Universidade do Minho
Escola de Engenharia
Carolina Santos Fernandes
15 Car olina Sant os F ernandes
Biodegradable
poly(trimethylenecarbonate)versus
non-biodegradable
poly(methyl methacrylate) beads as
antibiotic delivery devices in the treatment
of diabetic foot osteomyelitis
Biodegradable pol y(trime th ylenecarbonate) versus non-biodegradable pol y(me th yl me thacr ylate) beads as antibio tic deliver y de vices in the treatment of diabe tic foo t os teom yelitis
Universidade do Minho
Escola de Engenharia
Carolina Santos Fernandes
Biodegradable
poly(trimethylenecarbonate)versus
non-biodegradable
poly(methyl methacrylate) beads as
antibiotic delivery devices in the treatment
of diabetic foot osteomyelitis
Dissertação de Mestrado
Mestrado Integrado em Engenharia Biomédica
Ramo de Engenharia Clínica
Trabalho efetuado sob a orientação da
Professora Lígia Rodrigues
e da
Acknowledgements
I would like to thank Dr. Lígia Rodrigues for all the support, guidance and constant presence. I am extremely grateful for each and every advice and incentive that pushed me to do the best work that I could.
To Dr. Danielle, I would like to express that I am deeply thankful for the privilege to be working under her supervision during the development of my research work. I want to thank for all the help, inspiration, patience and understanding. It was a pleasure for me, to work along with such a wonderful person that besides all the teaching and besides sharing her knowledge with me, also always made me feel at home and integrated in a different country.
I would like to thank Dr. Miguel Gama for all the support and encouragement to go abroad and to embrace this experience.
To my Biomedical Engineering colleagues I would like to thank for your help, support and most of all, for your friendship.
Finally, I want to express special thanks to my family that are the most important persons in the world for me and to all my friends. Thank you for being so amazing and for being always by my side.
Abstract
Osteomyelitis is a frequent complication of diabetic foot ulcers and/or diabetic foot infection. It is defined as an infection of bone tissue and it is an aggravated condition in diabetic patient due to their poor blood flow and elevated blood glucose levels, which can increase biofilm formation. In general, osteomyelitis cannot be eradicated merely by intravenous administration of antibiotics. A successful treatment will include a combination of surgical procedures, usually surgical debridement of dead infected tissue and local antibiotic therapy. Non-degradable delivery devices are currently used for local antibiotic administration. The poly(methylmethacrylate) (PMMA) is widely used to develop gentamicin-loaded beads that are implanted on the local of infection. Even though these beads are routinely used to treat osteomyelitis, they present several disadvantages, including the requirement for a second surgery to remove the beads after treatment. Hence, the use of biodegradable devices for antibiotic delivery in osteomyelitis is a very attractive alternative. The poly(trimethylene carbonate) (PTMC) is an enzymatically biodegradable polymer that does not produce acidic degradation products. In the current thesis, the suitability of PTMC to function as an antibiotic delivery device for the local treatment of osteomyelitis was explored. Gentamicin-loaded PTMC beads were produced and their release kinetics was assessed. The inhibition of biofilm formation was also evaluated for these beads and compared with the commercially available PMMA Septopal® beads. Additionally, the glucose influence in biofilm growth was also studied, namely medium with and without 2.6 g/L concentration of glucose was used to grow the biofilm The results showed that the mass of biofilm (Enterobacter cloacae, Staphylococcus aureus) increased when glucose was present in the culture medium, which may explain the difficulties that are observed in the treatment of diabetic foot osteomyelitis. The antibiotic release profile and inhibition of biofilm formation by the PTMC beads was found to be similar to the PMMA beads. Therefore, PTMC seems a promising biodegradable carrier to be used in the local treatment of osteomyelitis.
Resumo
A osteomielite é uma complicação frequente em úlceras e/ou infeção do pé diabético. É definida como uma infecção do tecido ósseo, agravada em pacientes diabéticos devido ao baixo fluxo sanguíneo e elevado nível de glucose no sangue, o que pode contribuir para a formação de biofilme. Em geral, a osteomielite não pode ser erradicada meramente com administração intravenosa de antibióticos. Um tratamento bem-sucedido inclui uma combinação de procedimentos cirúrgicos, geralmente remoção cirúrgica do tecido infectado e morto, seguido de terapia antibiótica local. Dispositivos de administração não-biodegradáveis são actualmente utilizados para a administração de antibióticos no local infectado. O poli (metacrilato de metilo) (PMMA) é amplamente utilizado para desenvolver sistemas de libertação controlada de antibióticos, e estes são implantados no local infetado. Embora as partículas de PMMA sejam utilizadas com frequência no tratamento da osteomielite, estas apresentam várias desvantagens, incluindo a exigência de uma segunda cirurgia para as remover, após o tratamento. A utilização de dispositivos biodegradáveis para a libertação de antibióticos no tratamento da osteomielite é uma alternativa muito atraente. O poli (carbonato de trimetileno) (PTMC) é um polímero enzimaticamente biodegradável que não produz produtos de degradação ácidos. No presente trabalho foi explorada a adequação de PTMC para funcionar como um dispositivo de administração de antibiótico para o tratamento local da osteomielite. Foram produzidas partículas de PTMC carregadas com gentamicina e a cinética de libertação do antibiótico foi avaliada. Adicionalmente, a inibição da formação de biofilme foi estudada e comparada com as partículas de PMMA Septopal® comercialmente disponíveis. Além disso, a influência da glucose no crescimento de biofilme também fez parte deste estudo e por conseguinte, desenvolveu-se um biofilme em meio sem e com glucose com uma concentração de 2.6 g/L Os resultados mostraram que a massa de biofilme (Enterobacter cloacae, Staphylococcus aureus) aumentou na presença de glucose no meio de cultura, o que pode explicar a dificuldade observada no tratamento da osteomielite do pé diabético. O perfil de libertação do antibiótico e a inibição da formação de biofilme para as partículas de PTMC foi semelhante ao encontrado para as partículas de
PMMA. Nesse sentido, as partículas de PTMC parecem ser um transportador biodegradável promissor no tratamento local de osteomielite.
List of Contents
Acknowledgements iii Abstract v Resumo vii List of Contents ix List of Tables xiList of Figures xii
List of abbreviations xv
Scope xvi
Chapter 1. Introduction 1
1.1. DIABETIC FOOT ULCERS – AETIOLOGYAND PRECIPITATING FACTORS 1
1.2. CLASSIFICATION OF DIABETIC FOOT ULCERS 4
1.3. CLASSIFICATION OF INFECTION 6
1.4. OSTEOMYELITIS PATHOPHYSIOLOGY 8
1.5. TREATMENT OF OSTEOMYELITIS 10
LOCAL ANTIBIOTIC THERAPY 11
1.6. NON-BIODEGRADABLE ANTIBIOTIC DELIVERY SYSTEMS: PMMA BASED 14
1.7. PMMA CARRIERSTO TREAT OSTEOMYELITIS 16
BIODEGRADABLE ANTIBIOTIC DELIVERY SYSTEMS: PTMC BASED 17
Chapter 2. Materials and Methods 21
2.1. MICROORGANISMS AND GROWTH CONDITIONS 21
2.2. SEPTOPAL® 21
2.3. GENTAMICIN LOADED PTMC BEADS 22
2.4. RELEASEOF GENTAMICIN 23
2.5. MINIMUM INHIBITORY CONCENTRATIONAND GROWTH CURVES 24
2.6. BIOFILM GROWTH 25
2.7. BIOFILM MODEL 26
2.7.1. PREVENT BIOFILM 27
2.7.2. BIOFILMS TREATMENT 28
2.8. ANTI-BIOFILM EFFICACY 29
2.8.1. PREVENTIONOF BIOFILM GROWTH 29
2.8.2. TREATMENT OF BIOFILMS 29
2.9. STATISTICAL ANALYSIS 30
Chapter 3. Results and Discussion 32
3.1. RELEASE PROFILE FROM PTMC VERSUS PMMA BEADS 32
3.2. CLINICALLY ISOLATED STRAINS 37
3.3. BIOFILM GROWTH 38
3.3.1. PREVENTIONOF BIOFILM GROWTH 41
3.3.2 TREATMENT OF BIOFILMS 42
Chapter 4. Conclusions and Future Perspectives 52
List of Tables
Table 1. Wagner Ulcer classification system (Taken from [3]). ... 5 Table 2. University of Texas wound classification system (Taken from [3]). ... 5 Table 3. Antibiotic delivery carriers (Taken from [28]). ... 13 Table 4. Minimum inhibitory concentration values (µg/mL) for Staphylococcus .aureus and
List of Figures
Figure 1. Neuropathic ulcer in a typical position, i.e. under second metatarsal head and surrounded by callus (Taken from [6]).
(2)
Figure 2. Ischaemic diabetic foot ulcer (Taken from [7]). (2)
Figure 3. Neuroischemic ulcer (Taken from [7]). (3)
Figure 4. Infected ulceration and osteomyelitis (Taken from [39]). (7)
Figure 5. Chemical structure of the repeating unit of PMMA polymer (Taken from [29]). (14)
Figure 6. Chemical structure of poly(trimethylene carbonate) (Taken from [34]). (18)
Figure 7. Stainless steel molds used to prepare PTMC beads. (22)
Figure 8. Serial dilutions: A- Control wells; B- Gentamicin concentration 5 and 10 µg/mL wells (Adapted from [39]).
(28)
Figure 9. Cumulative gentamicin release from PTMC beads (containing 5 wt % gentamicin) in lipase solution and PMMA beads in PBS buffer. Results correspond to the average of 3 independent assays ± standard deviation.
(33)
Figure 10. Cumulative gentamicin release from PTMC beads (containing 7.5 wt % gentamicin) in lipase solution and PMMA beads in PBS buffer. Results correspond to the average of 3 independent assays ± standard deviation.
(34)
Figure 11. Cumulative gentamicin release from PTMC beads (7.5 wt % gentamicin) in lipase and cholesterol esterase solutions. Results correspond to the average of 3 independent assays ± standard deviation.
Figure 12. Cumulative gentamicin release from PTMC beads (7.5 wt% gentamicin) in cholesterol esterase solution and PMMA beads in PBS buffer. Results correspond to the average of 3 independent assays ± standard deviation.
(36)
Figure 13. Growth curves (A) Enterobacter cloacae; (B) Staphylococcus aureus, in NB medium with (+Glucose) and without glucose (-Glucose).
(38)
Figure 14. Enterobacter cloacae and Staphylococcus aureus biofilm growth after 24 h in NB medium without (- Glucose) and with 0.26 % of glucose (+ Glucose). (1) MTT staining; (2) Crystal violet staining. Results correspond to the average of 6 counts ± standard deviation.
(40)
Figure 15. CFU counts after 24 h of treatment with gentamicin 5 μg/mL and 10 μg/mL in NB medium without glucose (- Glucose) and NB medium with glucose (0.26 %) (+ Glucose). (A)
Enterobacter cloacae. Control (without treatment) presents CFU counts of 8.5 in medium
without glucose and 8.9 in medium with 0.26 % of glucose; (B) Staphylococcus aureus. Control (without treatment) presents CFU counts of 8.6 in medium without glucose and 8.8 in medium with 0.26 %. Results correspond to the average of 3 counts ± standard deviation.
(42)
Figure 16. CFU counts of 24 h treatment with gentamicin concentrations 500 μg/mL and 1000 μg/mL in NB medium without glucose (- Glucose) and NB medium with 0.26 % of glucose (+ Glucose). (A) Enterobacter cloacae – control (without treatment) CFU counts of 8.5 in medium without glucose and 9.3 in medium with 0.26 % glucose; (B) Staphylococcus aureus - control (without treatment) CFU counts of 8.7 in medium without glucose and 9.0 in medium with 0.26 % glucose. Results correspond to the average of 3 counts ± standard deviation.
(43)
Figure 17. CFU counts of 48 h treatment with gentamicin concentrations 500 μg/mL and 1000 μg/mL in NB medium without glucose (- Glucose) and NB medium with 0.26 % of glucose (+ Glucose). (A) Enterobacter cloacae – control (without treatment) CFU counts of 8.6 in medium without glucose and 8.9 in medium with 0.26 % glucose; (B) Staphylococcus aureus - control (without treatment) CFU counts of 8.8 in medium without glucose and 8.9 in medium with 0.26 % glucose. Results correspond to the average of 3 counts ± standard deviation.
(44)
Figure 18. CFU counting from elution media of PTMC with no content and elution media of PTMC loaded with 7.5 % gentamicin in cholesterol esterase solution without glucose (- Glucose) and with 0.26 % glucose (+ Glucose). (A) Enterobacter cloacae; (B) Staphylococcus
Figure 19. CLSM overlay images of 24 h old biofilms on polystyrene well plate. (A)
Staphylococcus aureus’s growth in NB medium without gentamicin (medium with and without
glucose). (B) Staphylococcus aureus’s growth in NB medium with gentamicin (medium with and without glucose).
(47)
Figure 20. Staphylococcus aureus CFU counting in the presence of PTMC and PMMA beads in medium without glucose (- Glucose) and with 0.26 % glucose (+ Glucose), after 24 h of treatment. PTMC control (without gentamicin) - 8.2 in medium without glucose and 8.3 in medium with 0.26 % glucose. PMMA control (without bead) - 8.0 in medium without glucose and 8.2 in medium with 0.26 % glucose. Results correspond to the average of 3 counts ± standard deviation.
(49)
Figure 21. Staphylococcus aureus CFU counting in the presence of PTMC and PMMA beads in medium without glucose (- Glucose) and with 0.26 % glucose (+ Glucose), after 48 h of treatment. PTMC control (without gentamicin) – 7.9 in medium without glucose and 8.1 in medium with 0.26 % glucose. PMMA control (without bead) - 8.1 in medium without glucose and 8.2 in medium with 0.26 % glucose. Results correspond to the average of 3 counts ± standard deviation.
List of abbreviations
OM Osteomyelitis
PMMA Poly(methyl methacrylate) PTMC Poly(trimethylene carbonate) EPS Extracellular polymeric substance MIC Minimal inhibitory concentration MMA Methyl methacrylate
PGA Polyglutamic acid PLA Polylactic acid TSB Tryptone soya broth PBS Phosphate buffer saline CFU Colony forming unit
MTT “3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide) CLSM Confocal laser scanning microscopy
Scope
Diabetic foot osteomyelitis treatment includes surgical removal of the infected bone and tissue, followed by implantation of an antibiotic delivery system that is able to provide high local antibiotic concentration for an extended period of time.
PTMC is a biodegradable polymer that presents promising characteristics in the treatment of diabetic foot osteomyelitis as an alternative to the currently used PMMA Septopal® beads.
The goal of this work is to evaluate the suitability of PTMC as an antibiotic delivery device to treat diabetic foot osteomyelitis and compare it to the commercially available option, namely the abovementioned PMMA Septopal® beads.
Pursuing this goal, PTMC beads will be created with the same size and gentamicin content as the PMMA Septopal® ones and the gentamicin release profiles from both these beads will be assessed during 14 days.
A biofilm model for diabetic foot ulcers using clinically isolated bacterial strains will be developed and the glucose influence in the biofilm growth will also be evaluated, given that diabetic patients have elevated blood glucose levels.
At last, the anti-biofilm efficacy will be established for both gentamicin carriers through the use of the biofilm model.
Chapter 1.
I
NTRODUCTION
Diabetes is known for its complications, it increases the risk for many serious health problems. Diabetic foot infections are one of the most common problems in diabetic patients and they are responsible for most of amputation cases in these patients.
1.1. D
IABETIC FOOT ULCERS–
A
ETIOLOGY AND PRECIPITATING FACTORSA diabetic foot ulcer is a thick wound located below the ankle in a diabetic patient. It commonly appears in pressure points such as plantar surfaces, metatarsal heads, heels and big toe. This wound is characterized by a loss of epithelium that extends through the dermis reaching deeper tissues. Chronic ulcers fail to progress through an orderly and timely sequence of repair, and so heal slowly [1].
Foot ulcers are the most common cause of diabetes associated hospital admissions and the lifetime incidence of foot ulceration is around 25 %. This condition is responsible for 85 % of major amputations in diabetic patients [2]. These patients have a 30-fold higher risk of lower limb amputation due to infection, as compared with patients without diabetes [3].
Furthermore, diabetic foot ulcers are associate with a negative psychological and social effect on the patients namely increased family tensions, reduced social activities, limited employment and financial burden [1].
Around half of all foot wounds will become infected during therapy which decreases the quality of life and contribute to a high morbidity and premature mortality among the diabetic patients [1].
Multiple causes contribute to diabetic foot ulceration, predominantly peripheral neuropathy and ischemia from peripheral vascular disease [4].
The peripheral neuropathy is present in more than 80 % of diabetic patients with foot disease, as a consequence of the metabolic disorders [5]. Neuropathy affects peripheral sensation, innervation of the small muscles of the foot and fine vasomotor control of the pedal circulation. The motor neuropathy is characterized by the alteration of the distribution of forces during walking leading to a reactive thickening of skin at sites of abnormal overload. Following, ischemic necrosis of tissues beneath the callus causes the breakdown of the skin and subcutaneous tissue, consequently forming a neuropathic ulcer (Figure 1) [6].
Diabetic patients are twice more prone to have a peripheral arterial disease, which is also a key risk factor for lower extremity amputation [7].
Macrovascular (atherosclerosis) and microvascular disease might cause the foot tissues to become ischemic. The ischemic foot loses its protective sweeting, is dry, red, thin with dystrophic nails and susceptible to the pressure from a shoe or even an adjacent toe leading to the appearance of an ischemic ulcer (Figure 2) [6].
Figure 2. Ischaemic diabetic foot ulcer
(Taken from [7]).
Figure 1. Neuropathic ulcer in a
typical position, i.e. under second metatarsal head and surrounded by callus (Taken from [6]).
Neuroischemia is the combined effect of diabetic neuropathy and ischaemia, by virtue of macrovascular disease and in some cases, microvascular dysfunction impair perfusion in a diabetic foot and a neuroischemic ulcer is formed (Figure 3) [7].
The initial injury to provoke an ulcer, can be caused by acute mechanical or thermal trauma when continuous or repetitive mechanical
stress is applied [6].
Impaired wound healing is a major factor that contributes to the augment of chronic foot ulceration and amputation. Diabetic patients have functional changes in the microcirculation and in cellular activity, the expression of the various growth factors and cytokines that are normally involved in tissue repair and wound healing. A normal process of
wound healing entails a complex interplay between connective tissue formation, cellular activity and growth factor activation; however these physiological processes are altered in these patients thus preventing the ulcer to heal [8].
Nosocomial infection rate has been reported to increase 2.7-fold when glucose blood levels are above 220 mg/dL. Diabetes mellitus has been linked to the development of these infections [9].
Elevated blood glucose causes endogenous glycation leading to cellular dysfunction in soft tissues and decreases cellular and humeral immune response [8].
Polymicrobial infections dominate diabetic foot infections and the diversity of bacterial populations in chronic wounds appears to be important to the chronicity of wounds. The biofilm mode of growth of the infecting organism represents another obstacle to heal a chronic wound [2].
Figure 3. Neuroischemic ulcer (Taken
Previous studies reported that biofilm formation in vitro increases with increasing glucose concentrations through the accessory gene regulator (agr) pathway [8]. Cellular environment containing glucose and at a low pH has been found to increase biofilm formation resulting from the decreased agr expression [10].
Even though the reported data suggests that biofilm formation increases in the presence of higher glucose concentration, only few concentrations were studied in the hyperglycemic range of 100 to 500 mg/dL and additional information about the dose response of biofilm formation to glucose levels in the clinically relevant range of 20 to 300 mg/dL, is still uncovered. The severity of anomalous glucose levels, affecting microbe pathogenicity is important to know specially when dealing with diabetic patients with an infected wound [8].
1.2. C
LASSIFICATIONO
FD
IABETICF
OOTU
LCERSA classification of foot ulcers is important in the selection of comparable populations for urgently needed multicenter trials. In this respect, two international working parties are trying to define a system that describes individual ulcers to develop a classification for audit and research. Even though there is no commonly accepted method for classification or description of foot ulcers yet [6].
Early and assertive diagnostic aids in the development of a proper management plan and can be crucial in preventing the formation of a chronical wound. There are some systems of ulcer classification based on a variety of physical findings such as size, depth, appearance and location. The classification system of Wagner Ulcer (Table 1) is the most well-known being based on wound depth and the extent of tissue necrosis.
Table 1. Wagner Ulcer classification system (Taken from [3]).
Grade Lesion
1 Superficial diabetic ulcer
2
Ulcer extension involving ligament, tendon, joint capsule, or fascia with no abscess or osteomyelitis
3 Deep ulcer with abscess or osteomyelitis 4 Gangrene to portion of forefoot
5 Extensive gangrene of foot
The major drawback of the Wagner’s classification is the fact that it does not consider the presence of ischemia or infection.
The University of Texas proposed a different classification addressing the ulcer depth and presence of infection and ischemia (Table 2). With increasing grade and stage of the wound, the difficulty to heal increases and vascular repair or amputation might be needed [4].
Table 2. University of Texas wound classification system (Taken from [3]). Stages Description
A No infection or ischemia B Infection present
C Ischemia present
D Infection and ischemia present
Grading Description
0 Epithelialized wound 1 Superficial wound
2 Wound penetrates to tendon or capsule
1.3. C
LASSIFICATIONO
FI
NFECTIONWhen an ulcer is formed, interior tissues are exposed to bacterial colonization, which can lead to an infection and this infection can further reach deeper tissues [11].
An infected ulcer must be diagnosed clinically when purulent secretions are present or at least 2 of the cardinal manifestations of inflammation are observed. An early diagnose and curing of the infection often contributes to healing of the ulcer [12].
Once an infection is installed, it typically takes the form of cellulitis, deep-skin and soft-tissue infections, acute osteomyelitis and chronic osteomyelitis.
Cellulitis is defined as an acute infection of the skin involving the subcutaneous tissues. Clinically it is characterized by erythema (>2cm in diameter) in association with purulent discharge, with or without lymphangitis. The diagnostic is generally based on the morphologic features of the lesion and the clinical setting. If drainage or an open wound is present, most probably there is an infection. The infection may be caused by indigenous flora colonizing skin and appendages as Staphylococcus aureus and Streptococcus pyogenes, or by a wide variety of exogenous bacteria [13,14].
Skin and soft-tissue infections are defined as a suppurative microbial invasion of the epidermis and subcutaneous tissues that induce either a local or systemic host response. This type of infections are characterized by induration, erythema, warmth and pain or tenderness and range from mild self-limiting furunculosis to life threatening necrotizing fasciitis [15, 16].
Osteomyelitis (OM) derivate from Greek words: osteon, myelos and itis and it refers to the infection of the bone and bone marrow. Even though bone is normally resistant to bacterial colonization, some events as trauma, surgery or prosthesis may disrupt the bone and lead to an infection [17].
leads to limb amputation; the risk for amputation in acute diabetic infections is four times higher with OM than with soft tissue infection alone. Furthermore, the presence of OM requires a longer duration of antibiotic therapy and a longer duration of hospital stay, thereby raising the hospitalization costs [18].
OM can be found in 15 % of patients with diabetic foot ulcers, and in 20 % of patients with diabetic foot infections. The risk of developing OM is higher at the presence of ulcers larger than 2 cm2 or a diabetic foot ulcer with exposed bone or joint [18]. Although efforts have been made to reduce incidence and consequences of OM, there is still a large proportion of the world population suffering from chronic OM, due to a vast increase in reconstructive orthopedic procedures and higher prevalence of diabetes mellitus, together with longer life-expectancies [19].
The most common sites of OM are in the forefoot (90 %) followed by the midfoot (5 %) and hindfoot (5 %). The bones usually more involved are the weight-bearing bones of the foot, particularly to what it is referred as the ‘tripod of the foot’ that includes the first metatarsal head, fifth metatarsal head, and the calcaneum [18].
Histologically, OM is characterized by the presence of leukocytes or inflammatory cells, such as lymphocytes and plasma cells, and by the presence of bone necrosis (Figure 4).
An inadequate treatment of the acute phase permits OM to persist and become chronic or “dormant” for a considerable period of time, only to manifest again at a later stage [19].
OM can be classified according to duration (acute or chronic), pathogenesis (trauma, contiguous spread, hematogenous, surgical), site, extent or type of patient. Although several
Figure 4. Infected ulceration and osteomyelitis
Mader is one of the most widely used. Under this system, OM is classified according to the anatomic involvement of the infected bone, in four types [20]:
Type 1 – Medullary OM, infection confined to the medullary cavity of the bone;
Type 2 – Superficial OM, involves only the cortical bone and often originates from a direct inoculation or a contagious focus infection;
Type 3 – Localized OM, the infectious process does not involve the entire bone diameter;
Type 4 – Diffuse OM, involves the entire thickness of the bone characterized for loss of stability.
OM of the foot is a very serious and common problem in diabetic patient and a high index of clinical suspicion is required for diagnosis [5].
1.4. O
STEOMYELITISP
ATHOPHYSIOLOGYThe pathophysiology of chronic OM is multifactorial beginning with the spread of bacteria that can reach the bone by hematogenous seeding, followed by direct inoculation, or airborne contamination [21]. Whatever the route of access, bacteria must be able to subsequently adhere to components of the bone matrix in order to start the infection [22].
The most common pathogens responsible for OM are the Staphylococcus species, followed by Enterobacteriaceae and Pseudomonas species. S. aureus is the main causative strain of chronic bacterial OM and around 90 % cases of OM associated with implants are caused by S. epidermidis [22].
of nasal and skin colonization with S. aureus, who is more likely to be present on the skin and take advantage of any disruptions [5].
S. aureus is known for its capacity to bind fibrinogen which can provide an explanation
for this microorganism ability to survive in body fluids. Bacteria clump together and are covered in a layer of fibrinogen, hence protected from the host defense mechanisms and antibiotics [22].
Diabetic foot infections are mostly polymicrobial with mixed positive and gram-negative bacteria, of both aerobic and anaerobic types [21].
Once bacteria are in contact with the bone and/or implant it may use different mechanisms to facilitate cell-cell and/or cell-implant adhesion. Bacteria become sessile, reduce their metabolic rate, and produce a biofilm that protects them from antimicrobials, opsonization and phagocytosis [21].
Biofilm is defined as a multistage process in which microbial cells adhere to a surface or to the surface of other organisms (initial reversible attachment), with subsequent production of an extracellular matrix that contains polysaccharides, proteins and DNA (extracellular polymeric substance – EPS) [2]. Cells embedded in this matrix, also referred to as ‘slime’, communicate with each other and show a coordinated group behavior. The biofilm cells are phenotypically and physiologically different from non-adhered (planktonic) cells. One of their typical properties is the increased resistance to antimicrobial agents [23].
An estimated 65-80 % of all infections is thought to be biofilm-related, being the biofilm formation often considered as the underlying reason why the treatment with antimicrobial agents fails [23] since the embedded organisms are extensively protected by their slimy layer [19].
The protective mechanism found in a biofilm appears to be distinct from the mechanisms responsible for conventional antibiotic resistance, making biofilm bacteria
800-1.5. T
REATMENTO
FO
STEOMYELITISIn the early 1900s, around 20 % of patients suffering from OM died, while the survivors remained with significant morbidity. Nowadays, the mortality and morbidity of these patients is lower due to surgical treatment together with prolonged antibiotic treatment [24].
A successful treatment of OM in the diabetic foot is a combination of antibiotic therapy and surgical procedures. A surgical intervention is almost always required due to the impaired blood supply of the affected regions and the low ability of the host immune system to clear infections [21]. The procedure includes surgical debridement, drainage of pus, excision of the osteomyelitic bone and/or minor amputations. Debridement is essential and is often the first factor in the elimination of an infection [24].
In addition, stabilization of the foot is also an important factor in the surgical management, since debridement often causes a large dead space that needs to be managed effectively to prevent the infection to resurge. The dead space management includes closed irrigation systems, local soft tissue flaps, vascularized free flaps, as a variety of methods for local antibiotic delivery [21].
Chronic OM cannot be eradicated alone by intravenous administration of antibiotics, since the infected necrotic focus in the bone, is usually surrounded by sclerotic avascular bone, which is almost unreachable using systemic antibiotics. Also, the necrotic bone is a suitable substrate for biofilm formation, reducing the antibiotic sensitivity of the infecting pathogens and making the infection difficult to treat [25].
Local delivery of antibiotics is a novel therapeutic procedure in chronic OM, allowing high antibiotic concentrations at the site of infection without systemic antibiotic toxicity [24], [19].
bacteriological and physico chemical point of view. Nevertheless, the increasing gentamicin resistance of staphylococci in bone infections requires the use of a different antibiotic.
Vancomycin is the antibiotic used as last resort for the treatment of clinically resistant bacteria, like methicillin-resistant S. aureus (MRSA) [26].
LOCAL ANTIBIOTIC THERAPY
Gentamicin-loaded bone cement beads were first created by Klemm in 1979 and these were used to fill the dead space after debridement of dead bone with a successful cure rate of 91,4% in more than 100 patients [21].
Systemic therapy has been left behind due to the numerous advantages achieved with the use of antibiotic-loaded beads. These beads are placed by a simple procedure and usually performed at time of the initial debridement of chronic OM or open fracture [21].
Currently, the standard procedure of OM treatment includes the traditional systemic antibiotic regimes associated with implant removal and/or tissue debridement, and bone void fillers absorbed with antibiotics, antibiotic-infused collagen sponges, or antibiotic-loaded bone cement [27]. Drawbacks of these systems are the fact that they require a second surgery for removal [21] and/or, they lack in controlled pharmaceutical release strategies needed for an effective local dosing and duration. Low and short antibiotic release can boost the development of antibiotic resistance and persistence infection [27].
In order to achieve the therapeutic drug concentrations in the infected area, high systemic doses are needed which can further worsen toxic side effects. Nonetheless, normal doses of systemic antibiotics may not be enough to breach the glycocalyx of the biofilm produced by the infecting bacteria [28].
The selection of the appropriate antibiotic has to be made by understanding the microbiology of bone and soft tissue infections [28]. Antimicrobial agents used in local delivery
spectrum; bactericidal potency low concentrations; elution from carrier in high concentrations for prolonged periods; low/no risk of allergy or delayed hypersensitivity; low influence on the mechanical properties of the carrier; and low drug serum protein binding [24], [27].
Furthermore, an ideal local antibiotic device system must provide reliable pathogen killing via immediate burst release within the first 24 hour period after administration, followed by sustained drug release above the tissue bed’s minimal inhibitory concentration (MIC) to address the remaining microbial threat [27].
Several antibiotic–releasing systems are currently marketed as bone void fillers, they release antibiotic over short periods of time and may degrade quickly [27].
Even though the ideal local antibiotic delivery system has not been discovered yet, a selection of very promising materials is currently in place. Table 1 lists the most successful carriers for the release of antibiotics according to the dosage prescribed [28].
Table 3. Antibiotic delivery carriers (Taken from [28]).
Carrier System Antibiotic Released References
Non-biodegradable
1. Bone cement
Gentamicin Baker & Greenham, 1988; Buchholz et al 1984,
Vancomycin Kuechle et al, 1990
Cefazolin Marks et al, 1976
Ciprofloxacin Tsourvakas et al, 2009
2. Bone cement beads
Gentamicin Buchholz et al, 1984; Mendel et al, 2005
Tobramycin Seligson et al, 1993
Cefuroxime Mohanty et al, 2003
Vancomycin Chohfi et al, 1998
Biodegradable
1. Plaster of Paris pellets
Gentamicin Santschi & McGarvey, 2003
Teicoplanin Dacquet et al, 1992
2. Collagen-Sponge Gentamicin Ruszczak&Friess, 2003 3. Fibrin-sealant Cefazolin Tredwell et al, 2006
Ciprofloxacin Tsourvakas et al, 1995 4. Hydroxyapatite blocks Vancomycin Shirtliff et al, 2002 5. Polylactide/polyglycolide
implants
Gentamicin Garvin et al, 1994b
Ciprofloxacin Koort et al, 2008
Vancomycin Calhoun &Mader, 1997 6. Dilactate polymers Fluoroquinolones Dounis et al, 1996;
Kanellakopoulou et al, 1999 7. Cancellous bone Vancomycin,
Ciprofloxacin
Witso et al, 2000 8. Calcium Sulfate Tobramycin Nelson et al, 2000 9. Calcium phosphate cement Teicoplanin Lazarettos et al, 2004 Miscellaneous
1. Fibres Tetracycline Tonetti et al, 1998
2. Chitosan Vancomycin Chevher et al, 2006
3. Biomedical polyourethanes Gentamicin, Ciprofloxacin Schierholz et al, 1997
A longer drug release profile with similar capability for bone integration could reduce the incidence of OM [27].
1.6. N
ON-
BIODEGRADABLEA
NTIBIOTICD
ELIVERYS
YSTEMS:
PMMA
BASEDThe poly(methyl methacrylate) (IUPAC name) also designated as PMMA is a non-biodegradable and biocompatible polymer. It is a synthetic homopolymer of methyl methacrylate monomer (MMA) (Figure 5), classified as hard, rigid, but brittle material, with a glass transition temperature of 105 °C [29].
The PMMA polymer is considered hydrophobic although it can become slightly hydrophilic when in contact with water as indicated by a decrease in the contact angle and a pronounced contact angle hysteresis. It is currently accepted as a non-toxic polymer with a very good toxicological safety record in biomedical applications and without references to severe adverse effects [29].
PMMA was first used by Charnley in 1958 to anchor joint replacements; it is commonly called bone cement and it is often used in orthopedics [19]. This polymer has the ability to successfully consolidate implants in bone in a handler and host-friendly manner, with limited adverse and rare allergic reactions [19].
Since 1970, PMMA has been used as a local delivery system of antibiotics in two different ways, namely as antibiotic-loaded bone cement applied in joint replacements and chains of antibiotic-loaded beads for musculoskeletal infections [29].
Figure 5. Chemical structure of the repeating unit of poly(methyl methacrylate) polymer (Taken
The first applications of PMMA as a particulate carrier material were on the development of nanoparticles for vaccination purposes and on beads to fill defects related to surgical resection of chronic OM [29].
Antibiotics most commonly used have been the aminoglycosides; gentamicin or tobramycin. Even though its use as a drug particulate carrier system has been somehow neglected due to the fact that it is (bio)inert and non-biodegradable, this polymer is still an eligible candidate as biomedical material because of its biocompatibility [29].
The selection of the antibiotic to be used in PMMA must be carefully considered since it has to present chemical and heat stability, since PMMA polymerization often reaches 80°C, and it has also to be able to diffuse through the pores of the polymer providing a local concentration that exceeds the MIC for the infecting organisms [30]. Additionally, the antibiotic must be available in the powder form since liquid antibiotics weaken the cement matrix and finally, it has to be water-soluble and hydrophilic to potentiate the release [19].
Gentamicin sulfate is the antibiotic most commonly used; it is an excellent additive to PMMA due to its broad spectrum of action, bactericidal properties, low rate of primarily resistant pathogens and good thermostability. Vancomycin and tobramycin are also eligible options, they are both water soluble and available in powder form [21]. Nevertheless the use of vancomycin in non-biodegradable carriers is limited, especially in bone cement applications because it shows poor release due to the antibiotic high molecular weight [26].
Antibiotic release from bone cement will depend on how the body fluid is capable of reaching the regions of the cement matrix. In the first hours or days after implantation it is observed a burst release, since the antibiotic at the cement surface is easily available. The dissolution fluid penetrates the bone cement dissolving the antibiotic within superficial regions, while the interior usually remains unreached [30]. Often, only 10 % of the total amount of antibiotic incorporated is released from bone cement [19].
1.7. PMMA
C
ARRIERS TOT
REATO
STEOMYELITISPMMA can be used in the treatment of OM as chains of beads or spacers [19].
Beads of PMMA can be used for all kinds of chronic OM and are commercially available as chains of gentamicin-loaded PMMA spheres (Septopal®) or handmade from commercially available antibiotic-loaded bone cement [19].
Although PMMA beads are frequently used for OM, they are not specialized in the therapy of resistant infections, presenting restrictions mainly regarding the use of various antibiotics [19]. Septopal® beads demonstrate a good antibiotic release due to a porous cement matrix and the addition of glycin yielding gentamicin tissue levels above MIC for non-resistant bacterial strains, for 30 days. These beads are routinely used to treat OM with no regard about the infecting bacterial strains that are present or their sensitivity to gentamicin. The augmenting of gentamicin-resistant staphylococci related with the use of PMMA beads has been raising concerns about the choice of such therapeutics. Another disadvantage of PMMA beads is the fact that they can provide a substratum for bacterial colonization, mainly because the amount of antibiotic release decreases over time thus becoming ineffective [19].
The major drawback of PMMA beads is the need for a second surgical procedure to remove the beads after treatment, since the polymer is non-biodegradable [21], [25]. Additionally, the beads do not release all of their antibiotic content, reportedly after 2 weeks in
situ, only 20-70 % of the total amount of gentamicin loaded is released and the concentration of
the antibiotic drops considerably afterwards [25].
The PMMA spacers are a temporary implant to maintain the joint space and soft-tissue tension, besides being used in joint replacement infections. They decrease the morbidity and the implantation of a new prosthesis, during the second surgery, is less complicated. for the patient [19].
The spacer can be modified by using commercially available mould kits. This enables the clinicians to alter the antibiotic composition and dosage according to the size of the implant [19].
The advantages of this method are the immediate treatment of the source of infection, reaching high antibiotic levels locally; maintenance of joint mobility; limitation of scar formation; absence of soft tissue contraction and easy re-implantation [31]. However, the antibiotic sustained release from the spacers is lower when compared to the beads, due to their lower surface area [19].
The use of low-dose antibiotic bone cement for handmade spacers or beads results in a sub-therapeutic antibiotic release that can contribute to the development of antibiotic resistance. On the other hand, the addition of more than 10 % of antibiotic in PMMA weakens significantly the weight-baring carrier and the toxicity risk increases when the overall antibiotic loading is too high [32]. Furthermore, unpredictable release rates are expected when there is an uneven distribution of the antibiotic and a variable surface area [19].
BIODEGRADABLE ANTIBIOTIC DELIVERY SYSTEMS:PTMC BASED
The use of biodegradable devices as antibiotic carriers to treat OM is very appealing since these carriers are able to fulfill the dead space resulting from the surgical debridement and, more importantly, they release all their entire antibiotic content and a second surgical removal after treatment is not required [26], [19].
The most frequently investigated biodegradable antibiotic carriers are the poly(lactic acid) (PLA) and/or poly(glycolic acid) (PGA). However, the use of these polymers as bone implants are controversial due to the bone resorption, that can occur as a result of a decrease in pH while the polymer degrades [26]. Further, some studies have reported an inflammatory foreign-body reaction, related with PLA and PGA implants [25]. Therefore, these polymers are not ideal for the local treatment of bone infections. Carriers that do not produce acidic
degradation products are highly preferable. This requirement is satisfied with the biodegradable poly(trimethylene carbonate) (PTMC) [26].
PTMC (Figure 6) is a resorbable, amorphous and flexible polymer with a glass transition temperature of approximately -17 °C. Also, it degrades in water, CO2 and propanediol, and it is
a relatively new polymer in biomedical applications [26], [33].
Figure 6. Chemical structure of poly(trimethylene carbonate) (Taken from [34]).
The degradation of PTMC in vitro at pH 7 and 37 °C is quite low compared to the in
vivo degradation, thus suggesting enzymatic involvement [25, 26]. In fact, enzymes play an
important role in the in vivo degradation which can be simulated in vitro by the use of a lipase solution. PTMC hardly degrades in vitro without the presence of lipase and it is known also to be susceptible to cholesterol esterase [26], [35].
PTMC degrades enzymatically in a uniform manner by surface erosion providing sustained high antibiotic release rates [25]. Enzymatic surface erosion by cholesterol esterase and superoxide anion radicals, mediated by macrophages, was only recently reported and PTMC appears to degrade completely in bone and assist in promoting bone regeneration [19], [25].
In vitro data suggests that molecular weight of PTMC can be adjusted to control the
release rate of various antibiotics with an ideal and sustained, zero order release profile [19].
after aqueous conditioning, which might induce conformational changes on the enzyme when absorbed at PTMC interface, reducing its activity [35].
Another possible explanation is the oxidation of PTMC as the primary in vivo degradation mechanism. Oxidative degradation occurs as a frustrated phagocytosis when macrophages attach to the material surface and secrete reactive oxygen species. Among these, superoxide anion is believed to be responsible for the in vivo oxidative degradation of PTMC. The third explanation proposed is the fact that macrophages preferentially attach to, or are more active on, PTMC with molecular weights greater than 100 Kg/mol [35].
In short, PTMC appears to be a promising biodegradable carrier in the local treatment of OM presenting good biocompatibility and the ability to degrade in a uniform manner potentially releasing all its antibiotic content. Finally, due to its surface erosion, PTMC is not expected to limit the release of high molecular weight drugs [25, 26].
Chapter 2.
Materials and Methods
2.1. M
ICROORGANISMS ANDG
ROWTHC
ONDITIONSEnterobacter cloacae, Proteus vulgaris, Enterococusfarcalis, Staphylococcus
epidermidis, Staphylococcus aureus isolated from diabetic patients with OM (Bio Trading
Benelux B. V., Mijdrecht) were cryopreserved on beads (-80 ºC). Each of these species was smeared on blood agar plates and incubated aerobically for 24 h at 37 ºC. After incubation the plates were stored in the fridge at 4 ºC for a maximum of two months. For each experiment, a colony was inoculated in 10 mL of tryptone soya broth (TSB; Oxoid, Basingstoke, UK) or in nutrient broth (NB; Oxoid, Basingstoke, UK) and incubated for 24 h at 37 ºC. After incubation the absorbance was measured at the wavelength of 600 nm using a Spectroinic 20 Genesys spectrophotometer (Spectronic Instruments, Beun, DeRonde, Abcoude). Pre-cultures were diluted to an absorbance value of 0.1 which corresponds to 1x108 bacteria/mL.
S. epidermidis ATCC 12228 (purchased from ATCC, UK and used as received) was
streaked on a blood agar plate and incubated at 37 ºC for 24 h. After incubation the plate was stored at 4 ºC. For indirect bacterial inhibition assay one colony was inoculated in 10 mL of TSB culture medium and incubated at 37 ºC for 24 h.
2.2. S
EPTOPAL®
Antibiotic–loaded poly(methylmethacrylate) (PMMA) beads are commercially available under the name of Septopal (Biomet Europe, Darmstadt, Germany) and were kindly provided by the University Medical Centre of Groningen (The Netherlands).
Each Septopal bead contains 2.8 mg of gentamicin sulfate (corresponding to 1.7 mg of gentamicin base). The gentamicin release profile of Septopal beads was determined and they were used for anti-biofilm efficacy test.
2.3. G
ENTAMICINL
OADEDPTMC
B
EADSHigh purity, polymerization grade 1,3 – trimethylene carbonate was purchased from Medisse (Medisse BV, Ede, The Netherlands) and was used to prepare poly(trimethylene carbonate) (PTMC) polymer with MW 556 g/mol and Mn 439 g/mol.
PTMC polymer, 1 g, was dissolved in 38 mL of tetrahydrofuran (purchased from Merck, Darmstadt, Germany, used as received) under magnetic stirring at 250 rpm for 24 h.
Septopal PMMA bead contains 2.8 mg of gentamicin sulfate, the weight of one PTMC bead is approximately 52 mg which corresponds to 5 % of gentamicin content in each bead.
To the dissolved polymer 0.055 g of gentamicin sulfate (purchased from Sigma-Aldrich, St. Louis, USA) was added, in order to create PTMC beads with the same gentamicin content as PMMA beads. After gentamicin addition the solution was mixed for 5 min in ultrasonic bath followed by magnetic stirring for 24 h.
The solution containing the antibiotic dispersion was precipitated into 400 mL of anhydrous hexane (purchased from Sigma-Aldrich, St. Louis,
USA, used as received). Once PTMC and gentamicin sulfate do not dissolve in hexane, this induces precipitation of the polymer - through which gentamicin is homogeneously distributed. The composite was then collected and dried under vacuum at room temperature in a Heraus oven (Jott. Wilten- Utrecht N.V.)
Figure 7. Stainless steel molds
PTMC beads with dispersed antibiotic particles incorporated were prepared by compression-molded at 70 ºC, using a stainless steel molds (see Figure 7) and a laboratory press (Carver Inc., Wabash, IN, USA). From this procedure were created 10 PTMC beads loaded with 5 % of gentamicin.
PTMC beads with 7.5 % of gentamicin and without antibiotic were also made following the previous procedure.
2.4. R
ELEASE OFG
ENTAMICINEnzymes seem to play an important role in in vivo degradation of PTMC which can be simulated in vitro by the use of a lipase solution. Cholesterol esterase is one of the enzymes responsible for in vivo degradation.
Gentamicin release was determined at 37 ºC in lipase solution from
Thermomyceslanuginosus (EC 3.1.1.3., minimum 100.0 units/g, purchased from Sigma-Aldrich,
used as received) and in cholesterol esterase from porcine pancreas (minimum 30.9 units/mg, purchased from Sigma-Aldrich, diluted to a final concentration of 1 U/mL).
Gentamicin-loaded PTMC beads (n=3) were immersed in 5 mL of lipase or cholesterol esterase solution and incubated at 37 ºC under 150 rpm stirring (shaker incubator Innova 4000, New Brunswick scientific) for 2 weeks. Liquid samples of 1mL were taken every 24 h and then stored at 4 ºC before analysis. After taking the sample the entire solution medium was replaced. This procedure was done to 5 % and 7.5 % gentamicin-loaded PTMC beads in lipase solution.
The release of PTMC beads 7.5 % gentamicin loaded was also performed in cholesterol esterase solution in order to compare to the release in lipase solution.
For comparison, the gentamicin release from PMMA (n=3) was determined in potassium phosphate buffer (PBS).
Gentamicin concentrations in lipase or cholesterol esterase solution could not be determined spectrophotometrically due to interference of the enzyme. An indirect bacterialinhibition assay was used to determine gentamicin concentrations in these samples. The same process was used to determine gentamicin release from PMMA beads.
Pre-culture of S. epidermidis ATCC 12228 was incubated for 24 h, this species is highly sensitive to gentamicin. The pre-culture was smeared on nine TSB agar plates with a cotton swab.
Gentamicin stock solution with a concentration of 5 mg/mL was made out of 0.005 g of gentamicin diluted in 10 mL of PBS. The stock solution was diluted 10 times for a solution with 500 µg/mL gentamicin concentration. This last solution was diluted 2 times to obtain a solution with 250 µg/mL gentamicin concentration and this was sequentially repeated until a concentration of 2 µg/mL. From each known gentamicin concentration solutions 15 µgL was dropped on TSB agar plates, and the plates were incubated for 24 h. The antibiotic inhibits bacteria growth during incubation which resulted in zones of inhibition. The areas of the inhibition were clear around the position of the samples droplets, the diameter was measured in two perpendicular directions and a calibration curve was made.
The previous procedure was repeated with the samples taken from PTMC in lipase and cholesterol esterase solution, also for PMMA beads in PBS. Gentamicin concentration in each sample was deduced by comparison with the inhibition zones corresponding to the known gentamicin concentrations and using the calibration curve.
2.5. M
INIMUMI
NHIBITORYC
ONCENTRATION ANDG
ROWTHC
URVESGentamicin sensitivity of each clinically isolated strains was evaluated with Etest® (purchased from bioMérieux, USA, used as received).
In 5 TSB agar plates each diluted pre-culture (24 h of incubation at 37 ºC) was smeared and the Etest was then placed in the middle of each plate. The plates were incubated for 24 h and MIC value was measured afterwards.
The species S. aureus, E. cloacae, S. epidermidis were sensitive to gentamicin (MIC-value below 4 µg/mL) and therefore selected to be used throughout this work.
In this study, it was also our intention to test the influence of glucose on biofilm growth since diabetic patients have elevated blood glucose levels.
Nutrient broth glucose free medium (10 mL) was used for pre-culture of each selected species and after 24 h of incubation they were seeded in NB agar plates and in NB agar plates with 2.6 g/L of glucose, a clinically relevant concentration. New Etest was performed to test if gentamicin sensitivity of this species was affected by the presence of glucose.
Growth curves of planktonic bacteria were created to assess the glucose influence on bacterial growth. Pre-cultures of the 3 species were made in 10 mL of NB medium for 24 h of incubation.
In sterile containers (Greiner, NL), 500 µL of each pre-culture was added to 22.5 mL of NB medium and NB medium with 2.6 g/L of glucose. The solutions were stored in the stirring incubator (Shaker incubator Innova 4000, New Brunswick scientific, 37 ºC, 150 rpm) and the absorbance was measured at the wavelength of 600 nm in spectrophotometer (Spectronic Instruments, Beun, DeRonde, Abcoude) at 0, 1, 3, 4, 5, 6, 23, 25, 26, 27 h.
2.6. B
IOFILMG
ROWTHAfter testing the glucose influence on planktonic bacteria this effect was tested in biofilm growth.
Wells were washed with buffer and 200 µL of NB medium with 2.6 g/L glucose and without glucose was added. Plates were incubated at 37 ºC for 24 h and 48 h (medium was refreshed after 24 h). The medium was removed and the wells were gently washed 3 times with 200 µL of PBS. Each well was stained with 200 µL of 1 % crystal violet for 30 min at room temperature. After staining, the plates were gently rinsed 3 times with demineralized water. Quantitative analysis of biofilm formation was performed by adding 200 µL of ethanol/acetone (80:20) to solubilize the crystal violet. The absorbance of this solution was measured using a FLUOstar Optima plate reader (BMG Labtech GmbH, Offenburg, Germany) at a wavelength of 575 nm. The absorbance is proportional to the amount of crystal violet, which is directly proportional to the amount of biofilm growth in each well. This procedure was done to 24 and 48 h plates.
Crystal violet stains the living and dead bacterial and also the slime of the biofilm, to measure only the living bacteria present in the biofilm, a metabolic activity assay (MTT staining) was performed after 24 and 48 h. After seeding 200 µL of pre-cultures for 1 h in the 96-well plate the supernatant was removed from the wells and these were washed with 200 µL of PBS. Biofilms were stained with 200 µL of MTT solution for 1 h at 37 ºC in the dark. MTT solution was previously made with 10mL of PBS, 0.005 g of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, Sigma Aldrich St. Louis, USA), 0.100 g glucose monohydrate (purchased from Merck, Darmstadt) and 100 µL of menadione. The wells were washed with 200 µL of demineralized water and MTT was solubilized in 200 µL of acid isopropanol (5 %, 1 M, purchased from Merck, Darmstadt, Germany, used as received). The absorbance was measured using a FLUOstar Optima plate reader (BMG Labtech GmbH, Offenburg, Germany) at the wavelength of 575 nm.
2.7. B
IOFILMM
ODELA biofilm model was developed to evaluate gentamicin concentration necessary to prevent biofilm growth and to treat the biofilm. Also, the influence of glucose on gentamicin sensitivity was studied.
S. epidermidis has a MIC value in NB medium lower than the value that is possible to
measure in Etest. Also, the MTT or crystal violet stain results from the biofilm growth of this species were inconclusive so we did not proceed with this strain to further develop the biofilm model. Therefore, the biofilm model was developed with S. aureus and E. cloacae.
2.7.1. PREVENT BIOFILM
For the prevention of biofilm growth, 1 mL of pre-cultures of S. aureus and E. cloacae were seeded for 1 h at 37 ºC in 6-well plates with flat bottom (culture multiwell plate, Sigma-Aldrich, St. Louis, USA).
A gentamicin stock solution of 1000 µg/mL was previously made with 0.02 g of gentamicin and 20 mL of NB medium with 2.6 g/L glucose and without glucose, this solution was diluted to make solutions with the concentration of 5 and 10 µg/mL.
Pre-cultures were removed from the wells and gentamicin solutions (with and without glucose) were added to the wells and the plates were incubated for 24 h. For the control wells only medium with and without glucose was added. After incubation, the number of bacteria in the biofilm was determined by CFU counts (serial dilutions and plate counting). Gentamicin solutions were removed from these wells and they were gently washed with 2 mL of PBS, then 1 mL of PBS was added and the bottom of the wells were scraped with a cell scraper. From the wells 400 µL of solution was removed, stored in Eppendorf´s and diluted according to Figure 8.
2.7.2. BIOFILMS TREATMENT
Gentamicin solutions of 500 µg/mL and 1000 µg/mL in NB medium with and without glucose were made using a previously prepared stock solution. Diluted pre-cultures were seeded in 6-well plates for 1 h (37 ºC), medium with and without glucose was added and then plates were incubated. After 24 h of biofilm growth, the medium was removed and gentamicin solutions were added for a 24 h treatment.
The treatment was also performed for a 48 h old biofilm, so the medium from these plates was refreshed after 24 h and the same procedure described above was made. Control wells containing only medium with and without glucose were used.
The number of bacteria was determined by CFU count with the same dilution scheme as the one illustrated in Figure 8.
Figure 8. Serial dilutions: A- Control wells; B- Gentamicin concentration 5 and 10 µg/mL wells
2.8. A
NTI-B
IOFILME
FFICACYAfter establishing the biofilm model, the anti-biofilm efficacy was evaluated. The prevention and treatment of biofilm were tested once again, but the gentamicin solutions were replaced with gentamicin-loaded PTMC beads and Septopal PMMA beads.
2.8.1. PREVENTION OF BIOFILM GROWTH
For the prevention of biofilm growth model, we used gentamicin-loaded PTMC beads and PTMC beads with no gentamicin content, were used as control.
The results of the release profile of loaded PTMC beads in cholesterol esterase, showed that one bead releases 585 µg of gentamicin in cholesterol esterase solution. In order to have a gentamicin concentration of 7.5 µg/mL approximately, 226.5 µL of cholesterol esterase was added to 78 mL of NB medium with and without glucose in sterile bottles. After 24 h of stirring incubation (37 ºC, 150 rpm), the content of the bottles was removed and stored at 4 ºC for further use.
In 12-well plates with flat bottom (culture multi-well plate, Sigma-Aldrich, St. Louis, USA) pre-cultures were seeded for 1 h. After removing the pre-cultures 2 mL of previously stored solutions were added to each well and plates were incubated for 24 h.
Biofilm was scraped from the bottom of the wells and the number of bacteria was determined by CFU count as previously described and confocal laser scanning microscopy images were taken.
2.8.2. TREATMENT OF BIOFILMS
After 1 h of incubation of pre-cultures in 12-well plates, 1 gentamicin-loaded PTMC bead was placed in the well and 2 mL of cholesterol esterase solution (1 U/mL) in NB medium with and without glucose (2.6 g/L) was added. The same procedure was made with PMMA
beads, although cholesterol esterase solution was replaced by NB medium with and without glucose.
After 24 h and 48 h of treatment, the biofilm was scraped from the bottom of the wells and the number of bacteria was determined by CFU count as previously described.
2.9. STATISTICAL ANALYSIS
Due to time restrictions and limited availability of materials for the development of the current work, only the gentamicin release profiles from PTMC and PMMA beads were determined in triplicate.
A confidence level of 95 % interval was applied for statistical significance and student's
Chapter 3.
Results and Discussion
In this chapter the results are presented and discussed, namely the gentamicin release profile from PMMA Septopal® beads and its comparison with the release profile of the produced PTMC beads, with different gentamicin concentrations and erosion mediums.
The results related to the biofilm model developed are also provided, for the treatment and prevention of biofilm formation with different gentamicin concentrations and at last, the anti-biofilm efficacy using the PTMC and PMMA beads for treatment and prevention of biofilm formation.
3.1. R
ELEASEP
ROFILE FROMPTMC
VERSUSPMMA
B
EADSPMMA beads constitute an effective drug delivery system for local antibiotic therapy in bone infection. However, after high initial gentamicin concentration release at the site of infection (approximately 2 weeks), this concentration drops considerably. Therefore, the efficacy of gentamicin is observed immediately after implantation of the beads [25].
PTMC degrades very slowly by hydrolysis in vitro, however in vivo the degradation of PTMC subcutaneously implanted was found to be very fast. This difference suggests that enzymes are involved in the degradation process [25]. PTMC is well-known for its susceptibility to lipase and cholesterol [33].
accompanied by a high rate of antibiotic release and has been found to be similar to the observed for Septopal® PMMA beads [26].
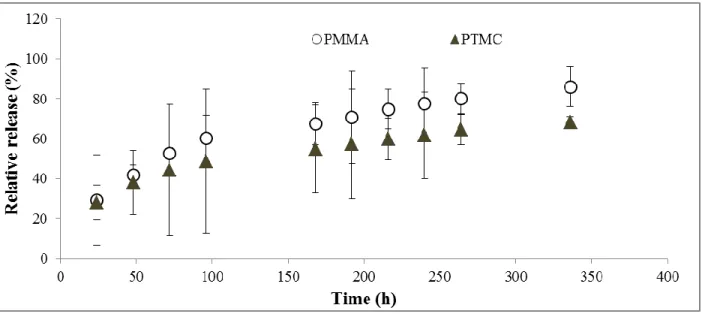
PTMC beads containing 5 wt% gentamicin were made and immersed in lipase solution, the release profile (Figure 9) shows a lower gentamicin release when compared with PMMA Septopal® beads (immersed in PBS).
Gentamicin release from PMMA beads are consequently higher than from PTMC beads with the same initial gentamicin content. However, the differences observed were not statistically significant (p>0.05). PMMA beads show a burst release, namely within the first 24 h about 30 % is released and this gradually increases to 85 % after 2 weeks.
In order to reach a similar gentamicin release from the two types of beads, new PTMC beads were made with higher gentamicin contents (7.5 wt% instead of 5 wt%). In this case, the release profile achieved for PTMC was found to be very close to the PMMA release (Figure 10), although the gentamicin content in PTMC is higher than in PMMA Septopal®.
Figure 9. Cumulative gentamicin release from PTMC beads (containing 5 wt % gentamicin) in lipase
solution and PMMA beads in PBS buffer. Results correspond to the average of 3 independent assays ± standard deviation.
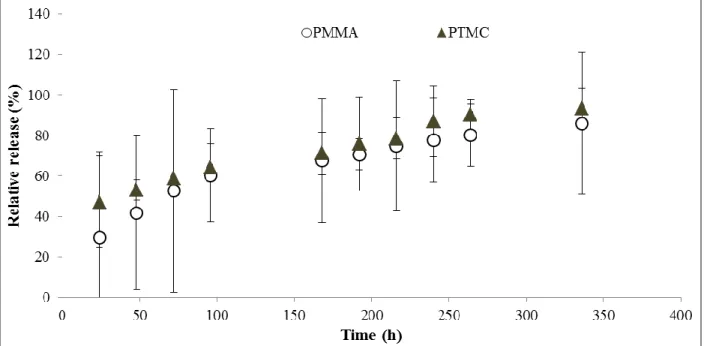
Cholesterol esterase is one of the enzymes that has been implicated in the in vivo degradation of PTMC [35]. In the Figure 11, a comparison between the release profile of gentamicin from PTMC beads (7.5 wt% gentamicin) immersed in lipase solution and immersed in cholesterol esterase solution is provided.
Figure 10. Cumulative gentamicin release from PTMC beads (containing 7.5 wt % gentamicin) in lipase
solution and PMMA beads in PBS buffer. Results correspond to the average of 3 independent assays ± standard deviation.
PTMC beads show higher gentamicin release in the presence of lipase solution than in cholesterol esterase. Even though, the lipase solution used is much more concentrated (100 U/g) compared to the cholesterol esterase solution (30.9 U/mg – which is approximately the same concentration that can be found in human blood). PTMC beads should have been made with a new adjusted gentamicin concentration to reach a higher release profile in the presence of cholesterol esterase.
Gentamicin-loaded PTMC beads in cholesterol esterase showed a lower release than PMMA beads (Figure 12). Gentamicin concentration should be adjusted once again, so we could have an antibiotic release from PTMC similar to the one observed from PMMA.
0 20 40 60 80 100 120 0 50 100 150 200 250 300 350 400 R e la ti v e r e le a se (% ) Time (h)
PTMC (Lipase) PTMC (Cholesterol esterase)
Figure 11. Cumulative gentamicin release from PTMC beads (7.5 wt % gentamicin) in lipase and
cholesterol esterase solutions. Results correspond to the average of 3 independent assays ± standard deviation.
![Figure 1. Neuropathic ulcer in a typical position, i.e. under second metatarsal head and surrounded by callus (Taken from [6])](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/20.918.170.445.689.916/figure-neuropathic-typical-position-second-metatarsal-surrounded-callus.webp)
![Figure 3. Neuroischemic ulcer (Taken from [7]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/21.918.536.824.294.516/figure-neuroischemic-ulcer-taken-from.webp)
![Table 1. Wagner Ulcer classification system (Taken from [3]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/23.918.252.718.157.379/table-wagner-ulcer-classification-taken.webp)
![Figure 4. Infected ulceration and osteomyelitis (Taken from [39]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/25.918.545.861.612.842/figure-infected-ulceration-osteomyelitis-taken.webp)
![Table 3. Antibiotic delivery carriers (Taken from [28]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/31.918.127.843.160.811/table-antibiotic-delivery-carriers-taken.webp)
![Figure 6. Chemical structure of poly(trimethylene carbonate) (Taken from [34]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/36.918.369.643.334.469/figure-chemical-structure-poly-trimethylene-carbonate-taken.webp)
![Figure 8. Serial dilutions: A- Control wells; B- Gentamicin concentration 5 and 10 µg/mL wells (Adapted from [39])](https://thumb-eu.123doks.com/thumbv2/123dok_br/17593181.819716/46.918.145.829.87.380/figure-serial-dilutions-control-wells-gentamicin-concentration-adapted.webp)