchange except for rigorous and honest work. The pleasure of working in such environment, both physical and intellectual, goes much beyond words can convey. Thanks for all the support, for showing me ways to look at life and science, for giving me the opportunity to think and work freely, and for being a GREAT friend! You once advised me not to follow leaders. I totally agree, but as Dylan says: “You don't need a weather man to know which way the wind blows”… Obrigado Professor, até já! (can I have the same desk?)
A big thanks to my dear landlord José E. Sucena, the sexiest man alive. For the help he gave me improving in knowledge and scientific reasoning, and for being so incredibly intelligent and incisive when I most needed it.
My dear friend Kadu, the immunology master who kindly “visited the greenhouse”, advising me in all sort of experiments. Thanks for everything, and for bringing so much knowledge (poker included) and happiness to the institute and to myself.
To my life partners and circus troupe (ariops!) Sara, Aninhas, Zuca, Patraquim, Alex, Picão, Marialva, Isa, Cocas, Palha, Nhé, Jorge, Bebinca and a few more, thank you for being my friends. You are the most promising scientists and the most incredible chunk of biological splendor I know.
For all my IGC friends, and especially to Carol, Rui, Lu, Dani, Jorge Costa, you are the best! All I accomplished was possible due to the support of my family. Thank you for absolutely everything, especially for the omnipresent love, outstanding food and for nurturing my genotype in the best way you could (the phenotype is clearly not good enough, but you tried…). A special thank you to my parents and brother, for guiding me through 24 years of profound happiness.
II. G
ENERAL
A
BSTRACT
In the words of the german mycologist Anton de Bary, symbiosis is defined as the living together of dissimilar organisms. The not unusual establishment of gradual physiological and/or developmental interdependence among organisms underlies a selective force attributable to symbiotic phenomena along extended co-evolutionary time. The commonly cited example of flowering plants´major radiation events in the Cretaceous stands as an impressive case of adaptive speciation linked to mutualistic co-evolution with insects. The existence of a whole biological Kingdom of photosynthetic organisms is regarded as the evolutionary derivation of a successful adaptation of the ancestral chloroplast as an endosymbiotic entity. Understanding the basal features of symbiotic systems stands as a fundamental topic in Biology, as it encompasses mechanisms that might have played roles in the evolution of major biological themes, such as multicellularity and immunity. Plants establish widespread, both phylogenetically and geographically speaking, mutualistic symbiotic interactions with large groups of Fungi. Among other important differences, according to whether the fungal hyphae penetrate or not the plant cell wall during proximate cellular interactions these relationships are classified as endo- or ectomycorrhizas. These comprise some of the most ubiquitous symbioses on Earth, with a large impact on agriculture and in shaping ecological niches. Addressing inter-specific cell-cell communication in these systems is crucial in order to properly understand its mechanistic basis and evolutionary paths. In the first chapter we address the hypothesis of the existence of a putative role played by ion dynamics in cell signaling between symbionts, as it has been shown to be the case for well characterized plant-microbe interactions. We describe an original process of ion flux modulation in ectomycorrhizal roots, elaborating on its relevance for plant nutrition and ontogeny. In the second chapter, we theoretically address the evolution of root endosymbioses, introducing useful evolutionary developmental biology concepts, such as homology and modularity, aiming at further clarifying the emergence of these systems. The transversal relevance of ionic behaviour in the establishment of biological connections stands out as the main conclusion of the overall work. Playing a crucial role in morphogenesis, modulation of ion fluxes and bioelectricity can provide an additional layer of regulation and fine-tunning of biological processes, particularly in information flow and processing during the establishment of symbiotic crosstalks.
III. R
ESUMOG
ERALDe acordo com o micologista alemão Anton de Bary, o termo “simbiose” pode ser definido como “a vida em comum de diferentes organismos”. O vulgar estabelecimento de interdependência fisiológica e/ou ontogénica entre organismos evidencia uma força selectiva que pode ser atribuída a fenómenos simbióticos, operando durante o tempo co-evolutivo. O exemplo comunmente citado dos grandes eventos de radiação das angiospérmicas durante o Cretáceo destaca-se como um caso de especiação adaptativa estreitamente relacionado com interacções mutualísticas com insectos. A própria existência de todo um Reino biológico composto por organismos fotossintéticos é vista como tendo resutado da derivação evolutiva da adaptação do cloroplasto ancestral ao ambiente endossimbiótico. A compreensão de aspectos basais do funcionamento de sistemas simbióticos destaca-se como um tema fundamental em Biologia, uma vez que relaciona mecanismos que poderão ter actuado ao longo da evolução de grandes inovações biológicas, como a multicelularidade e a imunidade. As plantas estabelecem relações mutualísticas com um variado número de grupos de fungos, com enorme dispersão geográfica e filogenética. Entre outras diferenças importantes que as distinguem, estas podem ser classificadas como endo- ou ectomicorrizas consoante a capacidade exibida pelas hifas dos fungos penetrarem ou não a parede celular vegetal. Correspondem a algumas das mais ubíquas simbioses na Terra, com grande impacto na agricultura e na modificação de nichos ecológicos. O estudo da comunicação interespecífica célula-célula nestes sistemas é fundamental, de forma a que possamos melhor entender as suas bases mecanísticas e percursos evolutivos. Assim, no primeiro capítulo debruçamo-nos sobre a hipótese da existência de um putativo papel desempenhado pela dinâmica iónica celular em fenómenos de sinalização entre simbiontes, tal como demonstrado em exemplos bem caracterizados de interacções planta-microorganismo. Descrevemos um processo de modulação de fluxo iónico em raízes ectomicorrizadas, elaborando em torno da sua relevância para a nutrição e desenvolvimento da planta. No segundo capítulo apresenta-se uma abordagem teórica à evolução de endossimbioses radiculares, introduzindo conceitos úteis provenientes da biologia evolutiva e do desenvolvimento, tais como os de homologia e modularidade, com o objectivo de melhor clarificar a emergência destes sistemas. A relevância transversal do comportamento iónico
no estabelecimento de conecções biológicas destaca-se como conclusão central do trabalho aqui descrito. Apresentando um papel crucial em fenómenos de morfogénese, a modulação de fluxos iónicos e bio-electricidade providenciam uma camada adicional de regulação e afinação de processos biológicos, particularmente no fluxo e processamento de informação durante a formação de interações simbióticas.
T
ABLE OFC
ONTENTSACKNOWLEDGMENTS….……….…..II GENERAL ABSTRACT….….……….….III RESUMO GERAL……….….IV 1. CHAPTER 1
ION CHOREOGRAPHIES IN ECTOMYCORRYZAL ROOTS………..1
1.1 ABSTRACT………..………2
1.2 INTRODUCTION………..………..3
1.3 RESULTS………..………..4
1.3.1 ECM COLONIZATION EFFECTS ON PLANT GROWTH PARAMETERS………..….4
1.3.2 H+ FLUX AND SURFACE PH SIGNATURES FOR ECM ROOTS….………..6
1.3.3 CA2+ AND ANION FLUX PROFILES……….7
1.3.4 TIME-COURSE VARIATION OF EXTERNAL CA2+ AND ANION CONCENTRATIONS……….7
1.3.5 PHARMACOLOGICAL ASSAYS ON H+, CA2+ AND ANION FLUXES……….7
1.3.6 SPECTRAL ANALYSIS OF THE ION FLUX OSCILLATIONS………....…...10
1.3.7 A DUAL EFFECT OF THE EXTERNAL PH AND CA2+ CONCENTRATION ON EXTRACELLULAR ION FLUXES…………...12
1.4 DISCUSSION……….……..13
1.4.1 THE CONTROL OF ROOT SURFACE PH IN ECM ROOTS BY EXTRACELLULAR H+ FLUXES IS LINKED TO PM H+-ATPASE ACTIVITY……….…….….……13
1.4.2 CA2+ EFFLUX SUPPRESSION AND INCREASE UPON CA2+ UPTAKE IN ECM ROOTS……….…….………..14
1.4.3 ACTIVATION OF ANION UPTAKE BY ECM FUNGUS……….……….16
1.5CONCLUDING REMARKS……….…….…17
1.6METHODS………...18
1.6.1 BIOLOGICAL MATERIAL, INOCULUM PRODUCTION AND IN VITRO SYNTHESIS OF ECTOMYCORRHIZAS…..………..18
1.6.2 MEASUREMENTS OF H+, CA2+ AND ANION FLUXES AND CURRENTS USING THE ION-SELECTIVE VIBRATING .PROBE SYSTEM……….19
1.6.3 INHIBITION WITH VANADATE (VO43-), GADOLINIUM (GdCl3) AND 4,4′-DIISOTHIOCYANATOSTILBENE-2,2′-DISULFONIC
ACID (DIDS)………….………..20
1.6.4 ION FLUX OSCILLATION ANALYSIS………....21
1.6.5 STATISTICAL ANALYSIS……….……….21
1.7REFERENCES………..22
1.8SUPPLEMENTARY DATA……….……….28
2. CHAPTER 2 ON THE EVOLUTION AND DEVELOPMENT OF ROOT SYMBIOTIC SYSTEMS….………..30
2.1ABSTRACT………..………..31
2.2 INTRODUCTION………32
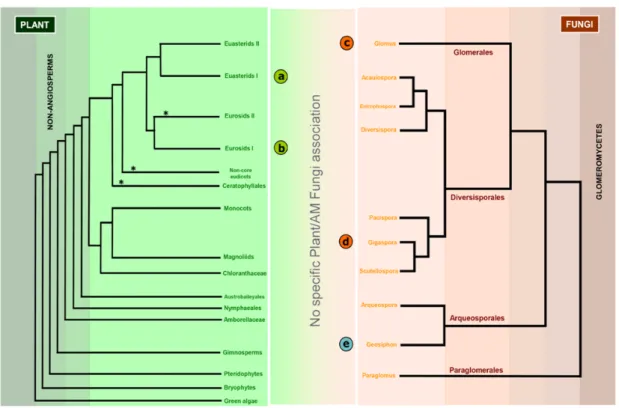
2.3 FOLLOWING THE ONTOGENY OF AM………..……....33
2.4 SETTING UP A RENDEZ-VOUS: DIFFUSIBLE SIGNALS IN PRESYMBIOSIS ………34
2.5 APPRESSORIA FORMATION………..37
2.6 PLANTS DETERMINE THE SHAPE OF HYPHAE TO COME…….……….……..38
2.7ESTABLISHING A “SYMBIOTIC SYNAPSE”………….………..…..39
2.8SIGNALING IN ROOT ENDOSYMBIOSES: COMMON GENES, DIFFERENT OUTPUTS……….41
2.8.1 NODULATION AT A GLANCE…..……….41
2.9LINKING AM TO NODULATION……….…….43
2.10HOW BACTERIA GOT EN ROUTE TO THE ROOT……..………..45
2.11CHOOSING BETWEEN STATES: CA2+ SETS THE FRONTIERS….………...46
2.12CONCLUDING REMARKS……….………..48
2.13REFERENCES……….………..50
3. C
ONCLUSIONS……….56CHAPTER I
1. I
ON CHOREOGRAPHIES IN ECTOMYCORRYZAL ROOTSWork published under the title:
A pH signaling mechanism involved in the spatial distribution of calcium and anion fluxes in ectomycorrhizal roots (2009) New Phytologist 181: 448–462
Ramos AC1, Lima PT1, Dias P1, Kasuya MCM2, Feijó JA1,3
Affiliations:
1Instituto Gulbenkian de Ciência, Centro de Biologia do Desenvolvimento, Oeiras, Portugal;
2Depto. Microbiologia, Universidade Federal de Viçosa, Viçosa-MG, Brazil;
3Depto. Biologia Vegetal, Faculdade de Ciências da Universidade de Lisboa,Portugal
Key Words: protons, anions, calcium, ectomycorrhizae, ion-selective vibrating probe, pH signaling, Pisolithus microcarpus
1.1
ABSTRACT
Mycorrhization is a typical example of a host-microorganism symbiotic interaction where the symbiont cell biology and the host immune response co-evolved several functional links. Here we address the role played by ion fluxes across the root, concerning nutrient uptake, osmoregulation, growth and signaling events. An ion-selective vibrating probe system was used to assess the net fluxes of protons (H+), calcium (Ca2+) and anions (A-) along non-mycorrhizal
and ectomycorrhizal (ECM) roots of Eucalyptus globulus colonised with Pisolithus sp. Our data showed that, from five root zones analysed, the main effect of fungal colonization was localised to the elongation zone, where strong variations in ion dynamics and rhizophere acidification capacity were observed. Additionally, ion fluxes exhibited periodic fluctuations. To verify whether these fluctuations sustained oscillations, we applied continuous wavelet time spectrum analysis and determined that H+ and A- fluxes from ECM roots show longer periods than
non-mycorrhizal roots. In contrast, Ca2+ oscillations are completely abolished following fungal
interaction. These results are interpreted in light of a working model in which nutrient uptake and stimulation of growth are mediated by ECM fungi and may be pH-dependent. Furthermore, the variations detected in ECM roots for H+ and A- fluxes suggest a main contribution from the
1.2 I
NTRODUCTION
Establishment of an effective ectomycorrhizal symbiosis encompasses a progression of complex and overlapping developmental processes in both the colonizing mycelium and the roots of host trees (Martin et al., 2007). During mycorrhizal symbiosis, host plants show enhanced growth and increased soil nutrient uptake ability, which are believed to be promoted by the fungal partner (Taylor & Peterson, 2005). The mechanisms by which this occurs are poorly understood, although a number of anatomical and physiological factors are clearly involved, namely an increase in the absorbing surface area promoted by the extraradical mycelium (Marchner & Dell, 1994; Gobert & Plassard, 2002); the synthesis and exudation of organic compounds (Ahonen-Jonnarth et al., 2000; van Scholl et al., 2006) and exoenzymes (Pasqualini et al., 1992; Courty et al., 2006) to the soil in order to solubilize nutrients; and the regulation of host root proteins involved in the nutrient transport across the plasma membrane (PM) (Lei & Dexheimer, 1988; Javelle et al., 2003; Muller et al., 2007).
Changing ion fluxes across the root plasma membrane implies alterations of transmembrane electrical potential, contributed by the electrogenic proton (H+) pumps, which
in turn controls ion transport systems (Tazawa, 2003). High H+- ATPase activities were found in
the PM of external hyphae and sheaths of ectomycorrhizal (ECM) fungi (Lei & Dexheimer, 1988). This enzyme was also found to be stimulated by external anion concentrations (Churchill & Sze, 1984; Ullrich & Novacky, 1990) and inhibited by Ca2+ (Lino et al., 1998). In
this context, an induction in the uptake has been demonstrated for Pinus pinaster ECM roots (Gobert & Plassard, 2002; Plassard et al., 2002; Boukcim & Plassard, 2003; Hawkins et al., 2008). This supports the notion that H+ transport, PM H+-ATPase activity and root surface
acidification work together in order to promote uptake (Ullrich & Novacky, 1990; Glass et al., 1992; Forde, 2000). Positive effects on ion uptake during mycorrhizal symbiosis have been described for nitrogen, phosphate and some tracer elements such as copper and zinc (Marchner & Dell, 1994), though previous results for calcium have been limited and difficult to interpret (Bucking et al., 2002). For example, in the root cortex almost 100% of the cell wall calcium content can be easily exchanged for an external 44Ca label (Peterson & Enstone, 1996;
Kuhn et al., 2000). Similarly, studies of nutrient mobilization in ECM symbiosis have been performed by radioisotope coupling with laser microprobe mass analysis (LAMMA),
energy-& Enstone, 1996; Bucking energy-& Heyser, 2000; Bucking et al., 2007). However, very few detailed studies aiming to determine the regulation of ion dynamics in the ECM symbiosis have been carried out.
There is a profound effect of pH in several biological processes, including nutrient uptake, cell growth and plant–microbe interactions (Feijó et al., 1999; Felle, 2001; Michard et al., 2008). Recently, we showed that extracellular H+ fluxes are involved in both presymbiotic
and symbiotic development of arbuscular mycorrhizal symbiosis (Ramos et al., 2008a,b). By contrast, the possible impact of pH changes was not yet established for ECM associations. Proton fluxes presumably generated by the PM H+-ATPase activity can modify the root surface
pH in ways that may trigger, for instance, modifications in the availability of free extracellular Ca2+ or anion transport. As a first step to test the role of ion fluxes in ECM associations, we
performed a systematic analysis of the different root ion fluxes in the presence and absence of fungal colonization. We measured these fluxes by means of ion-specific vibrating probes. Major alterations were observed in the growing zone of the root, and are compatible with the notion that pH modulates nutrient uptake. Furthermore, the major alterations detected in ECM roots for H+ and A– seem to be associated with root-specific fluxes, while the results for Ca2+ suggest a
significant contribution of the fungus in the overall ion choreography.
1.3 R
ESULTS
1.3.1 ECM
COLONIZATION EFFECTS ON PLANT GROWTH PARAMETERSFor ion flux analysis purposes (Fig. 1b), formation of ectomycorrhizas was performed under in vitro conditions (Fig. 1a) in order to produce E. globulus with a high degree of colonization by P. microcarpus isolate 90A (Fig. 1c,d). During the experiments, plants presented 78.3% of ECM root colonization (Table 1; Fig. 1d). In addition, significant and positive effects of ECM colonization were found both on plant height and on shoot and root fresh weights (P < 0.05; Table 2). No changes in the number of root tips, at the time of the analysis, were detected. A significant decrease in the length of root hairs was found in ECM roots (Table 1). Plant growth was strongly correlated with ionic fluxes as significant Pearson’s correlation coefficients were found between H+ fluxes and plant growth parameters (0.78; P < 0.008), root surface pH
(−0.82; P < 0.0001) and anion fluxes (−0.59; P < 0.002). Moreover, we also found significant correlation coefficients between root surface pH and plant growth parameters (−0.72; P < 0.0102).
Significantly different by Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001). For root tips, P = 0.051. nd, not determined.
PARAMETER ANALYZED CONTROL ECM
Fungal colonization (%) nd 78.3
Plant height (cm) 14.38 17.61*
Shoot fresh weight (mg/plant) 33.94 45.33** Root fresh weight (mg/plant) 12.2 15.75* Root hair lenght (µm) 386.52 152.39**
Root tips (nº) 12 17
Figure 1| (a) Ectomycorrhiza formation under in vitro germination conditions of Eucalyptus globulus seedlings and Pisolithus microcarpus before transplanting to hydroponic settings. The arrows show inoculum discs containing MNM medium and fungal mycelium. Bar, 9 mm. (b) Representation of a root apex during measurements with an ion-selective vibratingprobe. Bar, 170 µm. (c) Representation of alateral root (arrowhead) of E. globulus around P. microcarpus mycelium (arrow) under our experimental conditions. Bar, 450 µm. (d) Cross-section of E. globulus roots colonized by Pisolithus microcarpus. The arrow indicates the fungal colonization. Bar, 50 µm.
Table 1| Average values of fungal and plant growth parameters analyzed in nonmycorrhizal (control) or mycorrhizal roots of Eucalyptus globulus colonized by Pisolithus microcarpus (ECM), 10 d after transplanting to hydroponic conditions (n = 35)
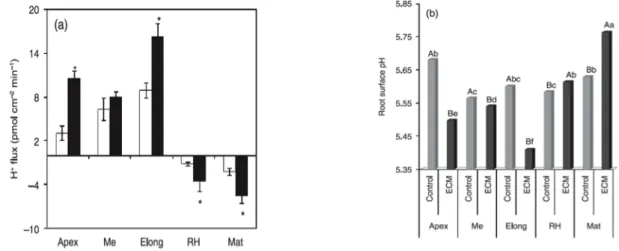
1.3.2 H
+ FLUX AND SURFACE PH
SIGNATURES FORECM
ROOTSA differential pattern of H+ fluxes was observed along the zones of eucalyptus roots (Fig. 2a). In
both nonmycorrhizal and ECM roots, the apex, meristematic and elongation zones were characterized as domains of significant H+ efflux. By contrast, root hair and mature zones were
characterized as domains of H+ influx (Fig. 2a). A sixfold stimulation on H+ effluxes was
observed at the elongation zone in the presence of colonizing P. microcarpus (P < 0.001). As expected, surface pH values along the root system showed a pattern consistent with the flux profile, and equally affected ECM colonization (Fig. 2b). The two domains described for H+
fluxes along the roots corresponded to patches of variable acidity, ranging from 5.56 in the meristematic region to 5.68 in the apex. In ECM roots, significant acidification was observed in the apex, meristematic and, most notably, elongation regions. The lowest pH value (< 5.4) was observed in the elongation zone. In root hairs and mature zones, pH values were found to be 5.6 and 5.8, respectively. These results support an ECM-driven increase in overall H+ influx. All
regions showed significant differences in the surface pH after the establishment of ECM. The global extracellular pH gradient increased by approx. 0.12 pH units in the control (Me vs Mat) to 0.4 pH units after ECM (Elong vs Mat) (Fig. 2b).
Figure 2| Proton fluxes (a) and root surface pH (b) along nonmycorrhizal (control, open bars) and ectomycorrhizal (ECM) roots of Eucalyptus globulus colonized by Pisolithus microcarpus (ECM, closed bars). Apex, meristematic (Me), elongation (Elong), root hairs (RH) and mature (Mat) indicate the zones analyzed. Bars represent the mean values + SE of five independent experiments (*statistical difference at P < 0.01). Negative values correspond to ion influx and positive values to effluxes. For H+ fluxes and surface pH, by two-way ANOVA
combined with Duncan’s test, the results showed that there was significant interaction between fungal treatment and root zones (P < 0.0001). For H+ fluxes, we found no statistically significant difference with fungal inoculation
at the meristematic zone. For pH data interpretation, bars followed by the same capital letter, in the same root region, are not significantly different by Duncan’s test at P < 0.05. Bars followed by the same lower-case letter, in different root regions, are not significantly different at P < 0.05 (n = 5).
1.3.3 C
A2+ AND ANION FLUX PROFILESInterestingly, the patterns of the Ca2+ and anion fluxes in control and ECM roots revealed a
quite different scenario. In all zones analyzed, the inoculation of eucalyptus plants induced an inhibition of the magnitude of Ca2+ fluxes (Fig. 3a). Furthermore, an inversion of flux direction
(efflux to influx) was observed in the elongation zone. On the other hand, a significant increase of anion influx was observed primarily at the elongation zone (P < 0.001) and, to a lesser extent, at the root hair zone (P < 0.01, Fig. 3b). The results also showed a significant inhibition of the anion influx at the meristematic zone (P < 0.01), while no significant changes were observed at the apex and mature zones (Fig. 3b).
1.3.4 T
IME-
COURSE VARIATION OF EXTERNALC
A2+ AND ANION CONCENTRATIONSAnalysis of the time-course variations in Ca2+ and anion concentrations in the medium with
nonmycorrhizal (control) and ECM roots after a 5 min exposure to the nutrient medium is presented in Fig. 3(c) and (d). These results indicate that ECM roots were more efficient than the control in taking up Ca2+ ions from the external medium (Fig. 3c). By contrast, control roots
seem to take up anions less efficiently than ECM (control change is nonsignificant) (Fig. 3d). This correlates well with the root surface pH values, since ECM roots showed a superior capacity to acidify the medium compared with the control (Fig. 2b).
1.3.5 P
HARMACOLOGICAL ASSAYS ONH
+, C
A2+ AND ANION FLUXESHighly significant changes in the ion fluxes were observed in the root system of E. globulus in the presence of P. microcarpus ECM fungus, notably in the elongation zone (Figs 2, 3). We further investigated the various fluxes in this region by detailed temporal analysis and pharmacological inference of the putative entities involved in their generation. All fluxes showed a clear oscillatory behavior in the elongation zone, irrespective of the conditions assayed. Changes in the oscillatory components of the ion fluxes were also induced by fungal colonization, mainly in the case of H+ and anion fluxes (Fig. 4; wavelet spectral analysis in Fig.
5). The addition of 100 µm orthovanadate, a P-type PM H+-ATPase inhibitor (Bowman, 1982;
Bowman et al., 1983), strongly inhibited all effluxes at the elongation zone (Fig. 4a). Ca2+ and
anion fluxes were differentially inhibited by 100 µm gadolinium and 50 µm DIDS, respectively (Fig. 4b,c). Gadolinium (Gd3+) is a widely used inhibitor for Ca2+ channels (Yang & Sachs,
1989; Hedrich et al., 1990; Klusener et al., 1995; Caldwell et al., 1998; Antoine et al., 2000, 2001) and DIDS is a commonly used Cl− blocker (Schroeder et al., 1993; Zonia et al., 2001,
2002; Messerli et al., 2004). Vanadate treatment led to an almost complete blockage of H+
fluxes (Fig. 4a, Table 2), and the observed differences between nonmycorrhizal and ECM roots were not significant (P > 0.05), suggesting that all effluxes detected were the result of the
% INHIBITION
TREATMENT Orthovanadate Gadolinium DIDS
Nonmycorrhizal 97.72 81.65* 100.00*
Ectomycorrhizal 82.6 75.41 73.83
For H+ fluxes, orthovanadate was applied to the final concentration of 100 μM. For Ca2+ and anion fluxes, 100 μM gadolinium
and 50 μM DIDS, respectively, were applied. n = 5. *At the same column, the mean values are significantly different by Student’s t-test at P < 0.01.
Figure 3| Fluxes of calcium (a) and anions (b) along nonmycorrhizal (control, open bars) and ectomycorrhizal (ECM) roots of Eucalyptus globulus colonized by Pisolithus microcarpus (closed bars). Apex, meristematic (Me), elongation (Elong), root hairs (RH) and mature (Mat) refer to theroot zones analyzed. Negative values correspond to ion influx and positive values to effluxes. Bars represent mean values + SE of five independentexperiments. (c, d) Fluctuations on external Ca2+ (c) and anion (d) concentrations in nonmycorrhizal (control, circles) and ECM
(squares) roots.For uptake analysis, roots were exposed for 5 min to Clark solution containing 0.2 mM Ca2+ (c)
and 1.5 mM anions (d). Scale bars represent the mean values + standard error (n = 5). *Means significantly different by Student’s t-test at P < 0.001. For Ca2+ and anion fluxes, by two-wayANOVA combined with Duncan’s
test, the results showed that there were significant interactions between fungal treatment and root zones(P < 0.0001). There were no significant effects of fungal inoculation for Ca2+ fluxes at the root hair zone, and for anion
fluxes at the apex and mature zones. *, P < 0.01; **, P < 0.001.
Figure 4| A representative graphical display of the standard output showing the oscillations in ion fluxes at the elongation zone of nonmycorrhizal (control) or mycorrhizal roots of Eucalyptus globulus colonized by Pisolithus microcarpus (ECM). (a) H+ flux oscillations in the absence and presence of 100 μM orthovanadate (VO
43-). (b) Ca2+
flux oscillations in the absence and presence of 100 μM gadolinium (Gd3+). (c) Anion flux oscillations in the
absence and presence of 50 μM 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS). Negative values correspond to ion influx and the positive values to effluxes.
plasma membrane H+-ATPase activity. Considering the stronger values of H+ effluxes in ECM
roots and the presence of different H+-ATPase isoforms in the fungal hyphae, presumably with
different sensitivities to vanadate, this degree of inhibition came as a surprise. Taken literally, one possible hypothesis is that the major proportions of these fluxes are actually generated by the root epidermis. The Gd3+ inhibition of Ca2+ fluxes showed a more complex pattern than that
influx in the elongation zone, a result which is difficult to interpret, as it implies a shift in the balance of functional carriers for Ca2+, which are presumably derived from different equilibrium
conditions. In the context of ECM, Gd3+ inhibits close to 80% of the Ca2+ influx, a result
consistent with the hypothesis that the majority of Ca2+ is taken up via Gd3+--sensitive channels,
some of which could be the result of fungal Ca2+ channels. This conclusion is supported by the
observation that there is an almost total inhibition of Ca2+ channels in nonmycorrhizal roots
(Figs 4b, 5). However, it should be pointed out that inhibition of an efflux by Gd3+ is not a
straightforward interpretable result, and calls for further study. Anion influxes seem to be proportionally inhibited by DIDS in the same way in both control and ECM roots. This supports the notion that most anion fluxes are root-generated (Fig. 4c; Table 2). Furthermore, it was observed that all inhibitors performed more effectively in control conditions, supporting the hypothesis that in ECM roots there is a greater variety of ion transporters, some of which being refractory to the broadband inhibitors used.
1.3.6 S
PECTRAL ANALYSIS OF THE ION FLUX OSCILLATIONSAs illustrated in the traces presented in Fig. 4, most of the continuous time-course measurements of fluxes showed components that were suggestive of sustained periodicity. To the extent that the spectral properties of these temporal variations could enlighten aspects of their regulation, we employed continuous wavelet time–frequency spectrum coupled to Fourier analysis to further dissect these properties. In all cases analyzed, we found evidence for underlying oscillations, sometimes with one single component, and in others with more than one component (Fig. 5a,c,e). More interestingly, they all showed some degree of modification upon colonization of Eucalyptus roots with the ECM fungus P. microcarpus (Fig. 5b,d,f). Results shown in Fig. 5 reveal that, in the control H+ flux oscillations, there is one dominant
period of c. 3.1 min, which lengthens to 5.3 min in the presence of the ECM fungus (Fig. 5a,b). This broadening of the major components of the oscillations were confirmed by Fourier analysis (P < 0.05; Fig. S1a,b). In addition, no significant oscillations were found in controls without a biological sample (not shown). By contrast, Ca2+ flux oscillations seem to show an
opposing trend after ECM (Fig. 5c,d). Firstly, they seem to have two major components in the control condition: a dominant one of c. 5.3 min (P < 0.01) and a second of c. 1.5 min. However, both disappeared in the presence of the fungus (Fig. 5c,d), giving rise to a number of small periods at the borderline of the S/N ratio of the system. The Fourier analysis confirmed
these results and showed that some of the high frequencies detected by continuous wavelet time–frequency spectrum analysis were not statistically significant at P < 0.05 (Fig. S1c,d). Finally, anion fluxes showed a third and different scenario. Control roots had at least one significant oscillation period of c. 0.6 min, thus characterized as being very fast, together with
Figure 5| Continuous wavelet time spectrum analyses of the H+ (a, b), Ca2+ (c, d) and anion flux oscillations
in the elongation zone of nonmycorrhizal (a, c, e) and mycorrhizal roots (ECM) of Eucalyptus globulus
colonized by Pisolithus microcarpus (b, d, f), as presented in Fig. 4. The frequencies are represented in min–1
and the periods in min. Wavelet analysis was coupled to Fourier analysis in order to dissect the frequency components, and shows the oscillatory pattern of the ion fluxes. No significant periods of ion fluxes were found in the medium without any biological sample (see also Fig. S2).
others considered nonsignificant by Fourier analysis at P < 0.05 (Fig. 5e, S1e). In the presence of the ECM fungus, however, there was a drastic change of behavior, giving rise to a longer period of c. 3.0 min (Fig. 5e,f, S1f ). Fourier analysis revealed the same short period of 0.6 min in ECM roots as above the level of system noise, but with a much-reduced significance. These results demonstrate that the ECM colonization changes the H+ and anion flux
oscillations, by increasing their periods by approx. double and sixfold, respectively, while for Ca2+ flux the oscillations are completely disrupted in the presence of the fungus. In addition, all
ion flux oscillations were fully inhibited by the respective inhibitors such as orthovanadate, gadolinium and DIDS (data not shown).
1.3.7 A
DUAL EFFECT OF THE EXTERNAL PH
ANDC
A2+ CONCENTRATION ON EXTRACELLULAR ION FLUXESIn systems showing prominent pH and Ca2+ dependency, the homeostasis of these ions seems
to be closely interrelated. We tested whether this was also the case at the elongation zone, by growing E. globulus roots in medium with three different Ca2+ concentrations (0, 0.5, 1 mm) for
5 d, and analyzing the H+ fluxes and root surface pH after that period (Fig. 6). The results showed that an increase in Ca2+ availability provoked a significant inhibition on the H+ effluxes in
the root elongation zone (Fig. 6a). Likewise, the root surface pH increased with the Ca2+
concentration. Also, at 0.5 mm Ca2+, almost all H+ effluxes were inhibited (Fig. 6a). pH also
induced some changes on Ca2+ efflux at the elongation zone, since under acidic conditions (pH
5.3) there was a significant increase in Ca2+ efflux (Fig. 6b). By contrast, under basic
conditions, a significant inversion of the Ca2+ efflux to one of influx was observed, suggesting
the presence of a pH-sensitive Ca2+ transport at the elongation zone.
Figure 6| (a) Extracellular H+ fluxes (bars) and root surface pH values (squares) at the elongation zone of
Eucalyptus globulus roots under three calcium concentrations (CaCl2). (b) Extracellular Ca2+ fluxes at the elongation zone with three different medium pH values. In this experiment, pH 5.7 was used as the control, since
1.4 D
ISCUSSION
This study presents the novel observation that different Eucalyptus root zones experience a differential modulation in their ion fluxes by the colonization of the ECM fungus P. microcarpus. Our experimental approach was efficient to produce plants with a high degree of fungal colonization at the stage of analysis. Thus, despite the inhibition of root hair growth, positive effects of ECM fungus on plant growth were observed (Table 1). This is a new aspect of host- pathogen interaction during ECM that reveals a potentially important aspect of coevolution between the fungal cell biology and the plant immune system, and one that may open for new paradigms of cell–cell communication through ion signaling pathways.
1.4.1 T
HE CONTROL OF ROOT SURFACE PH
INECM
ROOTS BY EXTRACELLULARH
+ FLUXES IS LINKED TOPM H
+-ATP
ASE ACTIVITYIn comparison with control uninfected plants, the highest rates of H+ efflux and acidic surface
pH were located at the elongation zones of ECM roots (Fig. 2). These effluxes are dependent on the PM H+-ATPase, as they were inhibited by 100 µm orthovanadate (inhibitor of P-type
plasma membrane H+-ATPase), a result which is conceptually sound since the elongation zone
is a specialized growing zone (Winch & Pritchard, 1999). In fact, it has been shown that this zone shows notably higher immunolocalization and higher activity levels of PM H+-ATPase than
the apical and meristematic zones (Jahn et al., 1998; Palmgren, 2001; see details in Enriquez-Arredondo et al., 2005). Using immunocytochemical approaches, Lei & Dexheimer (1988) found strong PM H+-ATPase labeling in root cortical cells of Pinus sylvestris- Laccaria laccata, in
external hyphae sheaths and Hartig nets. This localization supports the concept of a coupling mechanism between fungal and host H+ pumps in ECM roots (Fig. 2, Table 2). Indeed, it has
been demonstrated that for arbuscular mycorrhizal associations, some host PM H+-ATPase
isoforms show increased activity and gene expression after fungal colonization (Ferrol et al., 2002; Ramos et al., 2005; details in Rosewarne et al., 2007). The H+ efflux mediated by the
PM H+-ATPase is important for the regulation of cytoplasmic pH (Felle, 2001; Palmgren, 2001;
acidification of the apoplast (Hager, 2003). This effect is closely related to auxin-induced cell growth as proposed by the ‘acid-growth theory’ by Rayle & Cleland (1992). This implies that enhanced H+ efflux in ECM roots (Fig. 2a) results in an acidification of the apoplastic/ external
pH (Fig. 2b). Moloney et al. (1981) demonstrated that pH changes in the apoplast are crucial for root growth, since acidic buffering conditions act as stimulators whilst neutral or basic pHs act as inhibitors. Our results clearly show that when the H+ flux rate (Fig. 2a) and surface pH
values (Fig. 2b) are combined, highly significant Pearson’s correlation coefficients are obtained (−0.82; P < 0.0001). Other candidates that contribute to the control of extracellular H+ flux in
ECM are the presence of anions in the growth medium. These are reported to act as stimulators of the PM H+-ATPase (Churchill & Sze, 1984; Ullrich & Novacky, 1990; Glass et al.,
1992; Forde, 2000; Garnett et al., 2001). This concept is especially appealing taking into account the observed oscillatory behavior (Figs 5, S1), where the ECM colonization induced changes in the flux oscillations, leading to their increased periods. For Ca2+ flux oscillation, ECM
colonization abolished all significant periods observed in control roots (Figs 5b,c, S1). Combined with the reversion from efflux to influx in the elongation zone, this result could be interpreted as showing that the fungus contributes to the majority of the Ca2+ influx through
specific channels. These different activities would produce intricate temporal patterns impossible to synchronize on an organized oscillatory pattern. This being the case, the prediction would be that ectomycorrhizal plants should have an improved efficiency of Ca2+
uptake from the soil, a result partly confirmed in Fig. 3(c).
1.4.2 C
A2+ EFFLUX SUPPRESSION AND INCREASE UPONC
A2+ UPTAKE INECM
ROOTSCalcium has a paradoxical effect on PM H+-ATPase, as it has been reported to be an inhibitor
via a Ca2+-dependent phosphorylation pathway (Lino et al., 1998; Tazawa, 2003) and an
activator in guard cells (Assmann et al., 1985). An inhibition of the PM Ca2+ influx channels in
both animal (Yang & Sachs, 1989) and plant cells (Allen & Sanders, 1994; Klusener et al., 1995; Knight et al., 1996; Antoine et al., 2000, 2001) occurs by the addition of extracellular Gd3+ in a micromolar range. Despite its use for detection of Ca2+ stretch-activated channels
(Caldwell et al., 1998), Gd3+ is likely to inhibit other cationic channels as well, because of its
relatively broad effect. Our pharmacological analysis suggested that the Ca2+ influx in the
(Figs 3a, 4; Table 2). However, as the Ca2+ effluxes are largely governed by the chemical
potential gradient of Ca2+ generated by the PM Ca2+-ATPase, we hypothesized that the
suppression of effluxes in the control roots could represent an indirect dissipation of the Ca2+
gradient, as promoted by Gd3+ treatment (Fig. 4, Table 2). In addition, similar flux inhibition
profiles were obtained by Nemchinov et al. (2008) in Nicotiana benthamiana leaves. The authors proposed a model in which Gd3+-sensitive Ca2+ influxes and Ca2+ pumps are involved in
the signal transduction pathways of the hypersensitive response mechanisms (Nemchinov et al., 2008). As a passive Ca2+ efflux from the cell cytosol is thermodynamically improbable
(Shabala & Newman, 2000), an active mechanism must be involved. Two possible mechanisms of Ca2+ efflux might occur, one through Ca2+ release from the cell wall and the
other by Ca2+ extrusion via the PM Ca2+-ATPase (Lecourieux et al., 2006; Nemchinov et al.,
2008). It remains to be determined which of these two mechanisms is responsible for this event to occur. Alternatively, an increase in the activity of Ca2+ influx in ECM roots could reflect
an increased cytosolic concentration of this ion. Indeed, it has recently been demonstrated that the exposure of E. globulus root hairs to hypaphorine (an indole alkaloid secreted by P. microcarpus) led to an elevation of cytoplasmic Ca2+ concentration (Dauphin et al., 2007).
Thus, hypaphorine led to a reduction of the Ca2+ gradient across the plasma membrane, which
was correlated with the arrested growth of root hairs (Béguiristain & Lapeyrie, 1997; Dauphin et al., 2007). These results seem similar to our own observations (Table 1), where root hair length was reduced in ECM roots. Recently, Martin et al. (2008) published the genome of the ECM fungus Laccaria bicolor, in which numerous and diverse Ca2+ channels are found to be
encoded (see details at http:// genome.jgi-psf.org/Lacbi1/Lacbi1.home.html). Accordingly, we found ECM roots to have a higher uptake capacity of Ca2+ from the external medium (Fig. 3c).
In itself this would not necessarily lead to a major accumulation of Ca2+ in ECM of whole plants,
but clearly suggests a higher potential for ion uptake and storage in the cell wall (Peterson & Enstone, 1996; Kuhn et al., 2000) promoted by the fungus. In ECM associations, such as Suilus bovinus-Pinus sylvestris, an exposure to Ca2+ also led to an accumulation of this ion in
the interfacial apoplast in between symbionts and in the fungal sheath (Bucking et al., 2002). Depending on the fungal species, Ca2+ can also accumulate as calcium oxalate in the fungal
hyphae (Malajczuk & Cromack, 1982). In the light of this, calcium dynamics in ECM interactions needs to be more carefully investigated, not just using radioisotopes, but also by
means of an integration of techniques such as ion-selective vibrating probes, patch-clamp and imaging analyses.
1.4.3 A
CTIVATION OF ANION UPTAKE BYECM
FUNGUSIt is well known that an increase in the root surface concentration of H+ generates a
proton-motive force, which is necessary to drive the secondary transport of , SO42+, Cl−, Ca2+ and K+
(Portillo, 2000; Palmgren, 2001). Accordingly, we found that the changes in H+ efflux
attributable to ECM fungal infection in the elongation zone were strictly correlated to the root surface pH values (−0.82; P < 0.0001), and, significantly, correlations of root surface concentrations of H+ were found with both Ca2+ (−0.78, P < 0.001) and anion fluxes (0.66; P <
0.006). The correlation between Ca2+ and anions at the elongation zone (0.99, P < 0.001)
raised the possibility of an activation of anion influx by Ca2+, as demonstrated in other cells
(Hedrich et al., 1990). Since plant cells have adapted to low anion concentrations, anion uptake is generally coupled to the electrochemical gradient generated by the PM H+-ATPase
activity (Evans et al., 1980; Zimmermann et al., 1994; Garnett et al., 2001). Consequently, ECM roots possess strong anion influxes and H+ effluxes primarily at the elongation zone (Fig.
3b). Consistent with this, we observed high H+-ATPase activity in this root zone. It has been
reported that this enzyme is stimulated by anions in plant (Churchill et al., 1983; Churchill & Sze, 1984; Zimmermann et al., 1994) and animal cell membranes (Vieira et al., 1995). The induction of uptake in P. pinaster ECM roots, even at low external concentrations, was previously shown by Gobert & Plassard (2002). The H+ efflux and consequent root surface
acidification are necessary for the uptake mechanism to operate (Ullrich & Novacky, 1990; Glass et al., 1992; Forde, 2000), as this occurs via PM cotransporters (nH+/NO3-)
(Crawford,1995). This was already demonstrated for Eucalyptus nitens, where large H+ effluxes
were found in medium with NO3-. However, fluxes were quantitatively linked to H+ fluxes
(Garnett & Smethurst, 1999; Garnett et al., 2001, 2003). In addition, according to Garnett et al. (2003), negative correlation coefficients can be obtained between and H+ fluxes. Nitrate is
thus a strong candidate to be a component of the anion fluxes we observed, but unfortunately the technical limitations of the electrodes used do not warrant a straightforward conclusion in this respect (see the Materials and Methods section and Fig. S2a,b), with chloride probably playing also an important role. In normal conditions, the maintenance of the electrical membrane potential depends on the H+ efflux and influxes of anions and potassium (Felle,
2001; Tazawa, 2003). In ECM symbiosis, fungi have a high capacity to uptake potassium in their external hyphae (Rygiewicz & Bledsoe, 1984). One possible molecular basis for this was recently discovered in the same type of hyphae, where Corratgé et al. (2007) cloned the HcTrk1 transporter from Hebeloma cylindrosporum, and demonstrated it to encode for a single-file pore channel that cotransports Na+/K+ into the hyphae. Pharmacological analyses
suggested the presence of anion channels at the elongation zone, since the influxes were sensitive to DIDS. In guard cells, DIDS also inhibits anion uptake (Schroeder et al., 1993; Schwartz et al., 1995) similar to what was observed in this study (Table 2). Further studies should be focused on the proper discrimination of the specific anions involved on the observed response at the elongation zone of ECM roots.
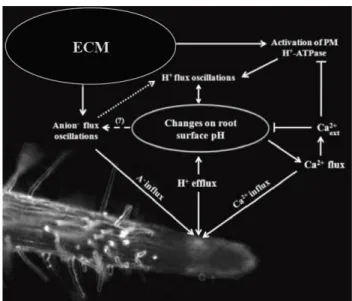
1.5 C
ONCLUDING REMARKSBased on our results, we propose a model for pH signaling in ECM roots, which is directly linked to nutrient uptake and plant growth (Table 1, Fig. 7). ECM fungi induce positive modulation of the H+ efflux rates and rhizosphere acidification, mediated by PM H+-ATPase
activities from both host and fungal partners. In turn, this stimulation triggers a pH signal that modulates Ca2+ transport and, indirectly, anion uptake (Hedrich et al., 1990). This hypothesis is
supported by our observation that external Ca2+ acts as a strong inhibitor of the H+ efflux and
root surface acidification in the elongation zone of eucalypt roots. By contrast, Ca2+ fluxes were
also affected by the medium’s pH, as has previously been reported in other plant cells (Foster, 1990). An increase in anion uptake and lower concentrations of external Ca2+ will thus occur,
which are reflected both in the promotion of plant growth and in PM H+-ATPase activity
(Zimmermann et al., 1994). The spectral analysis of the ion flux oscillations revealed itself to be an efficient parameter to compare biophysical effects of the ECM fungus in the fast oscillation components. This analysis can be used as an additional tool during the study of ion dynamics using the ion-selective vibrating probe technique, on the assumption that shifts in the main components of oscillations correspond to the activation/shift of a variety of molecular transporters.
1.6 M
ETHODS
1.6.1 BIOLOGICAL MATERIAL, INOCULUM PRODUCTION AND IN VITRO SYNTHESIS OF ECTOMYCORRHIZAS
Three agar discs containing mycelium of the ECM gasteromycete Pisolithus microcarpus isolate PT 90A were inoculated onto Petri dishes containing 20 mL of modified MNM (Marx, 1969) medium and incubated for 28 d at 28ºC. From the resulting colonies, 9 mm agar discs were cut off from the edge of actively growing colonies. Eucalyptus globulus Labill. Seeds were superficially sterilized with 5% sodium hypochlorite (v/v) for 15 min, rinsed with five changes of sterile water, and plated on modified Clark solution at quarter-strength (Clark, 1975) to which was added 2.9 µM thiamine-HCl and 1% sucrose in 0.5% (w/v) Phytagel (Sigma-Aldrich, Gillingham, UK). The use of Phytagel produced a clear and colorless medium, which is excellent for imaging and ion flux measurement with reduced electrical noise (Ramos et al.,
Figure 7| Proposed model for the pH signaling mechanism in ectomycorrhizal (ECM) roots and the differential modulation of anion (A−) and calcium (Ca2+) uptake
2008a). After 7 d, aseptically germinated seedlings were placed on the edge of 10-d-old ECM fungal mycelium grown on the same medium used for seedlings. These were left for 15 d in a controlled environment growth chamber, with 16 h of light (26ºC, 350 µmol m−2 s−1) and 8 h of
dark, for ectomycorrhiza formation. ECM plants were later transferred to hydroponic conditions in the same solution and growth chamber settings for 10 d. Subsequently, ion fluxes measurements were performed in secondary roots of intact plants. In addition, pieces of root system were washed and samples were subsequently collected for microscopic evaluation of mycorrhizal colonization, as described by Brundrett et al. (1996).
1.6.2 MEASUREMENTS OF H+, CA2+ AND ANION FLUXES AND CURRENTS USING THE ION
-SELECTIVE VIBRATING PROBE SYSTEM
A detailed description of the experimental setup of the ionselective vibrating probe technique utilized in this study has been well described (Kochian et al., 1992; Feijó et al., 1999; Shipley & Feijó, 1999; Zonia et al., 2002; Kunkel et al., 2006; Ramos et al., 2008a). In short, E. globulus plants colonized or not by ECM fungus P. microcarpus isolate PT 90A under hydroponic conditions, were placed in plastic Petri dishes (140x140 mm) filled with 30 mL of modified Clark solution at quarter strength, except for Ca2+ measurements, where 100 µM Ca2+
was used. Visual Minteq analysis was performed according to Parker et al. (1995) using the ion concentrations of the modified Clark solution applied in this study. We focused on secondary roots, as they are biologically and physiologically more significant than primary roots for nutrient supply to the plant. The volume occupied by secondary roots in the soil can reach 30–40% more than primary ones. Readings were taken in five defined root zones of nonmycorrhizal (control) and mycelium-covered roots: apex (tip), meristematic (100–150 µm); elongation (300–800 µm); root hairs (major presence of these structures); and finally mature zone (posterior to root hair zone). Ion-specific vibrating microelectrodes were produced as described by Feijó et al. (1999). Micropipettes were pulled from 1.5 mm borosilicate glass capillaries and treated with dimethyl dichlorosilane (Sigma-Aldrich). After silanization, they were backfilled with a 15–20 mm column of electrolyte (15 mM KCl and 40 mM KH2PO4, pH 6.0, for
H+; 100 mM KCl for anions; 100 mM CaCl
2 for Ca2+) and then frontloaded with a 20–25 µm
used Cl− electrodes to measure the anion fluxes given that this electrode has poor selectivity for
Cl− under our experimental conditions (Supporting information, Fig. S2a,b). Firstly the
measurement of chloride activity in the medium is slightly affected by the presence of other ions (Fig. S2a), but these changes should be expressed below noise level within the microvolt range usually measured on vibrating conditions for cellular fluxes. More importantly, the Cl−
electrode calibration with different anions showed that this electrode responds with a Nernstian slope to chloride and nitrate, and while sub-Nernstian to sulfate and phosphate, also exhibits a significant response within the concentrations used in this study (Fig. S2b). Last but not least, the background concentrations in the medium of the individual anions span various orders of magnitude, likewise affecting the signal-to-noise (S/N) ratio measurement of the fluxes in a way that is inversely proportional to the concentration. Taken together, these considerations make it almost impossible to discriminate the individual activities of every single anion, and therefore we have opted to refer to these fluxes as reflecting the global ‘anionic’ concentration rather than Cl− proper fluctuations. An Ag/AgCl wire electrode holder (World Precision Instruments,
Sarasota, FL, USA) was inserted into the back of the microelectrode and established electrical contact with the bathing solution. The ground electrode was a dry reference (DRIREF-2, World Precision Instruments) that was inserted into the sample bath. The microelectrodes were calibrated at the beginning and end of each experiment using standard solutions covering the experimental range of each ion, in order to obtain a calibration line. Both the slope and intercept of the calibration line were used to calculate the respective ion concentration from the mV values measured during the experiments.
1.6.3 INHIBITION WITH VANADATE (VO43-), GADOLINIUM (GdCl3) AND
4,4′-DIISOTHIOCYANATOSTILBENE-2,2′-DISULFONIC ACID (DIDS)
Inhibitor treatments were performed in Eucalyptus roots after determination of each ion flux at the elongation zone (n = 5). The data acquisition was stopped and the respective inhibitors (Sigma-Aldrich) were added in the Petri dishes with the following concentrations: plasma membrane H+-ATPase (100 µM orthovanadate), calcium channels (100 µM gadolinium) and
chloride channels (50 µM DIDS). Five to 10 min later, a background reference was taken and ion fluxes were again recorded. Interference caused by the inhibitors was controlled for by
direct incubation with ionophore-loaded probes. No significant interference of the inhibitors was found to occur for H+ and anions. For Ca2+, the interference was more pronounced with high
levels of gadolinium, which in the present study was used at lower concentrations (100 µM; Fig. S3).
1.6.4 ION FLUX OSCILLATION ANALYSIS
Frequency analyses were performed using AutoSignal v1.7 (Systat Software, Inc.). For each set of flux oscillations to be analyzed, a data trend removal was applied, consisting of a linear least-squares fit subtraction to remove the very low frequency trend of the data. Two distinct methods were then used to assess the frequency components of the oscillations: Fourier and Wavelet analyses. For Fourier analysis, a fast Fourier transform Radix 2 algorithm was used, ensuring that each data set was a continuous acquisition without breaks and with a constant sampling rate. Peaks were detected by a local maxima detection algorithm and considered relevant according to their significance levels (the higher the significance level, the less likely it is that a detected spectral signal will arise from random noise). Significance levels are given in the Results section. For wavelet analysis, a continuous wavelet time– frequency spectrum was obtained with a noncomplex Morlet wavelet (wave number, 12). A peak-type critical limit was used instead of the traditional confidence levels, as implemented in the software.
1.6.5 STATISTICAL ANALYSIS
All data was analyzed by one-way or two-way ANOVA in order to compare the mean values (considering ‘fungal treatment’ and ‘root region’ as factors), which were validated by convenient residual analyses and, when necessary, combined with Duncan’s test for multiple comparisons. To compare the control and fungal treatment (Table 1), we applied Student’s t-test for two independent samples and calculated confidence intervals for the mean difference, in order to guarantee a global 95% confidence level. The results are expressed as means with respective standard error, and the numbers of repetitions are given in each figure legend. All statistical analyses were conducted using the R program and the level of significance was set up at 5% (Ihaka & Gentleman, 1996).
1.7 R
EFERENCESAhonen-Jonnarth U, Van Hees PAW, Lundstrom US, Finlay RD. 2000. Organic acids produced by mycorrhizal
Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytologist 146: 557–567. Allen GJ, Sanders D. 1994. Two voltage-gated calcium release channels coreside in the vacuolar membrane of guard cells. Plant Cell 6: 685–694.
Antoine AF, Faure JE, Cordeiro S, Dumas C, Rougier M, Feijó JA. 2000. A calcium influx is triggered and propagates as a wavefront in the zygote after in vitro fertilization of flowering plants. Proceedings of the National Academy of Sciences, USA 97: 10643–10648.
Antoine AF, Faure JE, Dumas C, Feijó JA. 2001. Differential contributions of free cytosolic and extracellular fluxes of calcium to gamete fusion and egg activation in flowering plants. Nature Cell Biology 3: 1120–1124.
Assmann SM, Simoncini L, Schroeder JI. 1985. Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318: 285–287.
Béguiristain T, Lapeyrie F. 1997. Host plant stimulates hypaphorine accumulation in Pisolithus tinctorius hyphae during ectomycorrhizal infection while excreted fungal hypaphorine controls root hair development. New Phytologist 136: 525–532.
Boukcim H, Plassard C. 2003. Juvenile nitrogen uptake capacities and root architecture of two open-pollinated families of Picea abies. Effects of nitrogen source and ectomycorrhizal symbiosis. Journal of PlantPhysiology 10: 1211–1218.
Bowman BJ. 1982. Vanadate uptake in Neurospora crassa occurs via phosphate transport system II. Journal of Bacteriology 153: 286–291.
Bowman BJ, Allen KE, Slayman CW. 1983. Vanadate-resistant mutants of Neurospora crassa are deficient in a high-affinity phosphate transport system. Journal of Bacteriology 153: 292–296.
Brundrett M, Bougher NM, Dell B, Grove T, Malajczuck N. 1996. Working with mycorrhizas in forestry and agriculture. Camberra, Australia: Pirie Printers.
Bucking H, Hans R, Heyser W. 2007. The apoplast of ectomycorrhizal roots – site of nutrient uptake and nutrient exchange between the symbiotic partners. In: Sattelmacher B, Horst WJ, eds. The apoplast of higher plants: compartment of storage, transport and reactions. Dordrecht, the Netherlands: Springer-Verlag, 97–108.
Bucking H, Heyser W. 2000. Subcellular compartmentation of elements in nonmycorrhizal and mycorrhizal roots of Pinus sylvestris: an X-ray microanalytical study. II. The distribution of calcium, potassium and sodium. New Phytologist 145: 321–331.
Bucking H, Kuhn AJ, Schröder WH, Heyser W. 2002. The fungal sheath of ectomycorrhizal pine roots: an apoplastic barrier for the entry of calcium, magnesium, and potassium into the root cortex? Journal of Experimental Botany 53: 1659–1669.
Caldwell RA, Clemo HF, Baumgarten CM. 1998. Using gadolinium to identify stretch-activated channels: technical considerations. AmericanJournal of Physiology – Cell Physiology 275: 619–621.
Churchill KA, Holaway B, Sze H. 1983. Separation of two types of electrogenic H+-pumping ATPases from oat roots. Plant Physiology 73: 921–928.
Churchill KA, Sze H. 1984. Anion-sensitive, H+ pumping ATPase of oat roots: direct effects of Cl−, and a disulfonic stilbene. Plant Physiology 76: 490–497.
Clark RB. 1975. Characterization of phosphatase of intact maize roots. Journal of Agricultural and Food Chemistry
23: 458–460.
Corratgé C, Zimmermann S, Lambilliotte R, Plassard C, Marmeisse R, Thibaud JB, Lacombe B, Sentenac H. 2007. Molecular and functional characterization of a Na+-K+ transporter from the Trk family in the ectomycorrhizal fungus Hebeloma cylindrosporum. Journal of BiologicalChemistry. 282: 26057–26066.
Courty PE, Pouysegur R, Buee M, Garbaye J. 2006. Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biology & Biochemistry 38: 1219–1222.
Crawford NM. 1995. Nitrate: nutrient and signal for plant growth. Plant Cell 7: 859–868.
Dauphin A, Gérard J, Lapeyrie F, Legué V. 2007. Fungal hypaphorine reduces growth and induces cytosolic calcium increase in root hairs of Eucalyptus globulus. Protoplasma 231: 83–88.
Enriquez-Arredondo C, Sanchez-Nieto S, Rendon-Huerta E, Gonzalez-Halphen D, Gavilanes-Ruiz M, Diaz-Pontones D. 2005. The plasma membrane H+-ATPase of maize embryos localizes in regions that are critical during the onset of germination. Plant Science 169: 11–19.
Evans ML, Mulkey TJ, Vesper MJ. 1980. Auxin action on proton influx in corn roots and its correlation with growth.
Planta 148: 510–512.
Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK. 1999. Growing pollen tubes posses a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. Journal of Cell Biology 144: 483–496.
Felle HH. 2001. pH: signal and messenger in plant cells. Plant Biology 3: 577–591.
Ferrol N, Pozo MJ, Antelo M, Azcón-Aguilar C. 2002. Arbuscular mycorrhizal symbiosis regulates plasma membrane H+-ATPase gene expression in tomato plants. Journal of Experimental Botany 53: 1683–1687.
Forde BG. 2000. Nitrate transporters in plants: structure, function and regulation. Biochimica et Biophysica Acta
1465: 219–235. Foster JF. 1990. Influence of pH and plant nutrient status on ion fluxes between tomato plants and simulated acid mists. New Phytologist 116: 475–485.
Garnett TP, Shabala SN, Smethurst PJ, Newman IA. 2001. Kinetics of ammonium and nitrate uptake by eucalypt roots and associated proton fluxes measured using ion selective microelectrodes. Functional Plant Biology 30: 1165–1176.
Garnett TP, Shabala SN, Smethurst PJ, Newman IA. 2003. Simultaneous measurement of ammonium, nitrate and proton fluxes along the length of eucalypt roots. Plant and Soil 236: 55–62.
Garnett TP, Smethurst PJ. 1999. Ammonium and nitrate uptake by Eucalyptus nitens: effects of pH and temperature. Plant and Soil 214: 133–140.
Glass AD, Shaff JE, Kochian LV. 1992. Studies of the uptake of nitrate in barley. IV. Electrophysiology. Plant Physiology 99: 456–463.
Gobert A, Plassard C. 2002. Differential dependent patterns of uptake in Pinus pinaster, Rhizopogon roseolus and their ectomycorrhizal association. New Phytologist 154: 509–516.
Hager A. 2003. Role of the plasma membrane H+-ATPase in auxin induced elongation growth. Historical and new aspects. Journal of Plant Research 116: 483–505.
Hawkins BJ, Boukcim H, Plassard C. 2008. A comparison of ammonium, nitrate and proton net fluxes along seedling roots of Douglas-fir and lodgepole pine grown and measured with different inorganic nitrogen sources.
Plant, Cell & Environment 31: 278–287.
Hedrich R, Busch H, Raschke K. 1990. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO Journal 9: 3889–3892.
Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics 5: 299–314.
Jahn T, Baluska F, Michalke W, Harper JF, Volkmann D. 1998. Plasma membrane H+-ATPase in the root apex: evidence for strong expression in xylem parenchyma and asymmetric localization within cortical and epidermal cells. Physiologia Plantarum 104: 311–316.
Javelle A, Andre B, Marini AM, Chalot M. 2003. High-affinity ammonium transporters and nitrogen sensing in mycorrhizas. Trends in Microbiology 11: 53–55.
Klusener B, Boheim G, Liss H, Engelberth J, Weiler EW. 1995. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO Journal 14: 2708–2714.
Knight H, Trewavas AJ, Knight MR. 1996. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–50.
Kochian LV, Shaff JE, Ku" htreiber WM, Jaffe LF. 1992. Use of an extracellular, ion-selective, vibrating microelectrodes system for the quantification of K+, H+ and Ca2+ fluxes in maize suspension cells. Planta 188: 601–610.
Kuhn AJ, Schröder WH, Bauch J. 2000. The kinetics of calcium and magnesium entry into mycorrhizal spruce roots. Planta 210: 488–96.
Kunkel JG, Cordeiro S, Xu J, Shipley AM, Feijó JA. 2006. The use of noninvasive ion-selective microelectrode techniques for the study of plant development. In: Volkov V, ed. Plant electrophysiology – theory and methods. Berlin, Germany: Springer-Verlag, 109–137.
Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence signalling pathways. New Phytologist 171: 249–69.
Lei J, Dexheimer J. 1988. Ultrastructural localization of ATPase activity in the Pinus sylvestris/Laccaria laccata
ectomycorrhizal association. NewPhytologist 108: 329–334.
Lino B, Baizabal-Aguirre VM, González de la Vara LE. 1998. The plasma membrane H+-ATPase from beet root is inhibited by a calcium-dependent phosphorylation. Planta 204: 352–359.
Malajczuk N, Cromack K Jr. 1982. Accumulation of calcium oxalate in the mantle of ectomycorrhizal roots of
Pinus radiata and Eucalyptus marginata.New Phytologist 92: 527–531.
Marchner H, Dell B. 1994. Nutrient uptake in mycorhizal symbiosis. Plant and Soil 159: 89–102.
Martin F, Aerts A, Ahrén D, Brun A, Danchin EGJ, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V et al. 2008. The genome of Laccariabicolor provides insights into mycorrhizal symbiosis. Nature 452: 88–92.
Martin F, Kohler A, Duplessis S. 2007. Living in harmony in the wood underground: ectomycorrhizal genomics.
Current Opinion in Plant Biology 10: 204–210.
Marx DH. 1969. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic fungi and soil bacteria. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59: 153–163.
Messerli MA, Smith PJS, Lewis RC, Robinson KR. 2004. Chloride fluxes in lily pollen tubes: a critical reevaluation.
Plant Journal 40: 799–812.
Michard E, Dias P, Feijó JA. 2008. Tobacco pollen tubes as cellular models for ion dynamics: improved spatial and temporal resolution of extracellular flux and free cytosolic concentration of calcium and protons using pHluorin and YC3.1 CaMeleon. Sexual Plant Reproduction 21: 169–181.
Moloney MM, Elliott MC, Cleland RE. 1981. Acid growth effects in maize roots: evidence for a link between auxin economy and proton extrusion in the control of root growth. Planta 152: 285–291.
Muller T, Avolioa M, Olivia M, Benjdiab M, Rikirschb E, Kasarasa A, Fitza M, Chalotc M, Wipfa D. 2007. Nitrogen transport in the ectomycorrhiza association: the Hebeloma cylindrosporum-Pinus pinaster model. Phytochemistry
68: 41–51.
Nemchinov LG, Shabala L, Shabala S. 2008. Calcium efflux as a component of the hypersensitive response of
Nicotiana benthamiana to Pseudomonassyringae. Plant and Cell Physiology 49: 40–46.
Palmgren MG. 2001. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annual Review of Plant Physiology and Plant MolecularBiology 52: 817–845.
Parker DR, Chaney RL, Norvell WA. 1995. Chemical equilibrium models: applications to plant nutrition research. In: RH Loeppert, AP Schwab, S Goldberg, eds. Chemical equilibrium and reaction models. Madison, WI, USA: Soil Science Society of America, Inc, 163–200, 253–269.
Pasqualini S, Panara F, Antonielli M. 1992. Acid-phosphatase-activity in Pinus-pinea-Tuber-albidum
ectomycorrhizal association. Canadian Journalof Botany 70: 1377–1383.
Peterson CA, Enstone DE. 1996. Functions of passage cells in the endodermis and exodermis of roots.
Physiologia Plantarum 97: 592–598.
Plassard C, Guérin-Laguette A, Véry AA, Casarin V, Thibaud JB. 2002. Local measurements of nitrate and potassium fluxes along roots of maritime pine. Effects of ectomycorrhizal symbiosis. Plant, Cell &Environment 25: 75–84.
Portillo F. 2000. Regulation of plasma membrane H+-ATPase in fungi and plants. Biochimica et Biophysica Acta
1469: 31–42.
Ramos AC, Façanha AR, Feijó JA. 2008a. Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. NewPhytologist 178: 177–188.
Ramos AC, Façanha AR, Feijó JA. 2008b. Ion dynamics during the polarized growth of arbuscular mycorrhizal fungi: from presymbiosis to symbiosis. In: Varma A, ed. Mycorrhiza: structure function and biotechnology. Heidelberg, Germany: Springer-Verlag, 241–261.
Ramos AC, Martins MA, Façanha AR. 2005. ATPase and pyrophosphatase activities in corn root microsomes colonized with arbuscular mycorrhizal fungi. Brazilian Journal of Soil Science 29: 207–213.
Rayle DL, Cleland RE. 1992. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiology 99: 1271–1274.