The work presented in this thesis was performed at Molecular Energetics Group (FCUL) and Chemistry of Elements-f Group (CTN/IST), with financial support from the Foundation for Science and Technology, Portugal, (SFRH/BD/70201 / 2010).
To my husband, Teotónio!
"There must be no barriers for freedom of inquiry. There is no place for dogma in science. The scientist is free, and must be free to ask any question, to doubt any assertion, to seek for any evidence, to correct any errors."
This PhD thesis has been the most challenging academic task that I have faced. I would never have been able to complete my dissertation without tremendous support and precious help of the following people to whom I would like to express my deepest appreciation.
Foremost, I would like to express my sincere gratitude to my advisor Prof. Dr. Minas da Piedade for the continuous support of my PhD study and research, for his patience, motivation, enthusiasm, and immense knowledge. His supervision helped me in all the time of research and writing of this thesis (and to support my disorientation namely during the last months).
I would like to thank to the members of Molecular Energetics group at FCUL, namely (alphabetical order), Abhinav Joseph, Dr. Elsa Gonçalves, Prof. Dr. Martinho Simões, Dr. Ricardo Simões, Prof. Dr. Rui Borges, Dr. Rui Centeno. A special thanks to Dr. Carlos Bernardes for the support in all calorimetric experiments and discussion of ideas and to Dr. Filipe Agapito for theoretical calculations. At FCUL, I also thank Prof. Dr. Filomena Martins and her group members for the use of the solution calorimeter in her laboratory.
A special thanks to Dr. João Paulo Leal for having given me the opportunity to participate in this project and let me learn and experience so many techniques, namely mass spectrometry and glove-box techniques. I would like to thank to the other members of the Chemistry of Elements-f group at CTN/IST, namely (alphabetical order), Adelaide Cruz (for solvents distillation), Ana Parreira (for the friendship and the support when I felt so lonely), Ana Lucena, Dr. Bernardo Moreira, Dr. Joaquim Branco (for the opportunity to work with the glove-box, with all the dangers that I represented to that equipment), Dr. Joaquim Marçalo (for the chance to perform experiments in FT-ICR and in ESI-QIT mass spectrometers, even when my experiments cause long term stop in FT-ICR-MS), Dr. José Carretas, Dr. Leonor Maria, Dr. Teresa Gasche, Vânia Sousa (for all elemental analysis). I would like to thank to all other persons who have collaborated with this group during my visits in CTN/IST, specially Dr. Luís Ferreira (for the offer of the crucibles used in DSC experiments) and Dr. Maria Augusta Oliveira (for the great patience demonstrated when my compounds increase the water content inside the glove-box).
Prof. Dr. Hermínio Diogo, from IST, was a great support during my combustion experiments, particularly when I was almost to abandon the attempts to burn glass ampoules. In this specific case, Mr. Fernando Ferrinha was an enormous help to close those ampoules. But I would like to thank to other persons
(for the use of the densitymeter), Dr. Fátima Minas da Piedade (for the single crystal X-ray analysis), Dr. Maria João Ferreira (for 1H NMR analysis), Prof. Dr. Maria Conceição Oliveira and her student Ana Dias and Eng. Georgina Sarmento at the laboratory of analysis (for the GC-MS analysis).
I would like to thank to Prof. Dr. Luís Paulo Rebelo group (ITQB), namely Dr. José Esperança and Dr. José Nuno Canongia Lopes for providing most of the samples used in the mass spectrometry experiments.
My research would not have been possible without their help.
I also thank the Centre of Chemistry and Biochemistry (FCUL) and CTN/IST the opportunity to develop this research work and to Foundation for Science and Technology (FCT) for the financial support (SFRH/BD/70201/2010).
I extend my thanks to other persons who supported my life during this travel. I would like to thank my parents, my mother-in-law and sisters-in-law. They were always supporting me and encouraging me with their best food, accommodation and the cover design (thanks Gina). I thank my new “Lisbon family”: Dona São was like a grandmother and her grandsons, Francisco, Tomás e Vera, were like cousins. Thank you to Mariana, for the silly talks and good advices. Dr. Maria João Sottomayor was a great help to prepare the final discussion: I´m grateful for your opinion. Graça was amazing to correct my acknowledgments.
Finally, Teotónio, thank you very, very, very much. You are the only person who shared the same feelings with me, specially saudades, loneliness, sadness, lost, and some victories… Without you, I would never have been able to support all the sacrifices that I made!
O presente trabalho esteve focado em dois assuntos principais: (i) o estudo da energética de diferentes líquidos iónicos próticos (PILs); (ii) o estabelecimento de duas escalas quantitativas de afinidade em fase gasosa para catiões e aniões típicos de líquidos iónicos apróticos (AILs).
Os estudos termoquímicos dos PILs formados pelos catiões 1-alquilimidazólio (1-metilimidazólio, 1-etilimidazólio, 1-butilimidazólio e 1-octilimidazólio) ou 1,1,3,3-tetrametilguanidínio e pelos aniões etanoato, nitrato, cloreto ou perclorato foram realizados com base na combinação de diferentes técnicas calorimétricas, nomeadamente calorimetria Calvet, solução-reacção, combustão e DSC. Este estudo está distribuído por três capítulos.
No Capítulo 3, apresenta-se a metodologia usada para determinar a entalpia de formação em fase líquida do etanoato de 1-metilimidazólio,
o fHm
∆ {[Hmim][O2CCH3],l}=−(425.7±1.2) kJ∙mol‒1, com base em experiências de calorimetria de solução. Este resultado combinado com a entalpia de formação em fase gasosa da base 1-metilimidazole proposto neste trabalho, o
fHm
∆ (mim,g) = 126.5±1.1 kJ∙mol‒1, e com a entalpia de formação do ácido etanóico em fase gasosa, publicado na literatura, permitiu calcular a entalpia do processo de vaporização, [Hmim][O2CCH3] (l)
→ mim (g) + CH3COOH (g), o vapHm
∆ {[Hmim][O2CCH3]} = 119.4±3.0 kJ∙mol‒1. Este valor
está de acordo com o valor directamente obtido por calorimetria Calvet, o vapHm
∆ {[Hmim][O2CCH3]} = 117.3±0.5 kJ∙mol
‒1
, sugerindo, assim, que a vaporização de [Hmim][O2CCH3] envolve, essencialmente, uma transferência protónica, formando-se 1-metilimidazole e ácido etanóico. Este PIL foi escolhido para validar a metodologia usada, porque, embora seja um PIL de baixa ionicidade, o seu mecanismo de vaporização foi estudado por diferentes métodos e em diferentes gamas de pressão.
(polimorfo I) do nitrato de 1,1,3,3-tetrametilguanidínio,
o fHm
∆ {[Htmg][NO3],cr}=‒(311.8±2.3) kJ∙mol‒1 e a entalpia de fusão, o
fusHm
∆ {[Htmg][NO3]}=16.2±3.8 kJ∙mol‒1, com base em experiências de calorimetria de combustão e de DSC. A partir destes resultados, calculou-se
o fHm
∆ {[Htmg][NO3],l}=‒(295.6±4.4) kJ∙mol‒1. Foi ainda possível determinar os valores de entalpia de formação e de vaporização da base correspondente 1,1,3,3-tetrametilguanidina, o
fHm
∆ (tmg, l) = 7.7±2.8 kJ∙mol‒1 e o vapHm
∆ (tmg) = 50.0±1.2 kJ∙mol‒1, através de calorimetria de solução-reacção e de calorimetria Calvet, respectivamente. A partir da combinação destes valores, calculou-se o
fHm
∆ (tmg, g) = 57.7±3.0 kJ∙mol‒1, que está de acordo com o obtido por cálculos ab initio, o
fHm
∆ (tmg, g) = 58.4±4.0 kJ∙mol‒1. Também se analisou a natureza da transição de fase sólido-sólido observada para [Htmg][NO3] a 221.4±1.2 K por difracção de raios-X de cristal único, indicando os resultados estruturais que a transferência protónica completa entre o HNO3 e tmg ocorre apenas acima da temperatura da transição de fase.
O Capítulo 5 apresenta a extensão do estudo termoquímico de [Hmim][O2CCH3] e [Htmg][NO3] a outros PILs do tipo [HB]A (B = metilimidazólio, etilimidazólio, 1-butilimidazólio, 1-octilimidazólio e 1,1,3,3-tetrametilguanidínio; A = nitrato, cloreto, perclorato e etanoato), e a análise das respectivas energias de coesão, com base na combinação de calorimetria de solução e de Calvet. Este estudo sistemático demonstrou que a soma da acidez em fase gasosa, GA(HA), do precursor ácido HA e da basicidade em fase gasosa, GB(B), do precursor básico B, GA(HA)+GB(B), é um melhor indicador da energia de coesão dos PILs em estado condensado do que medidas baseadas em pKas aquosos, aq
a
pK , que são normalmente utilizadas. Além disso, o estudo da energética da vaporização/sublimação de PILs sugeriu que a decomposição nos seus precursores HA e B é significativamente mais favorecida do que a formação das espécies [HB]+ e A− em fase gasosa, embora não se possa desprezar a existência de espécie não dissociada [HB]A em fase gasosa.
gasosa usando duas técnicas de espectrometria de massa: espectrometria de massa de captura por quadrupolo com ionização por electronebulização (ESI-QIT-MS) e espectrometria de massa de ressonância ciclotrónica de ião com transformada de Fourier (FT-ICR-MS). Foi possível estabelecer duas escalas quantitativas de afinidade: uma correspondente a diferentes aniões ligados ao catião 1-butil-3-metilimidazólio, [C4mim]+ e outra referente a diferentes catiões coordenados ao anião bis(trifluorometilsulfonil)imida, [NTf2]−. Foi possível corroborar conclusões obtidas para séries mais limitadas de aniões, nomeadamente [NTf2]−, [PF6]−, [BF4]− e Br−. Verificou-se que a afinidade de aniões ao catião [C4mim]
+
depende do raio termoquímico, sugerindo que esta tendência pode também reflectir a energia de coesão dos líquidos iónicos em fase condensada. Finalmente, a análise dos resultados com base nos parâmetros empíricos de Kamlet-Taft mostrou ainda que a capacidade do catião em doar protões e a capacidade do anião aceitar protões para estabelecerem uma ligação de hidrogénio é um factor importante na “força” da interacção C+⋅⋅⋅A−.
No Capítulo 7 são apresentados os valores obtidos da determinação de entalpias de vaporização, ∆vapHmo, do 1-etilimidazole, 1-butilimidazole e 1-octilimidazole por
calorimetria Calvet. A análise estatística da variação da ∆vapHmo com o número de átomos
de carbono,n, da cadeia alquílica para uma série de 1-alquilimidazoles (n = 1 – 12) sugeriu que os dados apresentavam duas relações lineares para n ≤ 4 and n > 4. Combinando estes resultados de ∆vapHmo com os valores de entalpia de formação em fase
gasosa obtidos por cálculos teóricos (W1-F12 and CCSD(T)-F12) foi possível determinar a entalpia de formação da base em fase líquida.
Palavras-chave: líquidos iónicos próticos, líquidos iónicos apróticos, termoquímica, energia de coesão, fase gasosa, escala de afinidade, interacção catião-anião.
The present work was focused on two main subjects: (i) the study of the energetics of protic ionic liquids (PILs); (ii) the determination of gas-phase affinities for anions and cations typical of aprotic ionic liquids based on mass spectrometric measurements, by electrospray ionization quadrupole ion trap mass spectrometry (ESI-QIT-MS) and Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS). The thermochemical studies of PILs based on 1-alkylimidazolium (1-methylimidazolium, 1-ethylimidazolium, 1-butylimidazolium and 1-octylimidazolium) or 1,1,3,3-tetramethylguanidinium cations, and ethanoate, nitrate, chloride or perchlorate anions, were performed by using various calorimetric techniques, namely Calvet, reaction-solution, combustion calorimetry, and differential scanning calorimetry. The obtained results led to the corresponding enthalpies of formation in the solid and liquid phases. The analysis of the results indicated that the cohesive energy of PILs increases as the strength of the acid and base precursors, measured by their gas phase acidity, GA(HA) or basicity, GB(B), becomes larger. It also suggested that GA(HA)+GB(B) is a better indicator of the cohesive energy of PILs in the condensed state than the commonly used differences in the aqueous aq
a
pK of HA and [HB]+ species aq a
pK
∆ .
Two quantitative gas phase affinity scales were established and discussed: one corresponding to the affinity of various anions for 1-butyl-3-methylimidazolium, [C4mim]+ and another to the affinity of various cations for the bis(trifluoromethylsulfonyl)imide, [NTf2]
−
, anion. The analysis of the corresponding affinities in terms of empirical parameters that represent the polarity/polarizability and hydrogen bonding ability of molecules indicates that the C+⋅⋅⋅A− interaction strength depends on the H-bond donor ability of the cation and the H-bond acceptor ability of the anion.
It was also determined the enthalpies of vaporization of ethyl, butyl, and 1-octylimidazole by Calvet-drop calorimetry. Combining these results with high-level ab
other 1-alkylimidazoles, it was possible to determine the enthalpy of formation of the corresponding base in the liquid phase.
Keywords: protic ionic liquids, aprotic ionic liquids, thermochemistry, cohesive energy, gas phase, affinity scale, cation-anion interaction.
INDEX
Acknowledgments I Resumo III Abstract VII Index IX Index of tables XVIndex of figures XIX
List of abbreviations and symbols XXIII
Chapter 1
1. Introduction 1
1.1. Ionic liquids 3
1.1.1. History of ionic liquids 5
1.1.2. Protic ionic liquids 7
1.1.3. Aprotic ionic liquids 9
1.1.4. Analysis of ionic liquids in gas phase 10
1.2. Scope of the present work 13
References 15 Chapter 2 2. Methods 21 2.1. Calorimetry 24 2.1.1. Calvet microcalorimetry 22 2.1.2. Reaction-solution calorimetry 28 2.1.3. Differential scanning calorimetry 32
2.1.4. Combustion calorimetry 35
2.1.4.1. Calculations and corrections 38
2.2. Mass spectrometry 43
2.2.1. Fourier transform ion cyclotron resonance mass spectrometry
(FT-ICR-MS) 44
2.2.1.1. Description of the FT-ICR-MS technique 46
2.2.1.2. Sequence of events 49
2.2.1.3. Description of FT-ICR mass spectrometer used 51 2.2.2. Electrospray ionization quadrupole ion trap mass spectrometry
2.2.2.1. Description of QIT mass spectrometer used 54
2.3. Karl Fischer titration 55
References 59
Chapter 3
3. A general strategy for the experimental study of the thermochemistry of protic ionic
liquids: enthalpy of formation and vaporisation of 1-methylimidazolium ethanoate 65
Abstract 67
1. Introduction 68
2. Materials and methods 72
2.1. General 72
2.2. Materials 73
2.3. Calvet-drop vaporisation microcalorimetry 74
2.4. Solution calorimetry 75
3. Results and discussion 77
3.1. Calvet-drop vaporisation microcalorimetry 77
3.2. Solution calorimetry 79
4. Conclusion 82
Acknowledgments 83
References 84
Supplementary material 90
1. Enthalpy of vaporisation of [Hmim][O2CCH3] by drop-vaporisation Calvet
microcalorimetry 90
2.Solution calorimetry 93
3. Enthalpies of formation and vaporisation of 1-methylimidazole 96
References 99
Chapter 4
4. Thermochemistry of tetramethylguanidine and
1,1,3,3-tetramethylguanidinium nitrate 101
Abstract 103
1. Introduction 104
2. Materials and methods 105
2.1. General 105
2.2. Materials 107
2.3. Density of 1,1,3,3-tetramethylguanidine 108 2.4. Determination of the crystal structure of [Htmg][NO3] 109
2.5. Differential scanning calorimetry 110 2.6. Calvet-drop microcalorimetry 112
2.7. Combustion calorimetry 113
2.8. Solution calorimetry 115
3. Results and discussion 119 3.1. Density of 1,1,3,3-tetramethylguanidine 119 3.2. DSC characterization of the (cr II) → (cr I) and fusion phase transitions of
[Htmg][NO3]
120 3.3. Determination of crystal structure of [Htmg][NO3] 121
3.4. Entalpies of formation of solid (cr I) and liquid [Htmg][NO3] 126
3.5. Entalpies of formation of liquid and gaseous tmg and of gaseous Htmg+ 128 3.6. Cohesive energy of liquid [Htmg][NO3] 132
Acknowledgments 133
References 135
Supplementary material 141
1. Powder X-ray diffraction analysis of [Htmg][NO3] 141
2. Differential scanning calorimetry analysis of [Htmg][NO3] 142
3. Combustion calorimetry 143
4. Solution calorimetry experiments 148 5. Drop-vaporisation Calvet microcalorimetry experiments on tmg 150
References 153
Chapter 5
5. Thermochemistry of protic ionic liquids: Enthalpy of formation and vaporisation of [HB]A (B = 1-methyl-, 1-ethyl, 1-butyl and 1-octylimidazolium, and 1,1,3,3-tetramethylguanidinium; A = nitrate, chloride, perchlorate, and ethanoate)
155
Abstract 157
1. Introduction 158
2. Materials and methods 160
2.1. General 160
2.2. Materials 160
2.3. Differential scanning calorimetry 164
2.4. Solution calorimetry 165
3. Results and discussion 167
3.1. DSC measurements 167
3.2. Enthalpies of formation 171
3.3. Cohesive energy of [HB]A (l) 169
Conclusions 179
Acknowledgments 180
References 181
Supplementary material 186
1. Synthesis of protic ionic liquids 186 2. Differential scanning calorimetry (DSC) experiments. 188
3. Solution calorimetry. 192
4. Estimation of GB(C4im) and GB(C8im) 202
Chapter 6
6. Gas phase affinity scales for typical ionic liquid moieties by Cook’s kinetic method 203
Abstract 205
1. Introduction 206
2. Materials and methods 213
2.1. Materials 213
2.2. Electrospray ionization quadrupole ion trap mass spectrometry (ESI-QIT-MS) 214 2.3. Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) 215
3. Results and discussion 216
4. Conclusions 225
Acknowledgments 226
References 226
Supplementary material 230
1. Estimation of the thermochemical radius of [NTf2]− 230
2. Kamlet-Taft parameters of ionic liquids 231
References 232
Chapter 7
7. Thermochemistry of 1-alkylimidazoles 233
Abstract 235
1. Introduction 236
2. Materials and methods 237
2.1. General 237
2.2. Materials 238
2.3. Calvet microcalorimetry 238
2.4. Computational details 240
3. Results and discussion 241
3.1. Standard molar enthalpies of vaporization 242 3.2. Standard molar enthalpies of formation 244
4. Conclusions 248
Acknowledgments 249
References 250
Supplementary material 254
1. 1H NMR spectra (CDCl3) 254
2. Drop-vaporization Calvet microcalorimetry 256
3. Heat capacities 257
4. Variation of the standard molar enthalpies of vaporization of amines (R-NH2)
and nitriles (R-C≡N) with the number of carbon atoms, n, in the alkyl side chain.
260
References 264
Chapter 8
8. Conclusions 265
Annex 271
Annex. Calibration constant of combustion bomb 273 A.1. Calibration constant of the combustion bomb during the experiments with
PET and [Htmg][NO3]General
273
Chapter 1
Table 1.1 Properties of ILs. 5
Chapter 3
Table 1: Enthalpies of solution, reaction, vaporisation and formation. 77
Table S1: Results of the vaporisation enthalpy measurements on 1-methylimidazolium ethanoate, by drop-vaporisation Calvet microcalorimetry (Sample 1).
91
Table S2: Results of the vaporisation enthalpy measurements on 1-methylimidazolium ethanoate, by drop-vaporisation Calvet microcalorimetry (Sample 2).
91
Table S3: Results of the vaporisation enthalpy measurements on 1-methylimidazolium ethanoate, by drop-vaporisation Calvet microcalorimetry (Sample 3).
92
Table S4: Standard molar enthalpy of solution of 1-methylimidazolium ethanoate in
water, at 298.15 K. 94
Table S5: Standard molar enthalpy of solution of 1-methylimidazole in
⋅
3 2
CH COOH nH O , at 298.15 K. 95
Table S6: Standard molar enthalpy of solution of 1-methylimidazole in water, at
298.15 K. 95
Table S7: Standard molar enthalpy of solution of CH3COOH in
1-methylimidazole·nH2O, at 298.15 K.
96
Table S8: Standard molar enthalpies of combustion and formation of liquid
1-methylimidazole, at 298.15 K. 97
Table S9: Results of the vaporisation enthalpy measurements on 1-methylimidazole
(Sample A), by drop-vaporisation Calvet microcalorimetry. 98
Table S10: Results of the vaporisation enthalpy measurements on 1-methylimidazole (Sample B), by drop-vaporisation Calvet microcalorimetry. 98
Chapter 4
Table 1: Provenance and mass fraction purity of the materials used in this work. 109
Table 2: Crystal Data and Structure Refinement Parameters for [Htmg][NO3]. 111
Table 3: Densities of tmg at different temperatures. 120
Table 4: Bond distances, bond angles and torsion angles for [Htmg][NO3] forms I
and II. 124
Table 5: Hydrogen bonding and intermolecular interactions in the two polymorphs
forms I and II of [Htmg][NO3].
127
Table 6: W1-F12 Energy and enthalpy components and auxiliary data used to
Table S1: Indexation of the X-ray powder diffraction pattern of form I
[Htmg][NO3].
141
Table S2: Temperatures and enthalpies of fusion of [Htmg][NO3] obtained in a
Perkin Elmer DSC 7 apparatus. 142
Table S3: Temperatures and enthalpies of solid-solid phase transition obtained for
[Htmg][NO3] (heating mode) with a TA Instruments 2920 MTDSC apparatus.
142
Table S4: Temperatures and enthalpies of solid-solid phase transition obtained for
[Htmg][NO3] (cooling mode) with a TA Instruments 2920 MTDSC apparatus. 143 Table S5: Results of the combustion experiments on polyethylene ampoules at T =
298.15 K (po = 0.1 MPa). 144
Table S6: Specific volume of polyethylene as a function of temperature T = 298.15
K (po = 0.1 MPa). 146
Table S7: Results for typical combustion experiments at T = 298.15 K (po = 0.1
MPa). 147
Table S8: Standard molar enthalpy of solution of [Htmg][NO3] in HNO3 0.1 mol·dm
-3, at 298.15 K. 149
Table S9: Standard molar enthalpy of solution of tmg in an HNO3 0.1 mol·dm-3
solution, at 298.15 K. 149
Table S10: Results of the vaporisation enthalpy measurements on tmg by
drop-vaporisation Calvet microcalorimetry. 150
Table S11: Cartesian coordinates (in 10-10 m) of the atoms in the tmg structure optimized at the DF-B3LYP-D3/aug-cc-pVTZ level of theory. 152
Chapter 5
Table 1: Characterization of protic ionic salts. 162
Table 2: Results obtained from DSC experiments. 168
Table 3: Results of the solution calorimetry experiments, at 298.15K. 172
Table 4: Standard molar enthalpies of formation and fusion of the protic ionic
liquids studied in this work, at 298.15 K. a 173
Table 5: Enthalpy of formation of species B(g), HA(g), [HB]+(g) and A−(g), at 298.15K; gas phase basicity of base (GB) and acidity of acid (GA); and aq
a
pK . 176
Table 6: Thermochemical data calculated for the cohesive energy of [HB]A
according to processes (2) and (3). 177
Table S1: Enthalpy of fusion of 1-methylimidazolium nitrate.. 188
Table S2: Enthalpy of fusion of 1-ethylimidazolium nitrate, during the heating mode
(TA Instruments 2920 MTDSC apparatus). 189
Table S3: Enthalpy of fusion of 1-butylimidazolium nitrate (TA Instruments 2920
Table S5: Enthalpy of fusion of 1-octylimidazolium nitrate. 190
Table S6: Enthalpy of fusion of 1-methylimidazolium chloride. 190
Table S7: Enthalpy of fusion of 1-methylimidazolium perchlorate. 191
Table S8: Enthalpy of fusion of 1,1,3,3-tetramethylguanidinium chloride. 191
Table S9: Enthalpy of fusion of 1,1,3,3-tetramethylguanidinium perchlorate. 191
Table S10: Enthalpy of fusion of 1,1,3,3-tetramethylguanidinium ethanoate. 192
Table S11: Standard molar enthalpy of solution of 1-methylimidazolium nitrate in
HNO3:566H2O, at 298.15 K.
193
Table S12: Standard molar enthalpy of solution of 1-methylimidazole in
HNO3:522H2O, at 298.15 K.
193
Table S13: Standard molar enthalpy of solution of 1-ethylimidazolium nitrate in
HNO3:563H2O, at 298.15 K.
194
Table S14: Standard molar enthalpy of solution of 1-ethylimidazole in
HNO3:563H2O, at 298.15 K.
194
Table S15: Standard molar enthalpy of solution of 1-butylimidazolium nitrate in
HNO3:563H2O, at 298.15 K.
195
Table S16: Standard molar enthalpy of solution of 1-butylimidazole in
HNO3:563H2O, at 298.15 K.
195
Table S17: Standard molar enthalpy of solution of liquid sample of
1-octylimidazolium nitrate in HNO3:563H2O, at 298.15 K.
196
Table S18: Standard molar enthalpy of solution of 1-octylimidazole in
HNO3:563H2O, at 298.15 K.
196
Table S19: Standard molar enthalpy of solution of 1-methylimidazolium chloride in
HCl:555H2O, at 298.15 K.
197
Table S20: Standard molar enthalpy of solution of 1-methylimidazole in
HCl:522H2O, at 298.15 K.
197
Table S21: Standard molar enthalpy of solution of 1-methylimidazolium perchlorate
in HClO4:551H2O, at 298.15 K.
198
Table S22: Standard molar enthalpy of solution of 1-methylimidazole in
HClO4:513H2O, at 298.15 K.
198
Table S23: Standard molar enthalpy of solution of 1,1,3,3-tetramethylguanidinium
chloride in HCl:555H2O, at 298.15 K.
199
Table S24: Standard molar enthalpy of solution of 1,1,3,3-tetramethylguanidine in
HCl:510H2O, at 298.15 K.
199
Table S25: Standard molar enthalpy of solution of 1,1,3,3-tetramethylguanidinium
perchlorate in HClO4:549H2O, at 298.15 K.
200
Table S26: Standard molar enthalpy of solution of 1,1,3,3-tetramethylguanidine in
HClO4:521H2O, at 298.15 K.
Table S28: Standard molar enthalpy of solution of 1,1,3,3-tetramethylguanidine in
CH3COOH:596H2O, at 298.15 K.
201
Chapter 6
Table 1: [NTf2]− and Br− affinity differences for selected cations. 219 Table 2: Parameters of equation (16) and regression coefficients (R2) for α, β and π*. 223
Table S1: Kamlet-Taft parameters of some of the studied ionic liquids and the
corresponding mean values used in the present work. 231
Chapter 7
Table 1: Provenance and mass fraction purity of the materials used in this work. 242
Table 2: Thermochemical data for 1-alkylimidazoles, with n = (1 to 12), at T =
298.15 K and po = 1 bar. 242
Table 3. Parameters of equation (3) and regression coefficients (R2) for different n
ranges. 244
Table 4. Comparison of the standard (po = 1 bar) molar enthalpies of formation of selected alkanes obtained using the W1-F12 procedure with the corresponding experimental data at T = 298.15 K.
246
Table 5. Standard (po = 1 bar) molar enthalpies of formation of the Cnim (n = 1 to
8) compounds in the gas phase, at T = 298.15 K, calculated by using reaction (7) and equation (8).
247
Table S1: Results of enthalpy of vaporization measurements on 1-ethylimidazole by
Calvet microcalorimetry (po = 1 bar). 256
Table S2: Results of enthalpy of vaporization measurements on 1-butylimidazole by
Calvet microcalorimetry (po = 1 bar). 256
Table S3: Results of enthalpy of vaporization measurements on 1-octylimidazole by
Calvet microcalorimetry (po = 1 bar). 257
Table S4: Standard (po = 1 bar) specific heat capacities of liquid C8im obtained by
Calvet drop microcalorimetry. 258
Table S5: Standard (po = 1 bar) molar heat capacities of gaseous C2im, C4im, and
C8im.
259
Table S6: Parameters of equation (S5) and correlation coefficients (R2) for different
compounds and n ranges. 261
Table S7: Components of the W1-F12 enthalpies. 262
Table S8: CCSD(T)-F12a/3C(FIX)/cc-pVDZ-F12//DF-B3LYP-D3/aug-cc-pVTZ
enthalpies. 263
Table A.1: Results of the combustion experiments on benzoic acid at T = 298.15 K
Chapter 1
Figure 1.1: Commonly studied cations. 3
Figure 1.2: Commonly studied anions. 4
Chapter 2
Figure 2.1: Schematic representation of the Calvet microcalorimeter used. 25
Figure 2.2: Reaction-solution calorimeter and the calorimetric vessel used.
Schematic representation of the calorimetric vessel. 28
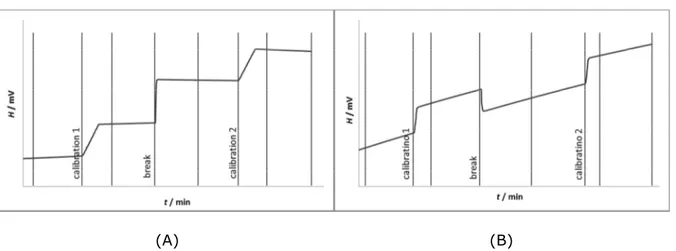
Figure 2.3: Representation of an exothermic and an endothermic solution
calorimetric experiments. 30
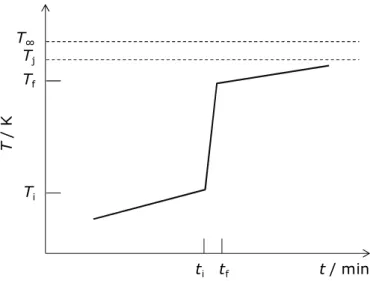
Figure 2.4: A DSC curve of an endothermic process. 33
Figure 2.5: The DSC 7 calorimeter from Perkin-Elmer used and the corresponding
calorimetric cell. 34
Figure 2.6: Combustion calorimeter used and some components. 36
Figure 2.7: Typical results from combustion calorimetry experiments. 39
Figure 2.8: Representation of the Washburn corrections of combustion calorimetric
results to standard states. 42
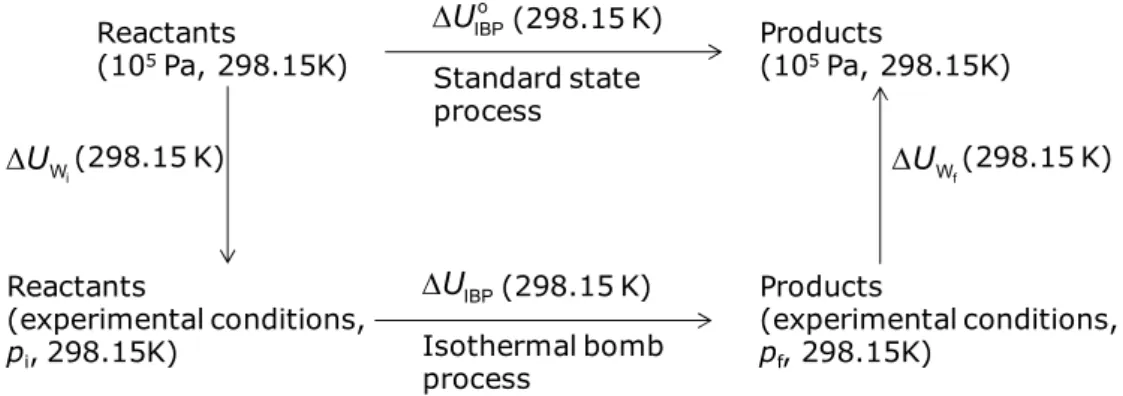
Figure 2.9: General scheme of a mass spectrometer. 43
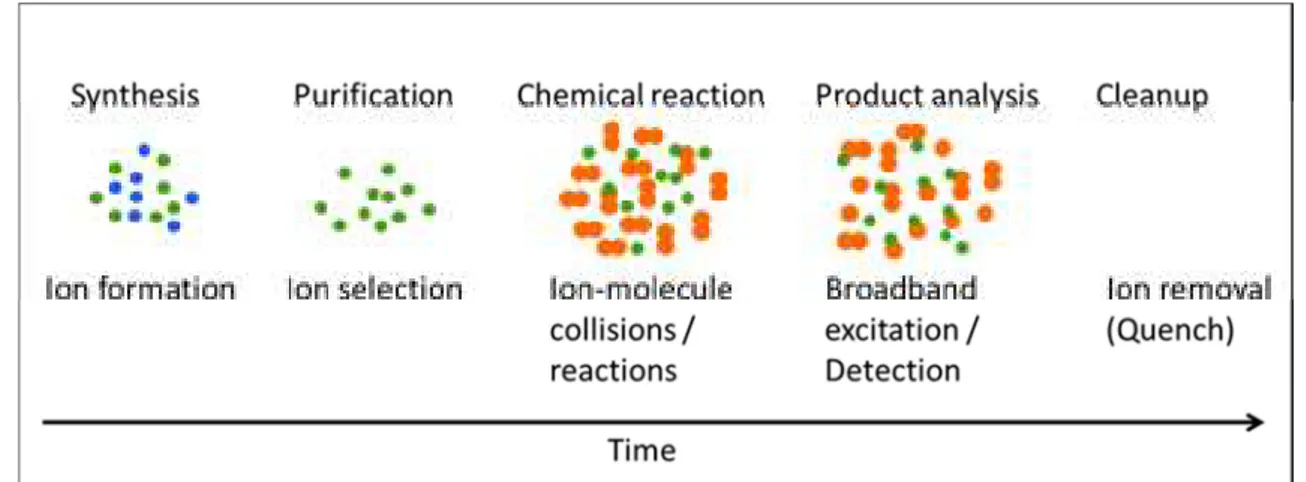
Figure 2.10: Comparison between the FT-ICR-MS event sequence and the
conventional chemical manipulation. 44
Figure 2.11: The three fundamental modes (cyclotron, magnetron and trapping) of
the ionic trajectory in an ion trap and the projection onto x-y plane. 46
Figure 2.12: Cyclotron motion in the plane perpendicular to the magnetic force
acting on a positive and negative ion. 47
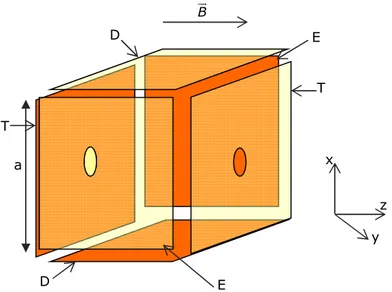
Figure 2.13: Trapped ion cell in FT-ICR-MS. 48
Figure 2.14: Fourier transform of a transient time domain signal into a frequency
domain signal of Lorentzian shape. 49
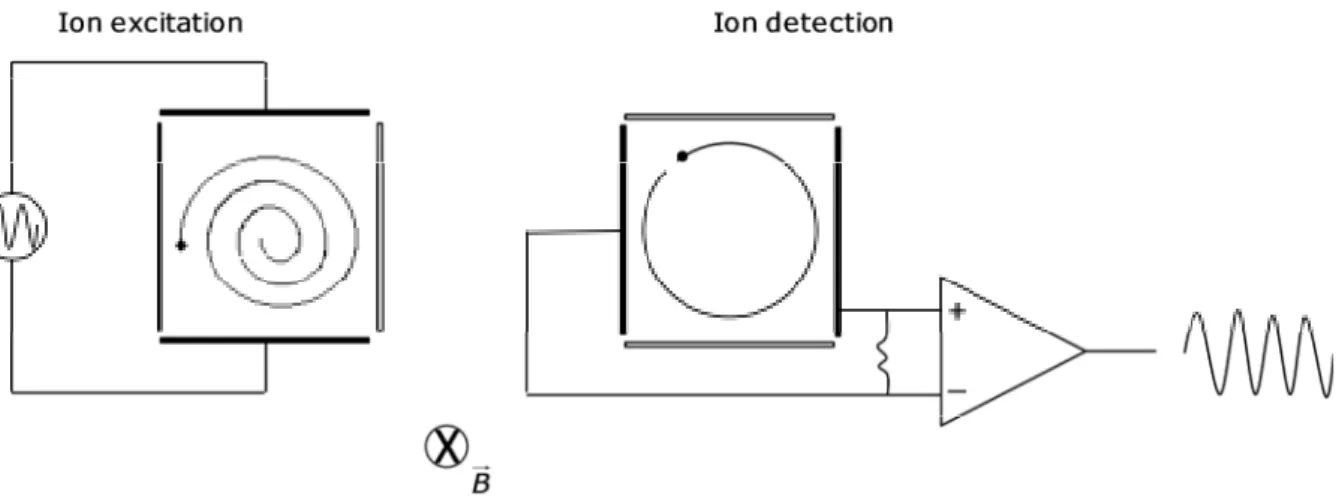
Figure 2.15: Ion cell in FT-ICR-MS. 50
Figure 2.16: FT-ICR mass spectrometer apparatus used. 52
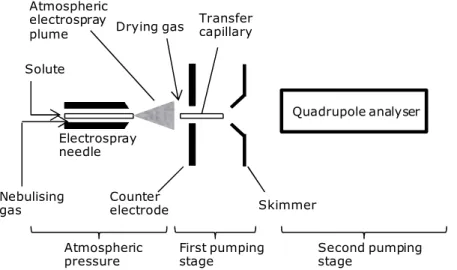
Figure 2.17: Scheme of an electrospray ion source. 53
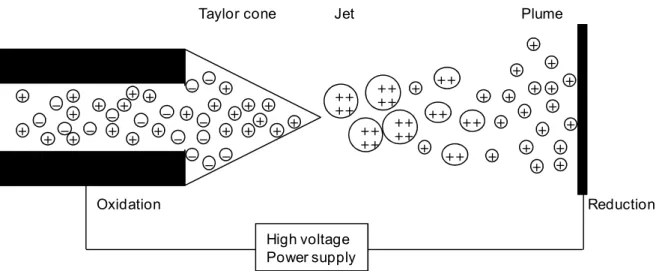
Figure 2.18: Representation of Taylor cone formation, ejection of a set and the
spray formation. 54
Figure 2.19: Representation of droplet formation. 55
Figure 2.20: Scheme of a quadrupole ion trap. 56
Chapter 4
Figure 1: Dihedral angles varied in order to locate the minima in the potential energy
surface of tmg. 117
Figure 2: Map of the tmg relaxed potential energy surface, obtained by rotating the
dihedral angles d1 and d2 in 15° increments. 118
Figure 3: Differential scanning calorimetry measured curves obtained for a
[Htmg][NO3] sample of mass m = 7.6320 mg in two consecutive cooling/heating runs
at a rate of 10 K∙min‒1.
121
Figure 4: Molecular diagrams for the two [Htmg][NO3] polymorphs with the atom
labeling schemes. 123
Figure 5: Hydrogen bonding patterns in the two [Htmg][NO3] polymorphs. 125
Figure 6: 3D packing diagrams for the two [Htmg][NO3] polymorphs. 126
Figure S1: Black and white version of figure 2. 151
Chapter 5
Figure 1: Differential scanning calorimetry measured curves obtained at a heating
rate of 10 K∙min‒1 for [HC
1im]A (A = NO3−, Cl−, ClO4−), [HC8mim][NO3], and [Htmg]A
(A = Cl−, [O
2CCH3]−, ClO4−).
167
Figure 2: Differential scanning calorimetry measured curves obtained for a
[HC2im][NO3] sample of mass 7.0380 mg in a consecutive heating/cooling runs at a
rate of 10 K∙min‒1.
169
Figure 3: Differential scanning calorimetry curves obtained for [HC4im][NO3] in a
series of consecutive cooling/heating runs at a rate of 10 K∙min‒1. 170
Figure 4: Effect of the alkyl side chain, n, of [HCnim][NO3] and Cnim on the enthalpy
of formation in the liquid state, o fHm(l)
∆ . 174
Figure 5: Dependence of cohesive energy according to process (2) and (3) with ∆pKa
and (GB+GA). 178
Chapter 6
Figure 1: [NTf2]− affinities of various cations obtained from ESI-QIT-MS and
FT-ICR-MS experiments. 217
Figure 2: [C4mim]+ affinities of various anions obtained from ESI-QIT-MS
experiments. 221
Figure 3: Variation of the [C4mim]+ affinity with the thermochemical radii of anions. 222 Figure 4: Variation of [C4mim]+ and [NTf2]‒ affinities for ILs studied in the present
work as a function of Kamlet-Taft parameters α, β and π∗. 224
Figure S1: Dependency of thermochemical radii of anions with the corresponding
Figure 1: Standard molar enthalpy of vaporization of 1-alkylimidazoles, at 298.15 K,
as a function of the number of carbon atoms in the alkyl side chain, n. 243
Figure 2: Standard molar enthalpy of formation of Cnim (n = 1 to 6) in the gas
phase, at 298.15 K, as a function of the number of carbon atoms in the alkyl side chain, n.
248
Figure S1: 1H NMR spectra of 1–ethylimidazole before and after distillation. The
arrows in the inset highlight peaks that are removed upon purification. 254
Figure S2: 1H NMR spectra of 1–butylimidazole before and after distillation. The arrows in the inset highlight peaks that are removed upon purification. 255
Figure S3: 1H NMR spectra of 1–octylimidazole before and after distillation. The arrows in the inset highlight peaks that are removed upon purification. 255
Figure S4: Standard molar enthalpies of vaporization of alkylamines and
n-alkylnitriles and n-alkylalcohols, at 298.15 K, as a function of the number of carbon atoms in the alkyl side chain.
Techniques
DSC Differential scanning calorimetry
ESI-QIT-MS Electrospray ionization quadrupole ion trap mass spectrometry
FT-ICR-MS Fourier transform ion cyclotron resonance mass spectrometry
NMR Nuclear magnetic resonance
Compounds
[allylmim]+ 1-allyl-3-methylimidazolium cation
Cnim 1-alkylimidazole
C4im 1-butylimidazole
[Hbim][NO3] 1-butylimidazolium nitrate [C4pyrid]+ 1-butylpyridinium cation
C2im 1-ethylimidazole
[Heim][NO3] 1-ethylimidazolium nitrate
C1im 1-methylimidazole
[HC1im]Cl 1-methylimidazolium chloride [HC1im][O2CCH3] 1-methylimidazolium ethanoate [HC1im][NO3] 1-methylimidazolium nitrate [HC1im][ClO4] 1-methylimidazolium perchlorate [Cnmim]+ 1-methyl-3-alkyl-imidazolium cation
[C2mim]+ 1-ethyl-3-methyl-imidazolium cation [C4mim]+ 1-butyl-3-methyl-imidazolium cation [C6mim]+ 1-hexyl-3-methyl-imidazolium cation [C1Cnpiper]+ 1-methyl-1-alkylpiperidinium cation
[C1C3piper] +
1-methyl-1-propylpiperidinium cation [C1C4piper]+ 1-butyl-1-methylpiperidinium cation [C1C3pyrol]+ 1-methyl-1-propylpyrrolidinium cation
C8im 1-octylimidazole
[HC8m][NO3] 1-octylimidazolium nitrate
tmg 1,1,3,3-tetramethylguanidine
[Htmg]Cl 1,1,3,3-tetramethylguanidinium chloride [Htmg][O2CCH3] 1,1,3,3-tetramethylguanidinium ethanoate [Htmg][NO3] 1,1,3,3-tetramethylguanidinium nitrate
[CnSO3] alkylsulfonate anion
[C1SO3]− methylsulfonate anion
[C2SO3]− ethylsulfonate anion
[C4SO3] −
butylsulfonate anion
[NTf2]− bis(trifluoromethylsulfonyl)imide anion
Cl− chloride anion
[chol]+ cholinium cation
[C2chol]+ ethylcholinium cation
[dca]− dicyanamide anion
[CH3CO2]− ethanoate anion
[PF6]− hexafluorophosphate anion
[NO3]− nitrate anion
[BF4] −
tetrafluoroborate anion
[SCN]− thiocyanate anion
[C(CN)3]− tricyanomethanide anion
[OTf]− trifluoromethanesulfonate anion
[phosp]+ trihexyl(tetradecyl)phosphonium cation
Thermodynamic and other quantities
+
C
A affinity of a species A for a cation C+
A
A− affinity of a species C for a anion A
− ad T ∆ adiabatic temperature A area ε calibration constant ρ density K equilibrium constant p
c massic heat capacity at constant pressure
C
I + or IA− intensity of the peak of the cation or anion,
respectively, in the mass spectrum
m mass
M molar mass
p pressure
k1 and k-1 rate constant for forward and reverse reaction, respectively
r
G
m∆
standard molar energy of Gibbs of reactiono fHm
∆ standard molar enthalpy of formation
o fusHm
∆ standard molar enthalpy of fusion
o rHm
∆ standard molar enthalpy of reaction
o solHm
∆ standard molar enthalpy of solution
o subHm
∆ standard molar enthalpy of sublimation
o vapHm
∆ standard molar enthalpy of vaporisation
o r
S
m∆
standard molar entropy of reactiono ,
p m
C standard molar heat capacity at constant
pressure
o , (s)
p m
C standard molar heat capacity at constant
pressure for solid state
o , (l)
p m
C standard molar heat capacity at constant
pressure for liquid state
T temperature
Tfus temperature of fusion
Ton onset temperature in DSC experiment
Tmax temperature of the maximum of the peak in DSC experiment
C
hapter
1
I
ntroduction
1.1. Ionic Liquids
1.1.1. History of ILs
1.1.2. Protic ionic liquids
1.1.3. Aprotic ionic liquids
1.1.4. Analysis of ionic liquids in gas phase
1.2. Scope of the present work
This chapter describes the scope of the present Thesis and gives an overview of the work that was carried out.
1.1.
I
onic Liquids
Ionic liquids, ILs, are a remarkable class of Coulombic fluids in which interest has burgeoned in the last decade due to the challenges and opportunities offered in terms of fundamental research and technological applications.1,2
ILs are conventionally defined as salts with a fusion temperature below the normal boiling temperature of water (373 K). This definition does not consider the nature of the ions: cations (C+) are generally organic compounds with low symmetry, such as imidazoliums or pyridiniums (Figure 1.1); anions (A−) are usually weak basic inorganic or organic compounds, for instance halides (Cl−, Br−), sulphates ([C2H5SO4]−), phosphates ([PF6]−), borates ([BF4]−) and others ([CF3SO3]−, [N(CF3SO2)2]−)3-6 (Figure 1.2). Thus, all ILs show two properties that define their nature: (i) they are liquids at or close to ambient temperature (its glass transition temperature and/or fusion temperature are lower than 373 K) and (ii) they contain ions and therefore exhibit ionic conductivity.5 Ionic liquids based on single and doubly charged species have been reported.7-11 ILs are also sometimes designated as room temperature molten salts (RTIL), low temperature molten salts, ambient temperature molten salts, ionic fluids, Coulombic fluids, and liquid organic salts.4,5
The practical difference between ionic liquids and molten salts (most common and
1,3-dialkylimidazolium N-dialkylpyrrolidinium N-alkylpyridinium
N-dialklpiperidinium tetraalkylammonium tetraalkylphosphonium
Figure 1.1: Commonly studied cations.
N N+ R3 R1 R5 R4 R2 N+ R2 R1 N+ R1 N+ R2 R1 N+ R4 R1 R3 R2 P+ R4 R1 R3 R2
Water-immiscible Water-miscible 6 PF − BF4 − CH CO3 2 − 2 NTf − a OTf−b CF CO3 2 − 1 2 3 4 BR R R R − N CN
( )
2 − NO3 − Figure 1.2: Commonly studied anions. Adapted from reference 12. a Bis(trifluoromethylsulfonyl)imide. b Trifluoromethanesulfonate.
most broadly applied term for ionic compounds in the liquid state) is that ILs may be handled as common solvents.4
Ionic liquids are normally subdivided into two major classes: aprotic (AIL) and protic (PIL). PILs contain an acidic proton on cationic species,13 while AILs do not contain acidic protons.13-16
AILs and PILs have both attracted considerable attention from industry and academia, because they have unusual properties when compared to molecular liquids, namely very large liquid temperature range, wide electrochemical window, highchemical stability, non-flammability, high heat capacity, negligible vapour pressure, high storage density and the ability to solubilise an ample variety of solutes (see Table 1.1).
In addition to ionic interactions, due to mutual electrostatic attraction or repulsion of charged particles ILs have hydrogen bonding, dipole-dipole and van der Waals interactions which are also present in conventional organic solvents. Thus ILs can dissolve both ionic and covalent materials. The presence of an alkyl chain on the cation makes them more soluble in less polar fluids.17 Therefore, they can act as catalysts and extraction solvents, promote synthesis or electrochemical reactions, work as liquid thermal storage media or heat transfer fluids. But the largest benefit is the possibility of tailor the physical and chemical properties, by changing different ions to design task-specific ionic liquids to meet different requirements.1,5,18-20
ILs are regarded as “green” alternatives to volatile organic solvents due to: (i) the low/negligible vapour pressure, limiting evaporation to the environment; (ii) the good
Table 1.1: Properties of ILs.21
Freezing temperature Preferably below 373 K Liquidus range Often > 473 K
Thermal stability Usually high
Viscosity Normally < 0.1 Pa∙s
Dielectric constant Implied < 30
Polarity Moderate
Specific conductivity Usually < 10 mS∙cm-1 Molar conductivity < 10 S∙cm2∙mol-1 Electrochemical window > 2V, even 4.5 V
Solvent and/or catalyst Excellent for many organic reactions Vapour pressure Usually negligible
solubilisation of a whole range of organic and inorganic materials; (iii) the possibility to provide a non-aqueous polar alternative for two-phase systems, because some ILs are immiscible with organic solvents; (iv) the opportunity to be recycled, increasing the performance in chemical transformations.1,19,22-25
This “green character” can, however, be suspicious because most ILs are water soluble and water sources are one of the most common paths for pollutants to enter the environment (accidental spills, effluents).26 Furthermore, many IL precursors are toxic and environmentally hazardous.27
1.1.1. History of ionic liquids
The term “ionic liquid” was first used during a Faraday Society Discussion Meeting by H. Bloom,28 albeit the object of this communication were pure molten alkali metal halide salts. Bloom said that molten salts “consist predominantly of ions. They differ, therefore, from all other classes of liquids in that they are the only group of pure liquids in which positively and negatively charged particles coexist and could therefore logically be called liquid electrolytes or ionic liquids”.28
This story started, however, in the nineteenth century, with the discovery of ethanolammonium nitrate (Tfus = 325–328 K), by S. Gabriel.29 Despite that, this
compound has received little attention, because, in respect to solvophobic behaviour, it showed a number of similarities with water.13
The first room-temperature ionic liquid, the protic ionic liquid ethylammonium nitrate formed by the neutralisation of ethylamine with concentrated nitric acid (m.p. 286–287 K), was reported by Paul Walden in 1914.30 Further research for applications of this IL were limited due to the explosive nature.24
A patent published in the 1930s described cellulose dissolution by using halide salts of nitrogen-containing bases (such as benzylpyridinium chloride, 1-ethylpyridinium chloride, etc.) above 403 K.12,31 The new obtained solution was suitable for chemical reactions, such as etherification and esterification, due to the presence of cellulose derivatives.12
Organic chloroaluminates are considered the first generation of ILs, after the mention in 1951 of the application of mixtures of aluminium(III) chloride and 1-ethylpyridinium bromide to the electrodeposition of aluminium.12 Due the fact that the selected system was chemically complicated and hence difficult to investigate, the detailed study of the chemical and physical properties of ionic liquids made from 1-butylpyridinium chloride and aluminium (III) chloride occurred only in 1975.1,12 Nevertheless, these compounds are easily hydrolysed or reduced, which requires the handling in an inert-gas atmosphere.
During this time, United States Air Force studied and patented different application of these ionic liquid as battery electrolytes4 and predicted that 1,3-dialkylimidazolium cations would be substantially more stable to reduction than the 1-alkylpyridinium cations.12 Therefore the opportunity of combining different ions to form air- and water-stable ILs became important in the 1990s, after the preparation and characterisation of a new range of air and water stable ILs based on the 1-ethyl-3-methylimidazolium cation and different anions, such as ethanoate ([CH3CO2]−), nitrate ([NO3]−) or tetrafluoroborate ([BF4]−).12 Since then, a wide range of ionic liquids has been developed containing hexafluorophosphate ([PF6]−), trifluoroethanoate ([CF3CO2]−), hydrogensulfate ([HSO4]−), alkylsulfate ([CnSO4]−), dicyanamide ([N(CN)2]−),
trifluoromethanesulfonate or triflate ([CF3SO3]− or [OTf]−), and bis(trifluoromethylsulfonyl)imide or bistriflamide ([N(CF3SO2)2]− or [NTf2]−).12
AILs have started to find diverse industrial use,12 but their benefit in certain applications, such as synthesis,31 catalysis,19 electrolytes,32 was severely restricted because they were assumed to be involatile. This view was shown to be erroneous in 2006, when it was demonstrated that a variety of ILs could be distilled at low pressure without decomposition. 33 This finding opened new perspectives in terms of exploring novel synthetic and purification routes of aprotic ILs, some of which with possible technological applications.12,34
The evolution of ionic liquids can be classified in the same way as technology. The first generation of ILs include the compounds with unique tunable physical properties, namely fusion temperature, density, viscosity, thermal stability, hydrophobicity. The ILs with targeted chemical properties, for instance chemical reactivity, high energy density, electrochemical window, coordination or solvation, combined with chosen physical properties corresponds to the second generation. The third and last generation, at this moment, consist of ILs with targeted biological properties, for example antibacterial, antifungal, antibiotic, local anaesthetic or emollient, combined with chosen physical and chemical properties.35 However, the number and types of ionic liquids are continually growing and their unique tunable properties present potentially limitless applications in a growing variety of disciplines.
1.1.2. Protic ionic liquids
Protic ionic liquids (PILs), based on a variety of proton transfer and association equilibria, are formed by proton transfer from a Brönsted acid, HA, to a Brönsted base, B, to yield, strictly speaking, a [HB]+[A]− species,13
If the equilibrium is less strongly shifted to the right, there will be some amount of neutral molecular species (HA Brönsted acid and B Brönsted base) in the ionic liquid which could be non-negligible. If this amount is considerable, due to the shifted of equilibrium to the left-hand side, the salt could no longer exist, which invalidates the designation of ionic liquid. Therefore, the preparation and utilisation of such PILs requires the knowledge of the position of this equilibrium in order to define the concentration of neutral species, to determine a “degree of dissociation” and “degree of proton transfer”, similar to the neutralization process that exist between HA and B in aqueous solution.3 PILs are conventionally considered pure if the neutral species lies below 1%, otherwise these PILs are probable best regarded as mixtures of ion in a molecular neutral media.3,36 The properties of the compound (transport properties, fusion temperature, etc.) are very dependent of the level of impurities.3
This equilibrium also explains the differences between PILs and AILs, namely the presence in PILs of acidic or labile protons that can form an hydrogen-bonding network13,36 and the non-negligible, measurable vapour pressures of many PILs at room temperature.33,37,38
The proton transfer can be improved by strong acids and/or strong bases. Although aq
a
pK is an indication of the strength of proton transfer from an acid to a base, it refers to aqueous solution,37,39 which may not be appropriate for PILs.13
Currently the lack of a method for determining the ionicity of PILs contributes for the discussion of the classification of this type of compounds as proper ionic liquids. At present, the “Walden rule” is used to assess the ionicity of ionic liquids. This relates the ionic mobility (represented by conductivity) to the fluidity of the medium through which the ions move.13,37 Ideally, e.g. in the absence of any ion-ion interactions, the slope of the graph should be unity and its position is established using solutions of aqueous KCl at high dilution.37 However, Walden plot is unable to distinguish between low ionicity in an IL, originated from ion pairing or from a low degree of proton transfer.40 Other techniques, like NMR,41,42 IR spectroscopy,42,43 and electrochemical measurements,44 were already used as probe the ionicity of PILs.
Albeit the low conductivity and stability, these liquids present low cost, simple preparation, synthesis and purification methods, and due to the exchangeable proton, PILs exhibit Brönsted acidity, which allows their use as solvents for acid catalysed reactions including Diels–Alder, Mannich and esterification reactions.40
1.1.3. Aprotic ionic liquids
As previously mentioned, aprotic ionic liquids (AILs) contain a different substituent (typically an alkyl group) at the site occupied by the labile proton in an analogous protic ionic liquid (PIL).13-16 They normally require synthetic strategies different from the simple acid-base reactions used to obtain most PILs.13
The large size and conformational flexibility of the ions lead to small lattice energies and large entropy changes that favour the liquid state. Negligible vapour pressure, non-flammability, wide solvating capability and high thermal, chemical and electrochemical stability are the key properties that generated an increasing interest in the scientific and industrial uses of ionic liquids, mainly as “green” alternatives to volatile organic solvents.23
Group I chlorides show fusion temperatures significantly above room temperature (Tm(LiCl) = 883 K, Tm(NaCl) = 1076 K, Tm(KCl) = 1045 K, Tm(CsCl) = 919 K).45 Even their mixtures (Tm(LiCl-CsCl) = 628 K, Tm(NaCl-KCl) = 931 K, Tm(CsCl-KCl) = 883 K)45 present fusion temperatures too high to allow their use as medium for organic chemistry. Fusion temperature is mainly influenced by the charge distribution on the ions, H-bonding ability, the symmetry of the ions and the van der Waals interactions.17 The reduction of fusion temperatures can be achieved by replacing the inorganic cations with organic cations, due to the cation asymmetry.12 There are many examples. This effect was pointed out when comparing the higher fusion temperatures of 1,3-dimethylimidazolium and 1,3-diethylimidazolium salts with those of the unsymmetrical 1-ethyl-3-methylimidazolium or 1-butyl-3-methylimidazolium cation analogues.12 N-butylpyridinium salts analogues of 1-ethyl-3-methylimidazolium salts melt at higher
temperatures, due to the fact that their cations possess a mirror plane, that is absent in 1-ethyl-3-methylimidazolium, which has only C1 symmetry (the ethyl group is not co-planar with the imidazolium ring).45,46 The same behaviour was observed for salts derived from the ammonium or pyrrolidinium cations: the symmetrical species were solids and the lower symmetry cations form liquids, at room temperature.47 Fusion temperatures were maximised for phosphonium salts with the more symmetrical cations and fall down as the chain gets longer.32,47
Strong hydrogen bonds in the lattice also influence the fusion temperature. Halide salts have much higher values46 than their tetrafluoroborate48 or hexafluorophosphate49 analogues. Bulky anions show a high degree of charge delocalization, which contributes to lower the lattice energy and the fusion temperature.5 Thus, the anions contribute more to the overall characteristics of AILs and determine air and water stability.50
ILs based on AlCl3 and organic salts, such as 1-butyl-3-methylimidazolium chloride, whose Lewis acidity can be controlled by varying the amounts of organic salt/AlCl3, are extremely hygroscopic and their handling is possible under a dry atmosphere. Phosphates based ILs are nearly neutral and air stable, but they react exothermically with strong Lewis acids (e. g. AlCl3) and water, which leads to the formation of HF. The anions more stable for such reactions are [CF3SO3]−, [N(CF3SO2)2]−, and the corresponding ILs are characterized by low fusion temperatures, low viscosities and high conductivities.51
The geometry and charge distribution of ions control molecular interactions. These are mainly long-range Coulomb forces between the net charges of the ions, but hydrogen bonding and dispersion forces can also play a crucial role on shorter range highly directional interactions.1,52
1.1.4. Analysis of ionic liquids in gas phase
After the finding that various families of ILs could be distilled at low pressure without decomposition, the nature of their vapours became a key question. For this
problem, the experiments of Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) were initially centred on uni-univalent AILs,38 which were found to distil under reduced pressure as neutral anion-cation pairs (NIPs), without any detectable amounts of isolated ions or higher aggregates (charged or uncharged) in the vapour phase. Therefore a C+A− species will distil as CA. This conclusion, which has also been supported by more recent studies,53-55 corroborated mass spectrometric results for the evaporation of thin films of ILs into high vacuum,56 and suggested that the detection of vapours containing isolated ions and higher aggregates in other mass spectrometric experiments was due to the fact that they did not reproduce thermal distillation conditions.55,57,58 The corresponding values of enthalpy of vaporisation depend mainly on the Coulomb interactions inside the liquid phase and the gas phase ion pair, because van der Waals interactions are less intense and increase with the alkyl chain length of cations.59,60
Similar studies for PILs confirmed the existence of another mechanism of sublimation/vaporisation: the vapour phase over a PIL is composed by dissociated neutral molecules precursors of the IL, which means that a [HB]+A− species will distil as B and HA).14,33,37,38,61-63 However it was already reported the presence of ion pairs in gas phase over PILs by IR and Raman spectroscopy64 and by IR spectroscopy and computational methods.55 This last analysis concluded that the presence of ions pairs in the gas phase of PILs is difficult to be probed experimentally, because the fraction of these species is very small.55 The investigation of the PILs 1-methylimidazolium ethanoate, [Hmim][O2CCH3],65 and 1,1,3,3-tetramethylguanidinium chloride, [Htmg]Cl,14 by FT-ICR-MS gave experimental evidence that, as previously assumed, the vaporisation of this class of compounds involves a proton transfer mechanism with the formation of two volatile neutral molecules. These studies also indicated that the association degree of the neutral gaseous species is controlled by a strongly pressure dependent equilibrium.14,65 Analogously to previous studies with AILs, the detection of vapours containing isolated ions by others techniques was due to the fact that they did not reproduce thermal distillation conditions.55,63,66
Mass spectrometric experiments were extended to a dicationic IL formed by a divalent cation and two univalent anions and showed that, analogously to the uni-univalent AILs, the vapour was also composed solely by the parent species, in this case a neutral ion triplet (NIT) consisting of one dicationic and two anionic moieties.7,8
Also examined was the reduced pressure distillation of molten salts composed of a Group 1 metal cation and a commonly used AIL anion, M[NTf2] (M = Li, Na, K, Rb, Cs; NTf2 = bis(trifluoromethylsulfonyl)imide). It was concluded67 that similarly to the studied AILs, the vapour phase essentially consists of NIPs, although, in the case of the Li and Na derivatives, a minor contribution from [M2(NTf2)2] neutral aggregates was observed. These substances therefore exhibit a behavior intermediate between that of AILs and Group 1 metal halides, MX (M = Li, Na, K, Rb, Cs; X = F, Cl, Br, I). Vapours originating from the latter, under low pressure, show significant fractions of aggregates larger than NIPs (particularly dimers), with the aggregation tendency decreasing as the sizes of the M+ or X− ions increase.68-70
While the mechanism of vaporisation/sublimation has been investigated by mass spectrometric studies for both PILs and AILs,14,38,54 the energetics of the process has been studied exclusively for AILs.71,72 Vapour pressures and enthalpies of vaporisation were determined by diverse methods such as Knudsen effusion,73-78 transpiration,79,80 mass spectrometry,56,81-84 UV-vis spectroscopy,85 calorimetry,86 and thermogravimetry.87 A review of these latter studies has recently been published.71 To the best of knowledge, prior to this thesis, there is only one reported attempt to experimentally determine the enthalpy of vaporisation of a PIL. This study involved 1-methylimidazolium nitrate, [Hmim][NO3], which melts at ±343 K and is therefore solid at ambient temperature. It was based on temperature-programmed desorption coupled with line-of-sight mass spectrometry (TPD-LOS-MS) experiments and vapour pressure-temperature measurements by the transpiration method.88
1.2.
S
cope of the present work
The rational design of ILs should considerably benefit from a better understanding of their properties at a molecular level. This is, however, a challenge, because the charge and the molecular and electronic structure of the ions create a complex interplay of interactions. Furthermore, theoretical analyses should be supported by reliable experimental data, which are lacking in several areas. This Thesis, which includes eight Chapters, was mainly focused on (i) the cohesive energies of ILs and their vaporisation energetics; (ii) the gas phase affinity of different IL moieties. They are described below:
− Chapter 1, the present chapter, describes the scope of the Thesis and gives a general introduction to ionic liquids, covering their history, classification, typical properties and potential uses.
− Chapter 2 includes the description of the main experimental techniques used. − Chapter 3 corresponds to the published work A general strategy for the experimental study of the thermochemistry of protic ionic liquids: enthalpy of formation and vaporisation of 1-methylimidazolium ethanoate (Phys. Chem. Chem. Phys. 2012, 14, 4440–4446), describing the methodology used to determine enthalpies of formation of protic ionic liquids, based solely on enthalpy of solution measurements.
− Chapter 4 refers to the published work Thermochemistry of 1,1,3,3-tetramethylguanidine and 1,1,3,3-tetramethylguanidinium nitrate (J. Chem. Thermodyn. 2014, 77, 179–189). This article describes the determination of the enthalpies of formation of the protic ionic liquid 1,1,3,3-tetramethylguanidinium nitrate based on combustion and Calvet-drop calorimetry. It also includes the determination of the enthalpy of formation of the precursor base 1,1,3,3-tetramethylguanidine, combining the previous results with enthalpy of solution measurements.
− Chapter 5 describes the investigation of the cohesive energies of a series of protic ionic liquids, using the methodology discussed in Chapters 3 and 4.
− Chapter 6 reports the mass spectrometry studies that led to the establishment of affinity scales of different anions for the cation 1-butyl-3-methylimidazolium, and of different cations for the anion bis(trifluoromethylsulfonyl)imide.
− Chapter 7 corresponds to the published work Thermochemistry of 1-alkylimidazoles (J. Chem. Thermodyn. 2015, 80, 59–64). It describes a combination of experimental a theoretical studies aimed at the determination of the enthalpies of formation in the gaseous and liquid states of 1-alkylimidazole compounds ranging from 1-methylimidazole to 1-dodecylimidazole. Energetic trends related to changes in the alkyl chain length are discussed.
− The overall conclusions of the Thesis are summarized in Chapter 8.
It should be noted that the abbreviations of compounds were uniformed for all chapters of this Thesis.
R
eferences
(1) Weingäertner, H. Angew. Chem. Int. Ed. 2008, 47, 654.
(2) Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; 2nd ed.; Wiley-VCH: Wienheim, 2007.
(3) MacFarlane, D. R.; Seddon, K. R. Aust J Chem 2007, 60, 3. (4) Wilkes, J. S. Green Chem 2002, 4, 73.
(5) Lovelock, K. R. J.; Villar-Garcia, I. J.; Maier, F.; Steinruck, H. P.; Licence, P. Chem. Rev. 2010, 110, 5158.
(6) Preiss, U.; Verevkin, S. P.; Koslowski, T.; Krossing, I. Chem. Eur. J. 2011, 17, 6508.
(7) Lovelock, K. R. J.; Deyko, A.; Corfield, J. A.; Gooden, P. N.; Licence, P.; Jones, R. G. Chemphyschem 2009, 10, 337.
(8) Vitorino, J.; Leal, J. P.; Licence, P.; Lovelock, K. R. J.; Gooden, P. N.; Minas da Piedade, M. E.; Shimizu, K.; Rebelo, L. P. N.; Canongia Lopes, J. N. Chemphyschem 2010, 11, 3673.
(9) Chang, J. C.; Ho, W. Y.; Sun, I. W.; Tung, Y. L.; Tsui, M. C.; Wu, T. Y.; Liang, S. S. Tetrahedron 2010, 66, 6150.
(10) Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. J. Chem. Eng. Data 2011, 56, 2453.
(11) Zhou, N.; Zhao, G. Y.; Dong, K.; Sun, J.; Shao, H. W. Rsc Adv 2012, 2, 9830.
(12) Plechkova, N. V.; Seddon, K. R. Chem. Soc. Rev. 2008, 37, 123. (13) Greaves, T. L.; Drummond, C. J. Chem. Rev. 2008, 108, 206.
(14) Vitorino, J.; Leal, J. P.; Minas da Piedade, M. E.; Canongia Lopes, J. N.; Esperanca, J. M. S. S.; Rebelo, L. P. N. J Phys Chem B 2010, 114, 8905.
(15) Cacace, F.; Depetris, G.; Pepi, F.; Angelelli, F. P. Natl. Acad. Sci. USA 1995, 92, 8635.