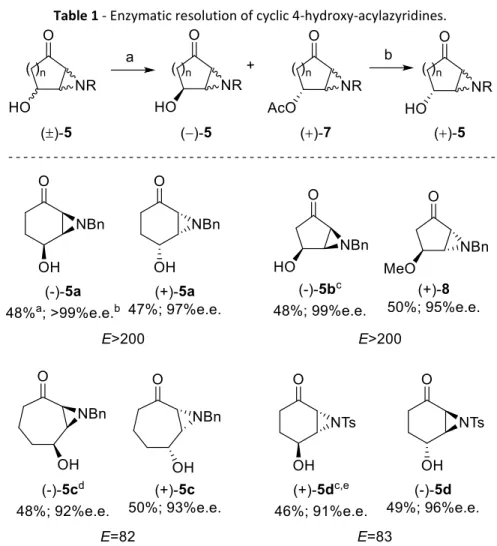
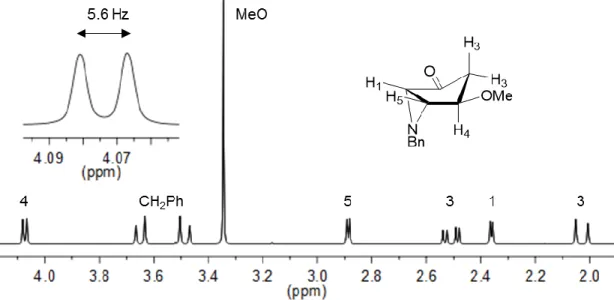
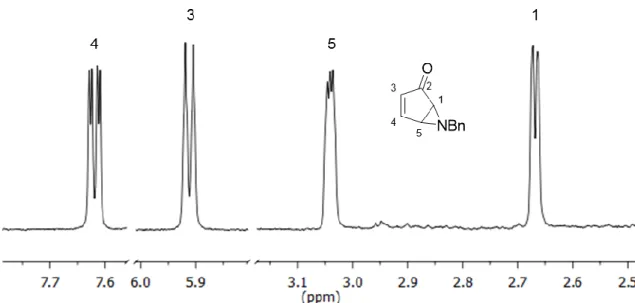
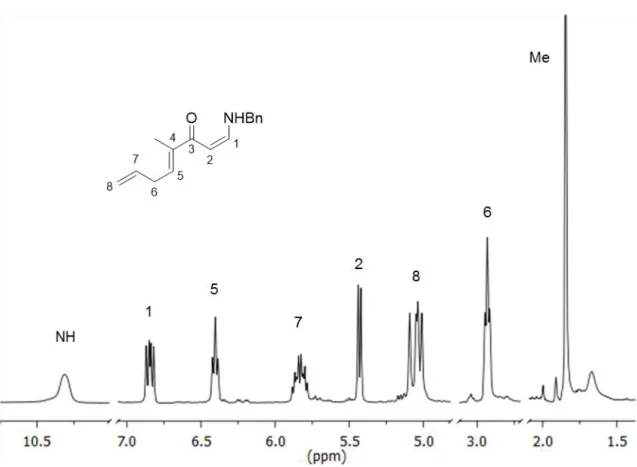
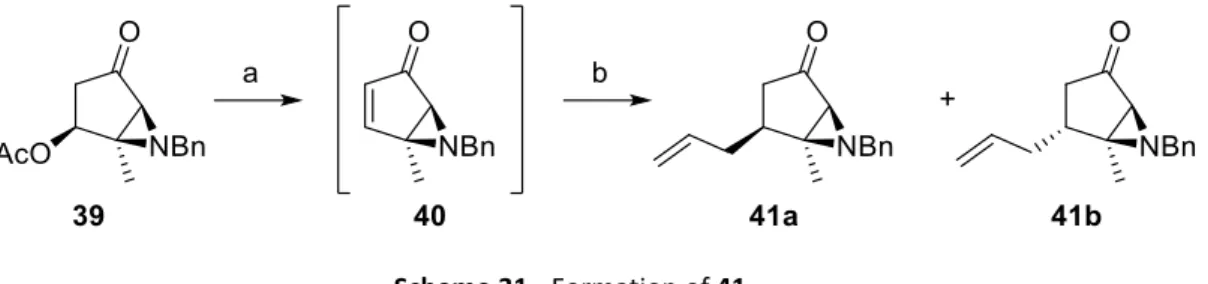
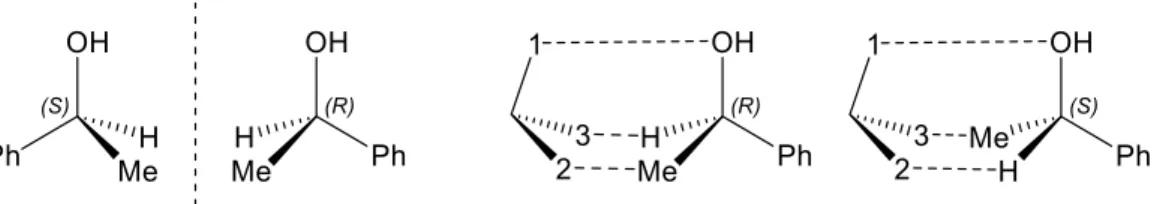
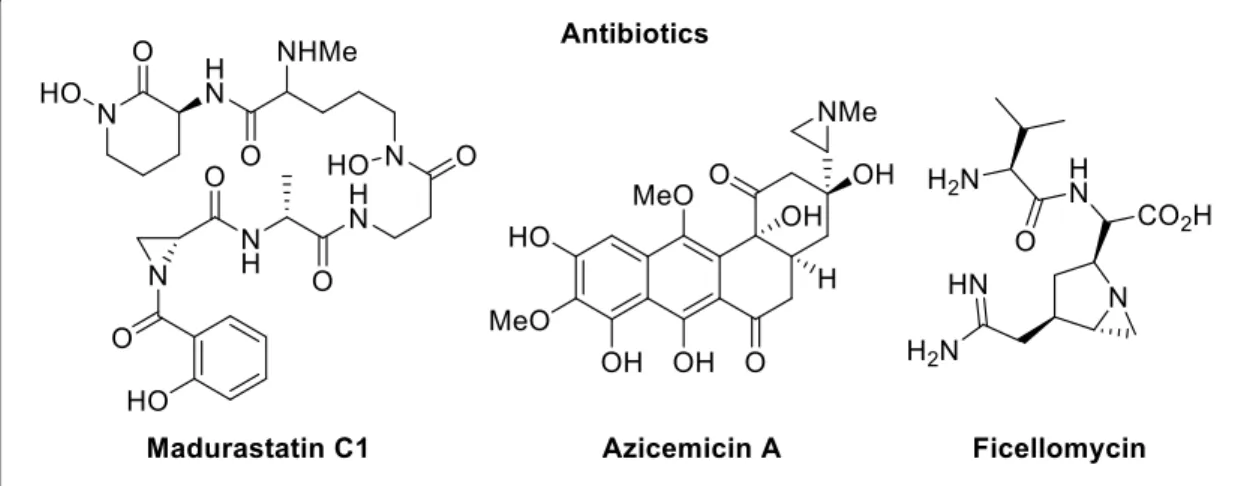
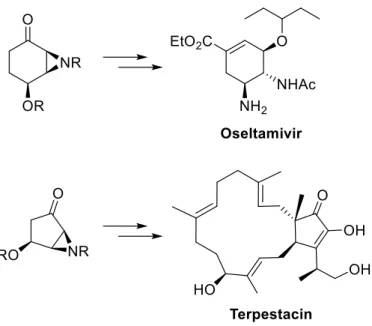
Aziridines and their asymmetric conversion to bioactive compounds
Texto
Imagem

Documentos relacionados
The organic layer was washed with water and brine, dried with MgSO 4 and concentrated in vacuo.. After stirring for 90 min at
The solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate (30 mL), washed with water (10 mL), diluted phosphoric acid solution (10 mL),
After 1 h at 0 °C, the reaction mixture was diluted with dichloromethane (30 mL) and the organic layer was washed with distilled water (10 mL), brine (10 mL), dried over anhydrous Na
Then the mixture was diluted with dichloromethane (20 mL) and the organic phase was washed with distilled water (5 mL) and brine (5 mL), dried over sodium sulfate and finally
The organic layer was separated and washed with saturated brine, and it was then dried over anhydrous magnesium sulfate.. The solvent was removed under reduced
The medium was extracted with ethyl acetate (30 mL) and the organic phase was washed with distilled water (2 × 10 mL) and dried over anhydrous Na 2 SO 4. thank FAPESP and CAPES
organic layer was separated, washed with brine, dried over magnesium sulfate and concentrated under reduced pressure. The residue was dissolved in dry methanol (1.8 mL), treated
Then, the reaction was extracted with ethyl acetate (4 × 100 mL), organic phase was dried over anhydrous MgSO 4 and the solvent was removed under reduced pressure.