OUTCOMES OF PRE-FERMENTATION AND
POST-FERMENTATION EXTENDED MACERATION FOR
ANTHOCYANIN AND TANNIN COMPOSITION OF RED
WINES FROM HOT IRRIGATED VINEYARDS OF SOUTH
EASTERN AUSTRALIA
DANAI IOANNIDOU
Dissertação para obtenção do Grau de Mestre em
Vinifera EuroMaster – European Master of Sciences of Viticulture
and Oenology
Orientador: Professor Christopher Ford
Co-orientador: Professor Jorge Ricardo da Silva
Júri:
Presidente: Olga Maria Carrasqueira Laureano, Investigadora Coordenadora do Instituto Superior de Agronomia da Universidade Técnica de Lisboa.
Vogais: - Christopher Ford, Professor, Universidade de Adelaide - Jorge Ricardo da Silva, Professor, UTL/ISA
- Manuel Malfeito, Professor, UTL/ISA
Acknowledgements
This thesis project was conducted at the Riverland Vine Improvement Committee (RVIC), the Adelaide University (S. Australia) and the Technological Educational Institute of Athens in Greece, by Danai Ioannidou, in fulfillment of the requirements for the degree of the European master of Viticulture and Enology.
I would like to sincerely thank my supervisor Dr. Christopher Ford, Senior Lecturer at the University of Adelaide, for his support from the beginning until the end of this project, and my co- supervisors, Dr. Olga Laureano and Dr. Jorge M. Ricardo-Da-Silva from ISA Lisbon, as well as Dr. Chatzilazarou Arhontoula from the Technological Educational Institute of Athens, for the knowledge that they offered me during my masters studies.
I would like to express my gratitude to Dr. Crystal Sweetman from Adelaide University, for the wine analysis, and to Dr. Yiannis Paraskevopoulos from the Technological Educational Institute of Athens for his support.
Also, I would like to thank the Riverland Vine Improvement Committee (RVIC), for the concession of the grapes and the necessary equipment.
Last but not least, many thanks to my family and friends who are always beside me supporting me.
Danai IOANNIDOU
Abstract
The aim of this study was to understand the effects of different maceration winemaking techniques
on the phenolic composition of red grapevine variety ‘Tempranillo’ (Vitis vinifera L.).
Experimental winemaking included 4 different treatments with 3 replicates. (5days pre-fermentation maceration at 11°C, fermentation without any pre-fermentation or post-fermentation extended maceration (control wines), and 3- and 6-days post- fermentation extended maceration on skins). Spectroscopic and RP-HPLC analysis were used to determine changes in phenolic components and between treatments during the alcoholic fermentation, and as they aged in the bottle.
It was determined if the wines made with pre-fermentation maceration had increased color intensity compared to the control wines, as well as if the wines that underwent extended maceration had increased concentration of (+)-catechin and (-)-epicatechin, increased color hue, decreased color intensity compared to the control wines.
Prolonging the maceration from 7 to 10 and 13 days, lead to significantly higher concentrations of total phenolics, and the monomeric flavan-3-ols, (+)-catechin and (-)-epicatechin, and lead to a significant decrease of the total anthocyanins.
Further investigation is needed in order to analyse the effect of the phenolic, tannin’s and anthocyannin’s composition on the quality of the wine in terms of color stabilization and mouth-feel. Post-fermentation EM reduced wine colour intensity and imparted a browner hue to the wine compared to the Control wine. This EM treatment is therefore unlikely to benefit winemakers who are seeking to produce highly coloured wines. However, post-fermentation extended maceration increased the concentration of wine flavan-3-ols ((+)-catechin and (-)-epicatechin). Therefore, the winemakers by varying the duration of post-fermentation EM may influence the desired balance between the extraction of these wine phenolics and the mouth-feel properties, taking into consideration the economic factor.
Resumo
O objetivo deste estudo foi compreender os efeitos das técnicas de maceração diferentes de vinificação sobre a composição fenólica de "Tempranillo" variedade de videira vermelha (Vitis vinifera L.).
Vinificação experimental incluiu quatro diferentes tratamentos com três repetições. (5 dias de maceração pré-fermentação a 11 ° C, a fermentação sem qualquer maceração pré-fermentação ou pós-fermentativa prolongada (vinhos de controle), e 3 - e seis dias de maceração pós-fermentativa prolongada em peles).
Análise espectroscópica e RP-HPLC foram utilizadas para determinar as alterações em componentes fenólicos e entre os tratamentos durante a fermentação alcoólica, e à medida que envelheciam na garrafa.
Determinou-se que os vinhos feitos com pré-fermentação maceração aumentou a intensidade da cor em comparação com os vinhos de controlo, bem como se os vinhos que foram submetidos a maceração estendido aumentou de concentração de (+)-catequina e (-)-epicatequina, o aumento da cor tonalidade, diminuição da intensidade da cor em comparação com os vinhos testemunha.
Prolongando a maceração 7-10 e 13 dias, resultam em concentrações significativamente mais elevadas de compostos fenólicos, e o monomérica flavan-3-óis, (+)-catequina e (-)-epicatequina, e levar a uma diminuição significativa do total antocianinas.
É necessária mais investigação a fim de analisar o efeito da fenólico, tanino e composição anthocyannin sobre a qualidade do vinho, em termos de estabilização da cor e sensação na boca. Pós-fermentação EM reduzida intensidade da cor do vinho e transmitido um tom acastanhado para a comparação com o vinho do vinho de controlo. Este tratamento de EM é, portanto, improvável para beneficiar os produtores de vinho que buscam produzir vinhos muito coloridos. Contudo, a pós-fermentação maceração prolongada aumentou a concentração de vinho flavan-3-óis ((+)-catequina e (-)-epicatequina). Por conseguinte, os produtores de vinhos, fazendo variar a duração da pós-fermentação EM podem influenciar o equilíbrio desejado entre a extracção dos fenóis do vinho e as propriedades de sensação na boca, levando-se em consideração o factor económico.
CONTENTS
Aknowledgments……….. 2
Abstract and key words………. 3
Resumo e palavras chave………... 4
Contents………....……… 5
List of tables, graphs and figures……… 7
Abreviations……….………….. 8
1. Introduction………..………. 9
1.1 Introduction: The importance of maceration in red winemaking………. 9
1.2 Grape’s and wine’s phenols………..………. 11
1.2.1 The Non-flavonoids……… ………… 13
1.2.2 The Flavonoids……….….... 14
1.2.2.1 Anthocyanins and polymeric pigments……….…….... 14
1.2.2.2 Flavan-3-ol monomers………...….. 15
1.2.2.3 Proanthocyanidins………...……… 15
1.3 Phenolic extraction from grape to wine………...……….…………. 15
1.3.1 Influence of pomace contact on extraction………...……… 16
1.3.1.1 Pre-fermentation maceration………...…. 17
1.3.1.2 Post-fermentation extended maceration………...… 17
1.3.1.3 Consequences of pre- and post-fermentation extended maceration for red wine ………..18
1.3.2 Measurement of red wine colour and phenolic composition…………..…………...……. 19
2. Materials and methods………...…… 20
2.1 Experimental winemaking: study of pre- and post-fermentation EM of red wine made from V. Vinifera Tempanillo………..……… 20
2.2 Red wine color and spectrometric measures……….…….. 23
2.3 Reversed-phase High Performance Liquid Chromatography (RP-HPLC)…………...…… 23
3. Results……….…...……… 24
3.1 Wine analysis results and discussion (Phenolic spectroscopic measures, RP-HPLC, and total anthocyanin measures)……….…...………. 24
3.1.1 Spectroscopic measures, and anthocyanins………...….………….. 24
3.1.2 HPLC analysis of wine………...……….……… 29
List of tables, graphs and figures.
Figure 2-1: Flow-chart; differences between winemaking treatments. (Pre-fermentation maceration Control, 3days post-fermentation extended maceration, and 6days post-fermentation extended maceration treatment).
Graph 3-1.1.a: Graph showing the Evolution of Color Intensity during ALF, from crush to end of ALF, resulted from the Spectral analysis.
Graph 3-1.1.b: Graph showing the Evolution of Color Hue during ALF, from crush to end of ALF, resulted from the Spectral analysis.
Graph 3-1.1.c: Graph showing the Evolution of Total Red Pigments during ALF, from crush to end of ALF, resulted from the Spectral analysis.
Graph 3-1.1.d: Graph showing the Evolution of Total Phenolics during ALF, from crush to end of ALF, resulted from the Spectral analysis.
Table 3-2: Spectral analyses of colour, SO2 resistant pigments, total phenolics and total anthocyanins for 2012 Tempranillo, at common pH:3.5.
Table 3-3: Spectral analyses of colour, SO2 resistant pigments, total phenolics and total anthocyanins for 2012 Tempranillo, at natural pH.
Table 3-4: Sum of the anthocyanins per treatment and the mean for the 3 replicates. The results are presented in mg/L.
Table 3-5: Sum of the tannins per treatment and the mean for the 3 replicates. The results are presented in mg/L.
Graph 3-6: Graph showing the differences between the treatments, resulted from the HPLC analysis of anthocyanins, and tannins ((+)-catechin, (-)-epicatechin), in the 2012 Tempranillo wines at 6 months post-crush.
Abbreviations
a.u absorbance units
C control maceration treatments
PFM5 pre- fermentation extended maceration of 5days, with plunging, held at 10oC for 5 days.
EM3 3days post-fermentation extended maceration treatment
EM6 6days post-fermentation extended maceration treatment
PFM pre- fermentation extended maceration
EM extended maceration MLF malo-lactic fermentation PMS potassium metabisulphite RS reducing sugars RT retention time SA South Australia TA titratable acidity VA volatile acidity
WCD wine colour density
WCH wine colour hue
1. Introduction
1.1 Introduction: The importance of maceration in red winemaking
The winemaking techniques influence the extraction of compounds from grapes, affecting the composition of red wines. Both temperature and duration of maceration, as the presence or absence of ethanol, are factors affecting the characteristics and composition of anthocyanins, as well as the phenomena of copigmentación (Gómez-Minguez and Heredia, 2004).
During alcoholic fermentation of red must, and post-fermentation extended maceration of wine, phenolics and other compounds from berry skins and seeds of the grapes are transferred to the wine by diffusion. Some of them are extracted during the early stages of maceration, whereas others are gradually extracted. Most grape phenolic compounds are contained in the skins and seeds (Kennedy et al. 2000, Souquet et al. 1996).
The skins are of greater importance as they contribute to an easier extraction of polyphenols into musts during maceration. (Meyer, J., and R. Hernandez, 1970).
Maceration of the skins during winemaking facilitates the release of phenolic components into the wine (Amrani Joutei and Glories 1995, Robinson 2006).
Although the skins contain much lower concentration of both total and polymeric phenols than seeds and stems (Kantz, K., and V. L. Singleton, 1991).
The two families of phenolic compounds include the non-flavonoids and the flavonoids. The flavonoids are of particular interest to this study, as they greatly influence wine colour and sensory characteristics (Auw et al. 1996, Somers and Evans 1974, Zimman et al. 2002). They include the anthocyanins, flavan-3-ol monomers, flavanols, and proanthocyanidins (also called condensed tannins). Thus, the composition and organoleptic properties of red wine are predominantly affected by maceration and the extraction of grape derived phenolic components and their subsequent reactions in wine (Basha et al. 2004, King et al. 2003, Sims and Bates 1994).
Diffusion is the process by which molecules spread from areas of high concentration, to areas of low concentration. Some of the factors that affect the diffusion of grape phenolic compounds into the wine during maceration are the temperature of the must, juice or wine, the duration of grape skin and seed contact, physical handling of the must including cap management technique, the extraction medium’s composition including alcohol level and sulphur dioxide concentration, inclusion of stems in the fermentation, yeast selection, the grape variety and maturity (Williams 1997, Arnous et al. 2002, Singleton and Trousdale 1983, Mazza et al. 1999, Soleas et al. 1998, Blanco et al. 1998, Bakker et al. 1998, Gomez-Plaza et al. 2000).
Winemaking practices influence the extraction of phenolic compounds. The temperature variations during processing influences extraction because temperature affects the permeability of the cells and membranes in grape berries. For the same reason, the use of pectolytic enzymes influence the extent
of phenolic extraction. (Romero-Cascales et al. 2008).
Other temperature treatments apart from cold soak, include fermentation under high temperature, must or grape freezing, heated maceration, and thermo-vinification. (Anli et al. 2000, Gerbaux et al. 1993, Sacchi et al. 2005, Okubo et al. 2003, Zimman et al. 2002, Gerbaux et al. 2003 Reynolds et al. 2001, Plaza et al. 2001)
Gerbaux did not observe any improvement in phenolic extraction as a result of pre-fermentation maceration, a vinification method in which wine musts are kept at low temperature for several days before alcoholic fermentation. However, in another study, pre-fermentation maceration at 10 degrees Celsius for 3 days increased the extraction of both anthocyanins and skin-derived proanthocyani- dins. (Cheynier et al. 2006).
In another study with Cabernet Sauvignon, pre-fermentation maceration extracted more color than was obtained in the control, but there was no significant difference in the total polyphenols between the finished wine of the control and that obtained from the pre-fermentation maceration (cold soak). (Okubo et al. 2003)
The lack of compatibility might be due to the differences in the conditions of the cold soak process.
Sacchi et al. (2005), summarize that temperature treatments including increased fermentation temperatures, thermovinification, the duration of grape skin and seed contact and, increase phenolic extraction (Ough and Amerine, 1961, Harbertson et al., 2002, Girard et al. 1997, Couasnon, 1999), whereas winemaking parameters such as yeast selection (Zini et al., 2002), and skin and juice mixing practices (Leone et al., 1983, Fisher et al., 2000) have variable effects on phenolic extraction.
The opportunity to extract phenolic compounds from the solid parts of the grape into the wine may be extended beyond the period of alcoholic fermentation. Winemakers can choose to extend the maceration period by crushing grapes and delaying juice inoculation with yeast for a number of days, or by delaying pressing off skins after the completion of alcoholic fermentation (Boulton et al., 1998, Watson et al., 1997, Heatherbell et al., 1996,).
Pre-fermentation maceration and post-fermentation EM, is done to increase the concentration of phenolics such as anthocyanins, flavan-3-ols and tannins in the wine. These compounds, present in the wine, undergo a variety of chemical reactions over the life of the wine that influence wine sensory properties (Yokotsuka et al., 1999, Gomez-Plaza et al. 2001, Reynolds et al. 2001, Scudamore-Smith et al., 1990). For example, during pre-fermentation maceration, extraction of monomeric anthocyanins occurs (Okubo et al. 2003, Reynolds et al. 2001). The monomeric anthocyanins in the wine undergo polymerisation reactions to form pigmented polymers (complexes with the tannins) that increase wine colour stability. (Somers, 1971, Somers and Evans, 1977, Auw et al., 1996, Bakker
and Timberlake, 1997). Extending the period of maceration after the alcoholic fermentation is complete provides more time for extraction of alcohol soluble compounds such as high molecular weight astringent and bitter tannins from the seeds (Singleton and Noble, 1976a, Cerpa-Calderón and Kennedy, 2008).
In order to understand more fully the consequences of maceration for red wine phenolics, the use of sensitive and specific experimental methods is required to detect and quantify changes in wine that have been induced by a variery of maceration tachniques (Gawel et al., 1997). Then conclusions will be performed regarding the efficacy of EM practices, in producing a final wine with the desired composition and organoleptic outcomes. Wineries can then use this information to decide on the economic advantages/disadvantages of utilizing such vinification practices.
Currently, pre-fermentation EM is believed to increase fruit flavours and aromatic quality, reduce bitterness and astringency, improve mouthfeel and body, and modify the varietal flavour of the resulting wine. In contrast, post-fermentation EM is believed to increase colour stability through the increased extraction of phenolics and subsequent polymerisation of tannins with anthocyanins. It is also thought to promote tannin polymerisation and result in a more complex, richer wine with better ageing capacity.
Ageing capacity is the ability of the wine’s organoleptic properties to improve with time (Watson et al., 1997, Sipiora and Granda, 1998, Gonzalez-Manzano et al., 2004, Auw et al., 1996, Yokotsuka et al., 1999).
In the research described in this thesis, the colour and the phenolic composition of the wines produced using pre-fermentation and post-fermentation EM, have been examined and compared to control wines, which received maceration only during alcoholic fermentation.
1.2 Grape’s and wine’s phenols
The phenols in grapes are present mainly in the seeds and skins (Cheynier ,2005).
The anthocyanins are restricted to the skin of red grapes, whereas the tannins are contained in the skin and seeds of grapes, and only a small amount in the pulp (Haslam, 1998).
The phenols, are very important constituents of red wines, which determine the organoleptic characteristics, which strongly define the wine style (Zoecklein et al, 1995). The anthocyanins responsible for red color, the tannins or proanthocyanidins for astringency, associated with the catechins or flavan-3-ols, responsible for the bitterness (Arnold et al, 1980), are the main phenolics in wine. These grape substances are diffused into the wine during maceration.
The main target of the maceration is to obtain wines with more color, body and complexity. Maceration length control, involves finding an optimum to obtain wines with improved sensory profile. But the same factors that favor the transfer of the desirable sensory substances can encourage
other bitter or astringent substances. Moreover, the evolutions of concentration substances transferred are not linear with time. This is because, during extraction, substances undergo side phenomena such as saturation and precipitation, adsorption on solids, the molecular condensation, oxidation and copigmentación (Ribéreau-Gayon, 1982, Sommers & Evans, 1979).
In the beginning the extraction is rapid, but from a certain point it becomes much slower. In the classical vinification, only about a third of the total phenols present in the grapes remains in the wine. The extraction of tannins (major constituents of phenols) follows a similar model (Boulton, 1995). Tannins can be destructed due to oxidation-condensation-precipitation, but the phenomenon is rather slow under normal conditions. During the early stages of the maceration, are preferably extracted skin tannins, rich in prodelphinidins, and poor in galloyllated tannins.
If maceration is prolonged, increases the extraction of seed tannins, strongly galloylated. The seed tannins are diffused more slowly and require a higher alcohol content to ensure dissolution. A significant extraction of (+)-catechins and (-)-epicatechins from the seeds is a feature of the vinification with extended maceration time. (Joutei Amrani and Glories, 1994, Boulton, 1995). The pattern of anthocyanin extraction during maceration is very different. Several studies demonstrated that the largest increase in anthocyanin extraction of red Vitis vinifera wines occurred early in fermentation (in the first 3 to 4 days of skin maceration), while little increase occurred after 10 days of skin contact and then show a decrease (Gómez-Plaza et al., 2001; Sacchi et al., 2005). After reaching a maximum value, the concentration of anthocyanins gradually decrease. (Amrani-Joutei and Glories, 1994, Ribéreau-Gayon, 1982, Somers and Evans, 1979).
The anthocyanins are very reactive and the evolution of the concentration during maceration is a combination of phenomena. They are rapidly dissolved into the juice due to their high availability, and the lack of alcohol is not an obstacle as they are water-soluble. The loss is partly due to adsorption on yeast and on the solid parts of grapes (like stems) (Ribéreau Gayon, 1982).
The anthocyanins are quickly involved in polymerization reactions with grape proanthocyanidins and acetaldehyde formed by the yeast. During fermentation, these polymers can be combined with proteins and become insoluble (Doco et al, 1996), or polysaccharides which partially precipitate with bitartrate crystals (Vernhet et al, 1999).
Many authors have determined that a longer skin contact usually gives wines with more color, more flavonoids, more tannins, less monomeric anthocyanins and more polymeric phenols and polymeric colour. (Scudamore-Smith et al, 1990, Kantz and Singleton, 1991, Kovac et al, 1992, Sims and Bates, 1994; Auw et al, 1996). The wine can become more astringent and more aromatic. Then, with a longer contact time the wine can become less fruity. The astringency is usually not becoming excessive over time, and may even decrease (Schmidt and Noble, 1983, Scudamore-Smith et al, 1990, Sims and Bates, 1994).
Asselin et al, (1999) experimenting with 4, 8 and 15 days of maceration of Cabernet Franc from different terroirs, determined that wines with 8 days were far superior in color and anthocyanins as the 4 and 15 days, on the other hand, wines with 15 days of maceration, had a higher content of total tannins, proanthocyanidins and tannins galloylated, which was associated with green tannins and herbaceous tastes.
The objective of this study was to determine the effect of time of maceration on phenolic composition and color and wine astringency of wine from Tempranillo grapes, from hot irrigated vineyards of South Australia, and understand the length of maceration that gives greater color and wine body, with less astringency, and determine which of the four maceration times, PFM5, control (0 days), 3 days EM, 6 days EM, is more appropriate to study the association between the phenolic profiles of the wines and their organoleptic characteristics.
According to Zoecklein et al. (1995), the major phenolic compounds families in Vitis vinifera wines are: i) non-flavonoids or phenolic acids (cinnamics, benzoics)
ii) flavonoids (anthocyanins, flavanols (proanthocyanidins or condensed tannins), flavonols, flavanonols, soluble tannin derivatives and other flavonoid derivatives), and tannins or polymerized phenols. These are located in the skins, seeds, and stems, and are higher in red wine than white wine. The flavonoid compounds in wine are mostly extracted from the skins and seeds of grapes during fermentation (Singleton and Esau 1969; Singleton and Noble 1973).
1.2.1 The Non-flavonoids
Non-flavonoid phenols are derivatives of hydroxycinnamic and hydroxybenzoic acids, such as coutaric (coumaroyl tartaric acid), caftaric (caffeoyl tartaric acid), gallic acid, and ellagic acid, as well as other lower molecular weight phenolics. Hydroxycinnamic acids, derived from p-coumaric, caffeic, ferulic and sinapic acid, exist in grapes as tartaric acid esters, but may be found in their free forms in wine due to enzyme acid hydrolysis during vinification (Ribereau-Gayon, 1965, Somers et al., 1987). Complex phenols, also referred to as tannins, are present in high proportion in seeds and stems. These are polymers of both flavonoid and nonflavonoid phenols, which can be hydrolyzable like the ellagitannins, or condensed, which are known as procyanidins (Zoecklein, 1995).
Caftaric acid, is the main substrate for enzymatic oxidation in wine. Through coupled oxidation reactions with anthocyanins, caftaric acid can contribute to anthocyanin oxidation and wine browning (Sarni et al., 1995).
1.2.2 The Flavonoids
The flavonoids may exist free or polymerized. These include monomeric flavan-3-ols, such as catechin, epicatechin, anthocyanins, and oligomeric and polymeric flavan-3-ols such as procyanidins. The main flavonoid species important to the chemical reactions and sensory properties of wine are the anthocyanins and flavanols (Singleton 1988; Singleton and Esau 1969; Singleton and Noble 1973). The catechins are associated with the wine astringency. The catechins and especially the proanthocyanidins (oligomeric- polymeric) are responsible for the astringency of the red wines, whereas the monomeric contribute to the bitterness (Singleton 1992). The astrigency and bitterness of the wine are mainly attributed to proanthocyanidins (Kovac et al., 1992). The extraction of proanthocyanidins and catechins increased progressively with the length of maceration (Vrhovšek et al., 2002). However, the astringency is diminished if the polymerization is increased (Haslam 1974, Jones et al., 1976, Oh et al., 1980).
The monomeric and oligomeric flavonoids are substrates of enzymatic as well as non-enzymatic browning in wines, and they brown more intensely than non-flavonoids (Jaworski and Lee, 1987, Lee and Jaworski, 1989).
1.2.2.1 Anthocyanins and polymeric pigments
The colour of the young red wines mainly depends on the extraction of anthocyanins from grape skin during maceration process (Gómez-Plaza et al., 2001)
The main pigment is malvidin in the form of 3-glucoside (in Vitis Vinifera), or esters of acetic, coumaric and caffeic acids, followed by delphinidin, cyaniding, petunidin and peonidin.
Anthocyanins are water-soluble pigments, which impart the red, purple, and blue coloration of grapes. They are contained in the skin vacuoles, and released into the wine during maceration and once in the wine, are involved in phenolic polymerisation reactions. At wine pH, the percentage of total anthocyanins in the red, flavylium form is less than 25% (Singleton 1988). When sulfur dioxide is added to wine it combines with the flavylium form, resulting in an additional equilibrium between the coloured form and the uncoloured bisulfite adduct. The colour of anthocyanin solution is also influenced by the presence of other components in the medium that cause colour shifts towards violet (bathochromic effect) and an increase in intensity (hyperchromic effect). Anthocyanin derived colour is an important sensory attribute of red wine because positive correlations exist between red wine colour and perceived wine quality (Somers, 1978, Somers and Evans, 1974).
Anthocyanins can bind to tannins or polymeric phenols, forming anthocyanin-tannin complexes. These complexes have been reported to stabilize the color of Vitis vinifera red wines and result in wines that taste less fruity and less astringent after aging (Scudamore-Smith et al., 1990).
In young red wines, colour is primarily due to the monomeric anthocyanins (Somers and Evans, 1977). As wine ages, polymerisation reactions occur and polymeric pigments become increasingly responsible for wine colour. Other factors that affect wine colour include pH, sulphur dioxide concentration, and co-pigmentation. The presence of other types of phenolic compounds also influences colour-related reactions in the wine (Nagel and Wulf, 1979, Ribéreau-Gayon and Glories, 1986). Anthocyanins progressively change the colour of wine as it matures. During red wine maturation, wine colour hue moves towards browner colours and colour density characteristically decreases. The changes are observed through a decrease in absorbance at 520 nm and an increase in absorbance at 420 nm (Somers and Verette, 1988).
1.2.2.2 Flavan-3-ol monomers
Flavan-3-ol monomers are present in the seeds of grapes (Romeyer et al., 1983, Singleton et al., 1966).
(+)-Catechin and (-)-epicatechin are the 2 main types of flavan-3-ol monomers in grapes.
Flavan-3-ol monomers are slightly bitter and astringent and polymerize to form procyanidins and condensed tannins in wine (Haslam, 1998). Long maceration times, high temperatures during maceration and high alcohol levels favour the extraction of these monomers from grapes into the wine (Meyer and Hernandez 1970, Oszmianski et al., 1986, Singleton and Draper, 1964).
1.2.2.3 Proanthocyanidins
Proanthocyanidins are tannins that consist of multiple flavan-3-ol monomers (like (+)-Catechin and (-)-epicatechin) joined together. Polymerisation is catalysed by low pH and can be complemented by cross-linking with acetaldehyde. These complex polymeric wine tannins are more stable against oxidative degradation compared to their monomeric constituents (Herderich and Smith 2005). Total tannin is significantly higher in the seeds than the skins (Souquet et al. 1996). Grape seed catechins and tannins are found mainly in the outer seed coat (Thorngate and Singleton, 1994), Gonzalez-Manzano et al., 2004).
Tannins are able to participate in polymerisation reactions, and can become red-coloured pigmented polymers and impart colour to the wine by acting as co-polymers with anthocyanin molecules. Tannins impart astringency and mouthfeel to red wine because of their ability to interact with proteins and precipitate with proteins involved in astringency perception.
1.3 Phenolic extraction from grape to wine
Phenolic extraction during winemaking is influenced by cell membrane disruption, solute solubility, and the surface area for extraction (Williams 1997, López et al., 2007). As such, various techniques
can be utilized to extract the desired amount of phenolics and achieve the right balance of wine sensory properties and colour stability. For skin and seed tannins, extraction is hindered by limited solubility (Watson et al. 1995, Gao et al., 1997). Increasing alcohol content, temperature, and duration of grape skin and seed contact with the juice/wine can increase tannin solubility, and therefore extraction (Singleton and Draper, 1964, Oszmianski et al., 1986). Cap management can also influence phenolic diffusion kinetics and play a particularly important role in determining the sensory properties of the final wine.
1.3.1 The influence of pomace contact on extraction
The extraction of anthocyanins and other flavonoids into the wine depends on their molecular size, and their location in the grape berry. Anthocyanins, phenolic acids, and skin-derived flavonols are very water-soluble and rapidly extracted (Koyama et al., 2007, Kelebek et al., 2006, Nagel and Wulf, 1979, Yokotsuka et al., 1999). Tannin is less water-soluble than the anthocyanins, thus tannin extraction increases with time on skins and increasing ethanol concentration (Gonzalez-Manzano et al., 2004). The smaller molecular size of anthocyanins compared to skin and seed tannins may enable them to diffuse more rapidly into the wine.
Mixing the cap with the juice/wine, which is primarily carried out to control cap temperature and prevent the cap from drying out, encourages extraction. Common methods used to mix the cap include punch-down, pumping-over, delestage (rack and return) and rotating the fermenter’s contents (if rotary fermenters are used). In addition, phenolic extraction is influenced by tank size and height-to-diameter ratio, as these variables also affect the concentration gradient required for extraction (Zoecklein et al., 1999).
Temperature can also be used to modify the phenolic extraction profile. Several studies have shown that pomace contact time and temperature during maceration have the greatest effects on extraction, especially in combination with high alcohol and sulphur dioxide (Oszmianski et al. 1986, Singleton and Draper, 1964). Maintaining the must and wine at a higher temperature (such as 30°C) during the period of skin contact can increase phenolic solubility and extraction (Ramey et al. 1986, Reynolds et al., 2001). Higher temperatures degrade cell walls and increase the permeability of the cells, increase the rate of diffusion and increase enzymatic and non-enzymatic reaction rates (Reynolds et al., 2001).
Extending the period of maceration beyond the time offered by alcoholic fermentation is an example of an old world winemaking technique. Kantz and Singleton (1991) have shown that extraction of monomeric and polymeric phenolics generally increases with increasing pomace contact time, and that the amounts extracted into the wine are proportional to the amounts present in the grape. Thus, EM provides the opportunity to extract additional skin or seed tannins, and pigmented tannins from
the marc that may provide desirable mouthfeel to wine and enhance colour stability.
1.3.1.1 Pre-fermentation maceration
Winemakers, to improve colour extraction and increase colour intensity, commonly adopt pre-fermentation EM, otherwise known as cold soak. Pre-pre-fermentation EM has also been suggested to increase fruit flavours and aromatic quality, reduce bitterness and astringency, improve mouthfeel and body, and modify the varietal flavour of the resulting wine (Heatherbell et al., 1996, Wollan, 1997). Pre-fermentation-induced increased wine colour is suggested to be due to the increased extraction of co-factors such as caffeic acid, which are responsible for enhanced co-pigmentation (Darias-Martin et al., 2001).
The term cold soak is used because the must is commonly chilled to temperatures of between 9 and 15oC to allow the skins some opportunity to macerate in a wholly aqueous environment. This is best achieved by preventing the onset of fermentation, and at the same time by minimising the chance of oxidative spoilage through the addition of sulphur dioxide, dry ice, and maintain cold temperatures. Generally, the juice and grape solids are left to soak for between 1 to 3 days before inoculation with a commercial yeast strain. Soaking allows, in the absence of ethanol, the diffusion of water-soluble grape compounds such as anthocyanins from the solid parts of the grape into the wine. Some winemakers exceed the length of the cold soak to even 10days.
1.3.1.2 Post-fermentation extended maceration
The post-fermentation EM, is usually carried out for a few days to even weeks, at temperatures 25 to 30 degrees Celsius, and in some cases the winemaker decides to perform a heated maceration at temperatures that can reach 38 degrees, depending on the needs for extraction and the wine style. In addition, it is known that the extension of maceration affects the transfer from the solid parts of grape berries to the must, influencing therefore wine phenolic composition. Extended maceration has been reported to improve the extraction of catechins (Ribereau-Gayon et al., 2003, Sun et al., 1999). In some cases, a decrease during the final stages of maceration was observed (Soleas et al., 1995).
During the extended maceration, alcohol-soluble compounds diffuse from the solid parts of the grape into the fermented juice. Thus, post-fermentation EM focuses on the further extraction of tannin, rather than anthocyanin. Alcohol level is an important aspect of extraction post-fermentation, especially for seed and skin tannins. Gonzalez-Manzano et al., (2004) have shown, using wine model systems, that the most readily extractable tannin was extracted when the alcohol level was above 12.5%.
through the increased extraction of phenolics and subsequent polymerisation of tannins with anthocyanins. It is also thought to promote tannin polymerisation and result in a more complex, richer wine with better ageing capacity (Watson et al., 1997, Sipiora and Granda, 1998, Gonzalez-Manzano et al., 2004, Auw et al., 1996, Yokotsuka et al., 1999).
1.3.1.3 Consequences of pre- and post-fermentation extended maceration for red wine
While pre-fermentation EM and its consequences for wine phenolic composition, particularly colour, have been studied by a number of groups, there are discrepancies between findings.
Budic-Leto and others (2003) and Watson and co-workers (1997) found that, in comparison to a control wine, a pre-fermentation EM treatment resulted in lower levels of anthocyanins, total phenols and tannins in Babic and Pinot noir wines. A study by des Gaschons and Kennedy (2003) compared a 4-day pre-fermentation EM with one lasting 10 days and found little quantitative difference in the tannin extracted.
Other studies have shown, however, that pre-fermentation EM can achieve a greater extraction of phenolic compounds, particularly anthocyanins, and increase the rate of pigmented polymer formation (Alvarez et al. 2006, Gomez-Miguez et al., 2007). In a study by Fulcrand et al., (2004) a 3-day pre-fermentation EM enhanced the extraction of both tannins and anthocyanins (without greatly altering their ratio). Cheynier et al., (1997a) also found that pre-fermentation EM increased the extraction of skin derived anthocyanins and tannins. Koyama et al., (2007) found that pre-fermentation EM (must held between 10-15°C for around 3 days) increased the anthocyanin to tannin ratio by 67%. However, they also found that this maceration technique significantly decreased the rate of pigmented polymer formation compared to a control wine.
Generally, it is accepted that post-fermentation EM increases total phenolic concentration, increases wine colour stability and affects wine bitterness and astringency. For example, Scudamore-Smith et al. (1990) noted post-fermentation EM wines as having less fruit flavour and being suited for longer cellaring due to increased astringency. Similarly, Gomez-Plaza et al., (2002) found that increasing the maceration time of Muscadine grapes from 4 to 10 days by a period of post-fermentation EM led to greater wine astringency.
Post-fermentation EM is also believed to affect tannin extraction more than anthocyanin extraction. For example, the study by Fulcrand et al., (2004) showed that a 3-week post-fermentation EM increased tannin extraction and reduced anthocyanin extraction compared to the control wine. Cheynier et al., (1997a) found that post-fermentation EM targeted extraction of phenolics from the seeds rather than anthocyanins from the skins. Reynolds et al., (2001), however, found that a 3-day post-fermentation EM increased anthocyanin concentration and wine color compared to a control wine. Reynolds et al., (2001) examined the influence of fermentation temperature with combinations
of no EM or pre- and/or post-fermentation EM (each for 3 days) on the composition and sensory properties of Shiraz. Wines made by a combination of pre-fermentation EM, higher fermentation temperatures (30°C as opposed to 15°C or 20°C), and post-fermentation EM had the most color and body of all treatment combinations.
Fulcrand et al., (2004) suggest that the increased maceration period offered by pre- and post-fermentation EM is more effective at extracting phenolics than the manipulation of temperature. Others may disagree due to the increased rate of phenolic extraction for red wine at higher temperatures.
1.3.2 Measurement of red wine color and phenolic composition.
There are a number of methods available to measure wine color and phenolic composition. Wine color and total phenolic content are parameters easily estimated by spectrophotometry (Bakker et al., 1986, Heredia et al., 2006, Somers and Evans, 1977, Versari et al., 2008), allowing changes in wine color and phenolic content to be monitored over the life of a wine. Analyses are based on the absorbance of light at characteristic wavelengths. Red pigment concentration is measured at a wavelength of 520 nm and yellow/brown pigment concentration, including oxidation products, is measured at 420 nm. Wine color density (WCD), has been defined as the sum of the optical density (OD) at 420nm and 520nm where OD420 is defined as the yellow/brown colored wine pigments/tannins and OD520 as the monomeric and polymeric red colored pigments. WCD is measured in absorbance units (a.u.) and describes the wine color intensity. Color hue (WCH) = A420 nm/ A520 nm, the ratio of yellow/brown to red pigments, is a measure of wine tint (Glories 1984, Somers and Evans 1977). Increasingly, the absorbance value of the sample at a wavelength of 620 nm (purple/blue pigment concentration) is being incorporated into WCD measures, especially of young wines because as wines age the absorbance at 620 nm decreases (Gomez-Plaza et al. 2006, Kelebek et al. 2006, Lorenzo et al. 2005, Parenti et al. 2004).
Wine color density and hue are just 2 of the characteristics of red wine used to describe its color; degree of red pigment coloration, estimate of the concentration of SO2 resistant pigments, and total red pigments are others (Somers and Evans, 1977).
The absorption of ultraviolet light at 280 nm enables the quantification of all phenolic compounds present in the wine (Somers and Evans, 1977). Measuring the absorbance of a wine at 280nm is one of the simplest methods for estimating total phenolic content because ultraviolet absorbance is not pH dependent (Harbertson and Spayd, 2006). By measuring wine absorbance at this wavelength, however, non-phenolic compounds will also absorb light and therefore are included in the estimation of total phenolic content (Somers, 1998). As such, Somers and Evans, (1977) used the expression OD280 – 4, as an index of total polyphenols in red wine. This substraction generates a more accurate
estimation of the wine’s total phenolics content (Somers, 1998).
The Folin-Ciocalteau spectrophotometric assay for determining wine total phenols (and other easily oxidized substances) is also widely used (Gollucke et al., 2008, Roginsky et al., 2006, Singleton et al., 1999, Vrhovsek et al., 2001).
A drawback of spectrophotometric assays is that they do not provide a breakdown of the phenolic compounds present in wine. Reversed-phase HPLC can isolate wine phenolics and is routinely used in grape and wine analysis to generate concentration values for flavan-3-ols including (+)-Catechin and (-)-epicatechin) (Auw et al., 1996, Ricardo-da-Silva et al., 1993, Downey et al., 2007, Oszmianski et al., 1986), and anthocyanins (Gomez-Miguez and Heredia, 2004, Parley et al., 2001, Bakker et al., 1986, Gao et al., 1997, Prajitna et al., 2007, Downey et al., 2007, Gomez-Plaza et al., 2006, Morata et al., 2007, Cortell et al., 2007).
Although RP-HPLC enables the quantification of complex compounds in 1 step, but the investigation of wine polymeric pigments and tannin’s concentration using this technique is limited, due to the fact that wine polymeric compounds have long retention times, making separation of high molecular weight polymeric procyanidins difficult to achieve (Peng et al., 2002, Versari et al., 2008). However, simple wine phenolics such as anthocyanins and flavanols, which elute from an RP-HPLC column as single peaks and without interference, are more easily detected and quantified using this method (Bakker et al., 1986).
2. Materials and methods
2.1 Experimental winemaking, study of pre- and post-fermentation EM of red wine made
from V. Vinifera Tempanillo.
Grapes from Tempranillo grape cultivar from the vineyards of the Riverland Vine Improvement Committee (RVIC) were sampled in order to determine the phenolic compounds, and then four different wines with three replicates were produced, from February 2012 through April 2012, in order to study the effects of pre-fermentation EM and post-fermentation EM.
The four wine treatments of Tempranillo included a 5 day cold soak kept in a 10 degrees Celsius room under dry ice (PF5), a Control (C), a 3days fermentation EM (EM3), and a 6days post-fermentation EM (EM6). Wines were analysed at 10days post crush.
Spectrophotometric, Reversed-phase High Performance Liquid Chromatography (RP-HPLC), were used to analyse treatments on the basis of color, concentrations of anthocyanins, phenolics, and (+)-catechin, (-)-epicatechin.
Before harvest, the grapes were tasted every week, from the middle of February, and every 2 days close to harvest. A sample of grapes was also taken, in order to measure the baumé, and decide the harvest date. Target baumé, was 14.5.
The grapes were picked by hand in small buckets on the 17th of March 2012, and were put into a container, where they stayed overnight at 10 degrees Celsius. Grapes samples were taken for analysis prior to crushing (baumé, pH, TA).
The next morning the grapes were destemmed and crushed, and equally distributed in 100liters plastic bins with lid. In the end of the process, each bin contained 50liters of crushed Tempranillo grapes.
12 plastic bins were used, representing four different treatments, with three replicates each.
Three of them represented the pre- fermentation maceration technique, and spent 5 days on skins, at 10 degrees Celsius. Then the three bins were left on the sun to warm up prior to the inoculation and the ALF begun. The wine was separated from the skins, immediately after the end of the ALF. Another three represented the control, which did not undergo any pre- or post- fermentation maceration.
Three bins represented the EM technique, and did not undergo any pre-fermentation maceration, but were left in contact with the skins for 3 days after the end of the ALF.
The last three bins, also represented the EM technique, and were left in contact with the skins for 6 days after the end of the ALF.
The four different treatments permitted to study the effect of the different macerations on the color and the tannic profile of the wines.
All the bins were plunged 10 times twice a day, and the temperature and must density were monitored periodically during the ALF. The fermentation temperature reached 26 degrees Celsius during the day, and dropped to 11 degrees at night. The aim though was successfully maintained, and the duration of the ALF was 7 days for all the treatments.
Figure 2-1: Flow-chart showing differences between winemaking treatments, where PF is the pre-fermentation maceration treatment, C is the control treatment, EM3 is the 3days post-pre-fermentation extended maceration treatment, and EM6 the 6days post-fermentation extended maceration treatment.
A unique doses of 50mg/L PMS (potassium metabisulfite) was added right after crushing.
All bins, received 35g/hl saccharomyces cerevisiae 796 yeast. After the beginning of the ALF, and after 2 baumé drop, 28g/hl Dynastart was added, and at 10 baumé there was a final addition of 28g/hl Bioactive. (In the middle of the ALF, there was a presence of Hydrogen Sulphite (H2S) odour, indication of lack of aminoacids and/or ammonia (NH3). After the addition of Bioactive, and some aeration during plunging, the odour dissapeared).
The target pH was 3.6, thus, some tartaric acid was necessary to add.
Samples for analysis were collected from the starting musts (0 days), and every 2 days during maceration, until the separation from the skins and the end of the ALF.
The samples were kept frozen, at -20 degrees Celsius until the day of analysis.
The control and the two EM treatments were separated from the skins, and the skins were pressed using a basket press. The final wine did not contain any pressings.
After the draining, the wine was kept into glass bottles, at 12 degrees Celsius in order to form the sediment, and was seeded with lactic acid bacteria (LAB) VP41 from Lallemand. Two days later, when 20% of the malic acid was degraded to lactic acid, SO2 (PMS) was added into the wines and the wine was racked allowing some natural aeration.
After settling, racking and bottling in 0.75L bottles with screw cap closure, the wines were stored at cellar temperature until the day of wine tasting at the Adelaide University, in June 2012.
2.2. Red wine color and spectrometric measures
During the period of maceration wine samplings from each treatment were taken, and at 5 months post-crush, samples of the wines were subjected to analysis of pH, TA, color density, color hue, degree of red pigment coloration, SO2 resistant pigments, total red pigments, total phenolics, and total anthocyanins The analysis was carried out according to the methods described by Iland et al., 2004a, p88-91) and developed by Somers and Evans (Somers and Evans, 1974, Somers and Evans, 1977).
During winemaking, the wine color density (color intensity), the hue, and the phenolics were determined by the method of Somers and Evans, using a spectrophotometer with 2mm and 10mm path-length cuvette. All values were converted to those obtained with a 10mm light-path cuvette. The wines were measured at natural wine pH, and at a common pH of 3.5.
The wine samples that were stored at -20 degrees Celsius, were left to warm up, and the measurements were taken at room temperature. Quartz cuvettes of 2mm and 10mm were used.
2.3 Reversed-phase High Performance Liquid Chromatography (RP-HPLC)
Tempranillo final wines, were analysed in order to compare the consequences of the EM treatments between treatments. By comparing each peak’s retention time with that of injected reference standards in the same chromatographic system (same mobile and stationary phase), a chromatographer may be able to identify each compound.
HPLC method:
The wine samples were centrifuged at 3,500 x g for 5 mins. The supernatant was filtered through 30mm, 0.45µm PVDF syringe filter (Agilent Technologies). The prepared wine samples were injected into a Hewlett Packard HP1100 high-performance liquid chromatograph with Agilent ChemStation software for LC 3D systems with auto-sampler.
The Column was Gemini 3µ C18 110A (150 x 4.6 mm) (from Phenomenex). The Guard column was Gemini C18 (4 x 3.0 mm) Security Guard cartridge.
The mobile phase was made with combinations of Solvent A and Solvent B. Buffer A consisted of 10% (v/v) formic acid, and Buffer B was 100% methanol. All chromatographic solvents were high-performance liquid chromatography (HPLC) ultra gradient grade.
Column was equilibrated in 83% Solvent A and 17% Solvent B. The column temperature was maintained at 40°C.
The flow rate of the solvent was 1 mL/min starting with 17% v/v of Buffer B, which was maintained for 1 minute and was then increased up too 35% of Buffer B over 14 minutes.
100% methanol was used to clean the column between runs. Diode Array Detector recorded absorbance at 520 nm and 280 nm.
The retention times were determined using standard solutions of anthocyanins glucosides (dissolved in 83% Solvent A and 17% Solvent B):
Malvidin-3-glucoside, Delphinidin-3-glucoside, Peonidin-3-glucoside, Cyanidin-3-glucoside, (+)-Catechin, and (-)-Epicatechin were identified and quantified from standards.
The absorbance of all anthocyanins was monitored and calibrated at 520 nm.
Standard curve series generated by serial dilutions of 1.0 mg/ml standards. Standard concentrations ranged between 0.05 to 1 mg/ml, (gr/L).
3. Results
3.1. Wine analysis results and discussion (Phenolic spectroscopic measures, RP-HPLC, and total anthocyanin measures)
Pre-fermentation extended maceration (EM) is reported to increase the color intensity and fruit flavor of red wines whereas post-fermentation EM is reported to improve colour stability and extract more tannins and total phenolics into the wine.
3.1.1. Spectroscopic measures: After the inoculation, there was a significant increase of the chroma (which is correlated to wine color density) of all treatments. After the inoculation, the increase was exponential, and continued increasing until the beginning of post-fermentation EM, which agrees with previous findings of Bakker. (Bakker et al., 1986).
The Tempranillo grape must, gradually acquired color from the beginning of fermentation to its end (7th day).
After the end of the ALF and during the post fermentation EM period, the color density of the Tempranillo started decreasing. The EM reduced the color intensity and slightly increased the color hue, resulting in a browner hue. Indeed, during red wine maturation, wine color hue moves towards browner colors and color density decreases. The changes are observed through a decrease in absorbance at 520 nm and an increase in absorbance at 420 nm (Somers and Verette, 1988).
to wine color density) from the Control, the EM3 and the EM6 treatments. The EM3 and EM6 treatments had a slightly higher Chroma from the Control treatment. (graph 3-1). Highest Chroma values for each treatment occurred during alcoholic fermentation, these values then slowly but significantly decreased. Although the wines were young at the time of analysis, these results indicate that post-fermentation EM is not necessarily resulting in increasing colour expression. Therefore, increasing the skin maceration time to more than 7 days had no significant effect on colour extraction, which agrees with previous findings (Ribereau-Gayon et al., 1994).
Graph 3-1.3, shows that the total phenolics increase with increased skin contact time. PF, EM3 and EM6 treatments showed higher values from the Control.
Graph 3-1.1.a: Graph showing the Evolution of
Graph 3-1.1.b: Graph showing the Evolution of
Color Hue during ALF, from crush to end of ALF, resulted from the Spectral analysis.
Graph 3-1.1.c: Graph showing the Evolution of
Graph 3-1.1.d: Graph showing the Evolution of
Total Phenolics during ALF, from crush to end of ALF, resulted from the Spectral analysis.
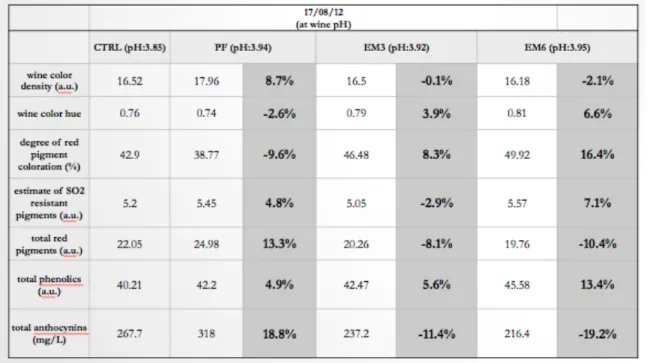
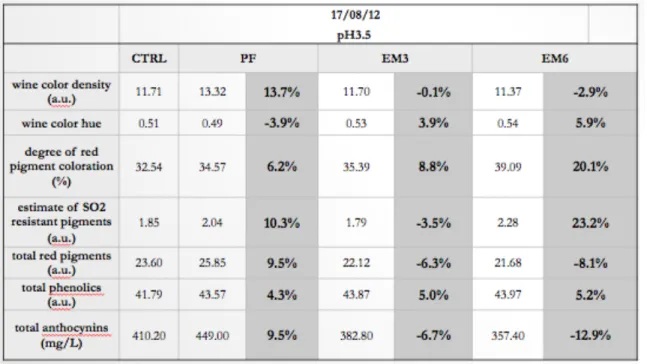
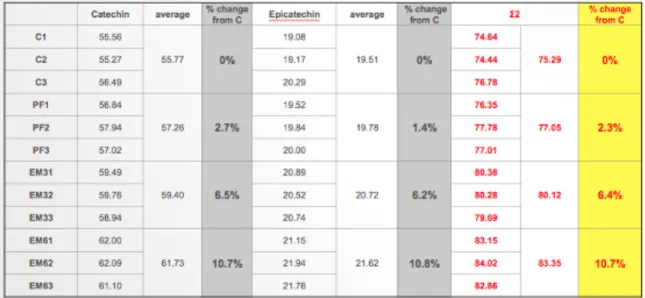
At 5 months post-crush, Tempranillo wines were analysed for color, total phenolics, total anthocyanins and for an estimate of the concentration of pigments resistant to SO2 bleaching. The values reported on tables 3-2 and 3-3, are the mean for the 3 replicates in each treatment. Total phenolics (Somers) and the estimate of SO2 resistant pigments are expressed as absorbance units (a.u.), total anthocyanins are expressed as mg/L. (table 3-3).
Results in Table 3-2 and 3-3 shows that the PF treatment was significantly lower in total phenolics than the EM treatments. There was no significant difference between treatments for the estimate of SO2 resistant pigments.
The values of these measurements for comparing multiple wines of the same pH (adjusted to 3.5 in this case), is of important use, because wine color is pH dependent. These color measurements would be much more useful however, when tracking the change in color over the life of a wine.
Anthocyanins are the primary phenolic compounds responsible for the color of red wines. These compounds are synthesized in grape skins during ripening.
On tables 3-2 and 3-3, and according to Somers Spectral analysis, the PF treatment showed the highest values of anthocyanins. Cheynier et al., (1997a) also found that pre-fermentation EM increased the extraction of skin-derived anthocyanins.
Furthermore, in this study, the spectroscopic results of anthocyanins, showed a degradation of anthocyanins during the EM. The EM6 treatment had the lowest amount of anthocyanins.
During fermentation and aging the concentration of monomeric anthocyanins decreases due to hydrolytic reaction and by binding to other phenolic constituents, particularly tannin. These anthocyanin-tannin complexes are known as polymeric anthocyanins and form the basis for stable wine color (ETS Laboratories, CA).
Table 3-2: Spectral analyses of colour, SO2 resistant pigments, total phenolics and total anthocyanins for 2012 Tempranillo, at natural pH, and percentage change from the Control.
Table 3-3: Spectral analyses of colour, SO2 resistant pigments, total phenolics and total anthocyanins for 2012 Tempranillo, at common pH:3.5, and percentage change from the Control.
3.1.2. HPLC analysis of wine
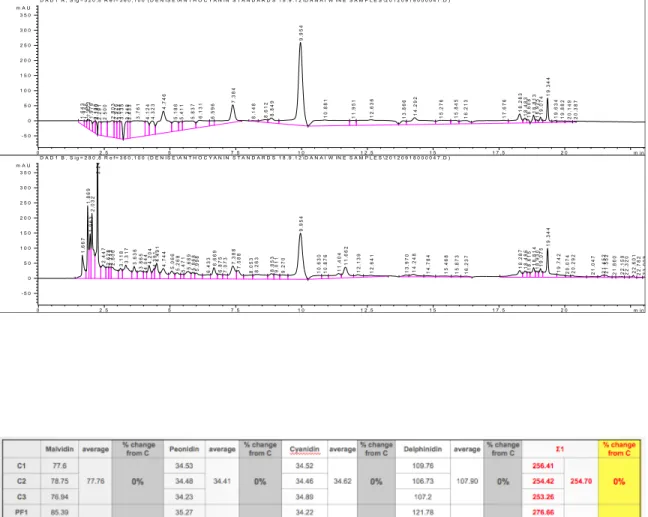
Wines were analysed for anthocyanins, (+)-catechin, (-)-epicatechin at about 6 months post-crush. The results are presented in tables 3.9 and 3.10.
RP-HPLC chromatograms of Tempranillo anthocyanins, (+)-catechin, and (-)-epicatechin for all treatments and 3 replicates, showing retention times (RT) and absorbance (mAU).
On the top is for anthocyanins at 520nm, and on the Bottom is for catechin & epicatechin at 280 nm. PF_replicate 1 m i n 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 1 0 0 0 1 0 0 2 0 0 3 0 0 D A D 1 A , S i g = 5 2 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 3 8 . D ) 1 .6 5 9 1 .8 0 9 1 .8 9 1 1 .9 9 9 2 .1 6 3 2 .3 1 1 2 .5 0 8 2 .8 2 2 2 .9 5 1 3 .0 5 9 3 .1 5 8 3 .3 8 2 3 .4 5 5 3 .6 4 2 3 .9 0 0 4 .1 4 1 4 .3 4 6 4.7 0 9 5 .2 0 1 5 .4 0 4 5 .7 8 8 6 .0 9 0 6 .5 3 1 6 .7 4 5 6 .9 5 8 7 .2 5 5 8 .1 0 0 8 .5 0 1 8 .7 5 8 9 .8 8 2 1 0 .8 2 4 1 1 .5 3 6 1 1 .9 0 3 1 2 .4 7 1 1 3 .7 2 3 1 4 .0 0 2 1 5 .0 3 2 1 5 .4 3 2 1 5 .7 9 3 1 7 .0 6 9 1 7 .8 2 2 1 8 .1 4 7 1 8 .3 3 0 1 8 .6 3 1 1 8 .7 5 4 1 8 .9 2 3 1 9 .2 4 1 1 9 .6 5 4 1 9 .7 9 1 2 0 .0 5 4 2 0 .2 6 6 2 0 .3 8 8 2 0 .8 3 8 m i n 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 1 0 0 0 1 0 0 2 0 0 3 0 0 D A D 1 B , S i g = 2 8 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 3 8 . D ) 1 .6 8 2 1 .8 8 9 1 .9 8 6 2.0 5 5 2 .2 7 0 2 .4 6 6 2 .6 4 9 2 .7 3 2 2 .8 2 1 3 .1 4 7 3 .3 3 8 3 .6 6 0 3 .9 0 2 4 .2 2 7 4 .5 2 1 4 .7 0 6 5 .0 6 4 5 .2 5 1 5 .4 7 9 5 .6 8 4 5 .8 4 6 5 .9 9 6 6 .3 5 8 6 .6 3 3 6 .8 2 4 7.25 6 7 .4 5 1 7 .5 5 5 7 .9 9 2 8 .2 3 6 8 .7 6 0 9 .0 0 4 9 .2 8 8 9 .8 8 2 1 0 .5 9 6 1 0 .8 3 5 1 1 .1 8 8 1 1 .3 9 8 1 1 .7 1 1 1 2 .0 8 7 1 2 .4 7 6 1 3 .0 3 1 1 3 .8 2 8 1 4 .0 1 8 1 4 .6 8 9 1 5 .0 2 4 1 5 .4 3 6 1 5 .6 8 9 1 5 .9 2 6 1 7 .0 7 6 1 7 .8 2 5 1 8 .1 4 9 1 8 .3 3 9 1 8 .6 3 2 1 8 .7 5 5 1 8 .9 2 4 1 9 .2 4 1 1 9 .6 4 7 1 9 .9 6 7 2 0 .1 8 6 2 0 .9 6 3 2 1 .3 8 2 2 1 .8 1 1 2 2 .1 1 6 2 2 .2 8 1 2 2 .5 7 4 2 2 .7 5 6 2 2 .9 8 5
PF_replicate 2
PF_replicate 3
m i n 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 5 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 3 5 0 D A D 1 A , S i g = 5 2 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 3 9 . D ) 1 .6 6 1 1 .8 1 0 1 .8 9 3 1 .9 9 3 2 .1 6 5 2 .3 1 3 2 .5 1 3 2 .8 3 5 2 .9 6 6 3 .0 7 4 3 .1 7 8 3 .4 0 3 3 .4 7 6 3 .6 7 8 3 .9 3 3 4 .1 7 0 4 .3 7 5 4 .7 6 3 5 .2 3 9 5 .4 4 9 5 .8 4 2 6 .1 3 4 6 .5 8 5 6 .8 0 3 7 .0 2 9 7 .3 1 6 8 .1 4 7 8 .5 2 3 8 .7 7 4 9 .8 6 1 1 0 .8 3 7 1 1 .9 1 6 1 2 .5 6 2 1 3 .8 0 9 1 4 .0 8 2 1 5 .1 0 5 1 5 .4 7 1 1 5 .9 7 2 1 6 .9 5 0 1 7 .6 4 2 1 8 .0 0 6 1 8 .2 2 1 1 8 .5 4 9 1 8 .6 9 8 1 8 .8 8 7 1 9 .2 1 1 1 9 .6 4 4 1 9 .8 1 4 2 0 .0 5 8 2 0 .2 6 0 2 0 .3 9 0 m i n 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 5 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 3 5 0 D A D 1 B , S i g = 2 8 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 3 9 . D ) 1 .5 5 6 1 .6 8 5 1 .8 9 1 1 .9 8 6 2 .0 5 5 2 .2 7 2 2 .4 7 7 2 .6 5 9 2 .7 3 7 2 .8 3 6 3 .1 6 3 3 .3 6 0 3 .6 8 4 3 .9 3 2 4 .1 0 1 4 .2 5 1 4 .5 4 4 4 .7 6 1 5 .1 0 0 5 .2 9 6 5 .5 2 6 5 .7 3 5 5 .8 9 9 6 .0 5 0 6 .4 1 7 6 .6 8 4 6 .8 8 3 7.3 1 7 7 .5 0 9 7 .5 9 8 8 .0 3 8 8 .2 8 2 8 .7 7 5 9 .0 5 7 9 .3 0 1 9 .8 6 1 1 0 .5 9 1 1 0 .8 4 2 1 1 .4 4 0 1 1 .7 3 5 1 2 .1 1 4 1 2 .5 6 9 1 3 .9 0 1 1 4 .1 0 7 1 4 .7 5 6 1 5 .1 0 2 1 5 .4 7 7 1 5 .6 9 7 1 5 .9 5 3 1 6 .9 4 1 1 7 .6 3 8 1 8 .0 1 0 1 8 .2 2 9 1 8 .5 5 0 1 8 .7 0 0 1 8 .8 8 7 1 9 .2 1 1 1 9 .6 6 4 1 9 .8 2 7 1 9 .9 5 8 2 0 .1 8 2 2 0 .9 5 4 2 1 .3 8 5 2 1 .8 1 5 2 2 .1 1 9 2 2 .2 8 6 2 2 .5 7 7 2 2 .7 5 7 2 2 .9 8 4 m in 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 1 0 0 0 1 0 0 2 0 0 3 0 0 D A D 1 A , S ig = 5 2 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 4 0 . D ) 1 .6 6 0 1 .8 0 7 1 .8 9 0 1 .9 7 9 2 .1 5 9 2 .3 0 4 2 .4 9 3 2 .8 0 9 2 .9 4 4 3 .0 5 3 3 .1 5 2 3 .3 8 1 3 .6 1 8 3 .8 7 6 4 .1 3 6 4 .3 4 9 4.7 2 3 5 .2 2 0 5 .4 2 7 5 .8 2 7 6 .1 3 9 6 .5 8 4 6 .8 0 2 7 .0 2 2 7 .3 1 4 8 .1 5 3 8 .5 3 7 8 .7 8 5 9 .8 6 0 1 0 .7 7 1 1 1 .8 4 8 1 2 .4 6 8 1 3 .7 7 9 1 4 .0 9 9 1 5 .1 8 2 1 5 .5 4 2 1 5 .8 6 1 1 7 .1 8 1 1 7 .9 8 6 1 8 .2 9 4 1 8 .4 3 6 1 8 .7 0 4 1 8 .8 1 6 1 8 .9 7 4 1 9 .2 7 0 1 9 .5 4 6 1 9 .6 8 4 1 9 .8 1 4 2 0 .0 7 1 2 0 .2 9 6 2 0 .3 9 7 m in 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 1 0 0 0 1 0 0 2 0 0 3 0 0 D A D 1 B , S ig = 2 8 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 4 0 . D ) 1 .6 8 3 1 .8 8 8 1 .9 8 0 2 .0 4 9 2 .2 6 6 2 .4 6 0 2 .6 4 2 2 .7 3 2 3 .1 4 3 3 .3 3 2 3 .6 5 2 3 .8 7 2 4 .0 5 3 4 .2 2 1 4 .5 2 3 4 .7 2 0 5 .0 7 9 5 .2 7 2 5 .5 0 5 5 .7 1 9 5 .8 8 4 6 .0 4 0 6 .4 1 1 6 .6 8 4 6 .8 8 2 7 .3 1 5 7 .5 0 8 7 .6 0 8 8 .0 5 2 8 .2 9 2 8 .7 8 6 9 .0 6 5 9 .3 1 6 9 .8 6 0 1 0 .5 5 2 1 1 .1 0 8 1 1 .3 7 4 1 1 .6 7 9 1 2 .0 3 4 1 2 .4 7 6 1 3 .8 7 8 1 4 .1 0 6 1 4 .7 5 0 1 5 .1 7 0 1 5 .5 6 0 1 5 .8 0 8 1 7 .9 9 3 1 8 .2 9 6 1 8 .4 4 8 1 8 .7 0 5 1 8 .8 1 7 1 8 .9 7 4 1 9 .2 7 0 1 9 .6 7 4 1 9 .9 9 4 2 0 .2 0 5 2 0 .9 8 1 2 1 .4 1 3 2 1 .5 1 6 2 1 .8 3 4 2 2 .1 3 7 2 2 .3 0 3 2 2 .5 9 3 2 2 .7 7 4 2 3 .0 0 0
C_replicate 1
C_replicate 2
m in 2 .5 5 7 .5 1 0 1 2 .5 1 5 1 7 .5 2 0 m A U - 5 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 D A D 1 A , S ig = 5 2 0 ,8 R e f = 3 6 0 ,1 0 0 ( D E N IS E \A N T H O C Y A N IN S T A N D A R D S 1 8 .9 .1 2 \D A N A I W IN E S A M P L E S \2 0 1 2 0 9 1 8 0 0 0 0 3 5 .D ) 1 .6 3 7 1 .7 8 6 1 .8 7 0 1 .9 7 0 2 .1 3 5 2 .2 2 7 2 .2 9 7 2 .4 6 0 2 .7 7 9 2 .9 1 2 3 .0 1 7 3 .1 0 7 3 .3 2 7 3 .4 0 2 3 .6 1 3 3 .8 6 6 4 .0 8 5 4 .2 8 5 4.6 5 1 5 .1 3 9 5 .3 4 5 5 .5 3 2 6 .0 3 2 6 .4 9 8 6 .9 2 6 7 .2 4 4 8 .0 4 0 8 .4 8 3 8 .7 2 4 9 .8 3 1 1 0 .7 1 8 1 1 .7 9 7 1 2 .4 5 9 1 3 .7 2 4 1 4 .1 2 2 1 5 .2 6 9 1 5 .6 7 2 1 6 .0 0 1 1 7 .4 6 2 1 8 .1 6 9 1 8 .3 7 7 1 8 .5 1 4 1 8 .7 6 1 1 8 .8 7 0 1 9 .0 3 0 1 9 .3 1 9 1 9 .7 1 8 1 9 .8 7 0 2 0 .1 2 7 2 0 .3 3 9 m in 2 .5 5 7 .5 1 0 1 2 .5 1 5 1 7 .5 2 0 m A U - 5 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 D A D 1 B , S ig = 2 8 0 ,8 R e f = 3 6 0 ,1 0 0 ( D E N IS E \A N T H O C Y A N IN S T A N D A R D S 1 8 .9 .1 2 \D A N A I W IN E S A M P L E S \2 0 1 2 0 9 1 8 0 0 0 0 3 5 .D ) 1 .5 3 3 1 .6 6 2 1 .8 6 7 1 .9 6 0 2 .0 2 7 2 .2 4 5 2 .4 3 2 2 .6 1 6 2 .6 9 4 2 .7 9 1 3 .1 0 0 3 .2 8 7 3 .6 0 7 3 .8 7 0 4 .1 7 9 4 .3 6 3 4 .4 6 5 4 .6 4 7 5 .0 0 7 5 .1 9 1 5 .4 0 3 5 .6 2 5 5 .7 9 8 5 .9 2 2 6 .3 4 4 6 .5 9 3 6 .7 9 1 7.2 4 4 7 .5 1 2 7 .9 3 5 8 .1 5 6 8 .4 7 1 8 .7 2 7 8 .8 9 8 9 .1 6 5 9 .3 1 3 9 .8 3 1 1 0 .5 6 0 1 1 .2 4 3 1 1 .5 6 7 1 2 .0 0 1 1 2 .4 7 4 1 3 .2 1 0 1 3 .8 6 5 1 4 .0 9 9 1 4 .3 4 3 1 4 .6 8 4 1 5 .2 8 0 1 5 .6 9 0 1 8 .1 7 2 1 8 .3 7 8 1 8 .5 2 3 1 8 .7 6 2 1 8 .8 7 3 1 9 .0 3 0 1 9 .3 1 9 1 9 .7 3 0 2 0 .0 4 4 2 0 .2 6 5 2 1 .0 2 1 2 1 .4 4 0 2 1 .5 4 2 2 1 .8 6 2 2 2 .1 5 6 2 2 .3 1 4 2 2 .5 9 3 2 3 .0 1 2 m i n 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 5 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 D A D 1 A , S i g = 5 2 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 3 6 . D ) 1 .6 3 8 1 .7 8 7 1 .8 7 1 1 .9 6 9 2 .1 3 6 2 .2 2 1 2 .2 8 7 2 .4 5 5 2 .7 8 0 2 .9 0 9 3 .0 1 2 3 .1 0 6 3 .3 2 6 3 .4 0 0 3 .5 9 9 3 .8 5 5 4 .0 8 7 4 .2 8 5 4.6 5 4 5 .1 6 0 5 .3 6 2 5 .5 5 1 6 .0 5 3 6 .5 2 4 6 .9 5 6 7 .2 6 6 8 .0 7 4 8 .4 8 5 8 .7 2 6 9 .8 0 9 1 0 .6 8 8 1 1 .4 2 3 1 2 .3 8 1 1 3 .6 5 4 1 4 .0 1 6 1 4 .9 8 7 1 5 .5 4 4 1 5 .8 9 3 1 7 .3 4 2 1 8 .1 0 6 1 8 .3 3 7 1 8 .4 7 9 1 8 .7 4 3 1 8 .8 6 0 1 9 .0 2 5 1 9 .1 8 1 1 9 .3 1 3 1 9 .6 1 7 1 9 .6 8 4 1 9 .8 6 4 2 0 .1 2 1 2 0 .3 2 5 2 0 .4 6 9 m i n 2 . 5 5 7 . 5 1 0 1 2 . 5 1 5 1 7 . 5 2 0 m A U - 5 0 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 D A D 1 B , S i g = 2 8 0 , 8 R e f = 3 6 0 , 1 0 0 ( D E N I S E \ A N T H O C Y A N I N S T A N D A R D S 1 8 . 9 . 1 2 \ D A N A I W I N E S A M P L E S \ 2 0 1 2 0 9 1 8 0 0 0 0 3 6 . D ) 1 .5 3 7 1 .6 6 3 1 .8 6 8 1 .9 6 0 2 .0 2 7 2 .2 4 4 2 .4 3 4 2 .6 1 5 2 .6 9 5 2 .7 8 3 3 .1 0 0 3 .2 8 5 3 .6 0 6 3 .8 6 0 4 .1 7 7 4 .3 6 6 4 .4 6 8 4 .6 5 1 5 .0 1 8 5 .2 0 6 5 .4 2 3 5 .6 4 6 5 .8 1 7 5 .9 4 7 6 .3 6 7 6 .6 1 9 6 .8 1 9 7.26 5 7 .5 3 4 7 .9 6 7 8 .1 9 3 8 .4 7 7 8 .7 2 8 8 .9 3 6 9 .1 8 7 9 .8 0 8 1 0 .5 1 7 1 1 .2 4 8 1 1 .5 7 3 1 1 .9 6 0 1 2 .4 0 0 1 3 .7 9 2 1 4 .0 1 1 1 4 .2 5 9 1 4 .6 0 4 1 5 .1 4 4 1 5 .5 5 6 1 8 .1 0 9 1 8 .3 3 9 1 8 .4 8 9 1 8 .7 4 3 1 8 .8 6 1 1 9 .0 2 5 1 9 .3 1 4 1 9 .7 2 1 2 0 .0 3 2 2 0 .2 5 5 2 1 .4 4 2 2 1 .5 4 0 2 1 .8 5 6 2 2 .1 5 5 2 2 .3 1 6 2 2 .5 9 3 2 3 .0 0 1
C_replicate 3