ContentslistsavailableatScienceDirect
Industrial
Crops
and
Products
j ou rn a l h o m epa g e :w w w . e l s e v i e r . c o m / l o c a t e / i n d c r o p
Essential
oils
of
Amazon
Piper
species
and
their
cytotoxic,
antifungal,
antioxidant
and
anti-cholinesterase
activities
Joyce
Kelly
R.
da
Silva
a,
L.C.
Pinto
b,
R.M.R.
Burbano
b,
Raquel
C.
Montenegro
a,b,
Elsie
F.
Guimarães
c,
Eloisa
Helena
A.
Andrade
d,
José
Guilherme
S.
Maia
d,e,∗aProgramadePós-Graduac¸ãoemBiotecnologia,UniversidadeFederaldoPará,66075-900Belém,PA,Brazil bLaboratóriodeCitogenéticaHumana,UniversidadeFederaldoPará,66075-900Belém,PA,Brazil cInstitutodePesquisasJardimBotânicodoRiodeJaneiro,22460-030RiodeJaneiro,RJ,Brazil dProgramadePós-Graduac¸ãoemQuímica,UniversidadeFederaldoPará,66075-900Belém,PA,Brazil
eProgramadePós-Graduac¸ãoemRecursosNaturaisdaAmazônia,UniversidadeFederaldoOestedoPará,68035-110Santarém,PA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received9January2014
Receivedinrevisedform22March2014 Accepted4April2014
Availableonline28April2014
Keywords:
Piperhispidum,PiperanonifoliumandPiper aleyreanum
Essentialoilcomposition
Cytotoxic,anti-cholinesterase,antifungal andantioxidantactivities
a
b
s
t
r
a
c
t
ThehydrodistilledoilsofPiperhispidum,Piperaleyreanum,andPiperanonifolium,collectedintheCarajás NationalForest,Parástate,Brazil,wereanalyzedbyGCandGC–MSandthen,evaluatedtheir antioxi-dant,antifungal,anticholinesteraseandcytotoxicactivities.Intotal,87constituentswereidentifiedin thePiperoils.Thesesquiterpeneswerethemosthighlyrepresentedclassesandthemaincompounds foundinthePiperoilswereselin-11-en-4-␣-ol,-elemene,-selinene,␣-selinene,bicyclogermacrene, -caryophyllene,␣-humulene,and␦-elemene.Allanalyzedoilsshowedpowerfulantifungalactivity, withthedetectionlimit(DL)from0.1to1.0gagainsttheCladosporiumcladosporioidesand Cladospo-riumsphareospermumfungi,aswelltheyhavenolyticeffectagainstthemiceerythrocytes.In the anticholinesteraseevaluation,theoils ofP.anonifolium(DL=0.01ng)andP.hispidum(DL=0.01ng) werehundredtimesmorepotentthanthestandardphysostigmine(DL=1.0ng).Moreover,theoilof P.aleyreanumshowedhighinvitrocytotoxicactivityagainstthehumanmelanomacelllineSKMEL-19 (IC50=7.4g/mL)andsignificantantioxidantactivity(DPPH=412.2mgTE/mL).Thehighercellgrowth inhibitioninducedbytheoilofP.aleyreanumisprobablyduetoelemene(-,␦-,and␥-elemene),aswell asotherstructurallyrelatedcompounds,whichwerepreviouslyreportedintheproliferationinhibition, stimulationofapoptosisandinductionofcellcyclearrestinmalignantcells.
©2014ElsevierB.V.Allrightsreserved.
1. Introduction
Piperaceaecomprisesaboutfivegeneraandmorethan2000 species (Jaramilloand Manos, 2001).The genusPiper ismainly distributed in the tropical and subtropical regionof theworld andhasbeenextensivelyinvestigatedasthesourceofnew nat-uralproductswithpotentialantifungal,antitumoral,antioxidant, antiplasmodial, and tripanocidal properties (Lago et al., 2009). Moreover,Piperspeciesareusedtotreatdiseases,includingfever, jaundice,rheumatism,andneuralgiainthefolkmedicineofvarious countries(Christophe,2006).
∗ Correspondingauthorat:ProgramadePós-Graduac¸ãoemQuímica, Universi-dadeFederaldoPará,66075-900Belém,PA,Brazil.Tel.:+559181467067; fax:+559132353043.
E-mailaddress:gmaia@ufpa.br(J.G.S.Maia).
Thespecies PiperhispidumSw.,Piperanonifolium Kunth,and
PiperaleyreanumC.DC.,arecommonlyfoundintheBrazilian Ama-zon.P.hispidumisknownbythevernacularnamesof“matico”and “aperta-ruão”,anditsleafinfusionisusedinthefolkmedicineas diureticandanti-hemorrhagic(Andradeetal.,2009).Inaddition, ithasbeenreportedfortheirantifungal(Navickieneetal.,2000), insecticidal(Santosetal.,2010),andantimicrobialandcytotoxic (Moraleset al.,2013)activities.P.aleyreanum is knownby the indigenousname“paninixpu”,anditsessential oilismentioned asimmunomodulator,analgesic,andantidepressantinlocalfolk medicine.Recently,theoilofP.aleyreanumshowed antinocicep-tiveandanti-inflammatoryactivities,aswellasagastro-protective activity(Limaetal.,2012).P.anonifolium isknownas“pimenta longa”,and thereareonlyfewchemicalstudiesreportingmono andsesquiterpenesfromtheiressentialoil(Andradeetal.,2005, 2009).
Chemical studies have shown that Piper has many classes of compounds,suchasunsaturated amides, flavonoids,lignans,
aristolactams,longandshortchainesters,steroids,andalkaloids (Navickieneetal.,2000).Moreover,theoilsofPiperintheAmazon haveshowedterpenoidandphenylpropanoidcompoundsasmajor constituents,alwayswiththepredominanceofoneovertheother (Silvaetal.,2011;Andradeetal.,2011).
Theaimofthisstudywastoidentifythecompositionofthe oilsof P.hispidum, P.anonifolium and P.aleyreanumand evalu-atetheircytotoxic,antifungal,antioxidantandanticholinesterase properties.
2. Materialsandmethods
2.1. Plantmaterial
TheaerialpartsofP.aleyreanumC.DC.(MG189271),P. anoni-foliumKunth(MG170246)andP.hispidumSw.(MG189262)were collectedintheCarajásNationalForest,Municipalityof Parauape-bas,Parástate,Brazil,duringtherainyseason(February2008).The plantswereidentifiedbyDrElsieGuimarãesfromJardimBotânico doRiodeJaneiro,RiodeJaneiro,Brazil,andtheyweredepositedin theherbariumofEmilioGoeldiMuseum,Belém,Parástate,Brazil.
2.2. Plantprocessingandextractionoftheessentialoils
The aerial parts of the plants (leaves and thin stems) were air-dried, ground and submitted to hydrodistillation using a Clevenger-type apparatus(100g,3h). Theoils were dried over anhydroussodiumsulfate,andtheirpercentagecontentswere cal-culatedonthebasisoftheplantsdryweight.Themoisturecontents of the samples were calculated after phase separation using a Dean–Starktrap(5g,60min)andtolueneasthesolventphase.
2.3. Oil-compositionanalysis
TheanalysisoftheoilswerecarriedonaGC–MSThermoFocus DSQII,underthefollowingconditions:DB-5ms(30m×0.25mm; 0.25mm film thickness) fused-silica capillary column; pro-grammed temperature,60–240◦C (3◦C/min);injector tempera-ture,250◦C;carriergas,helium,adjustedtoalinear velocityof 32cm/s(measuredat100◦C);injectiontype,splitless (2mLofa 1:1000hexanesolution);splitflowwasadjustedtoyielda20:1 ratio;septum sweepwasa constant 10mL/min; EIMS,electron energy,70eV;temperatureoftheionsourceandconnectionparts, 200◦C. Thequantitativedataregardingthevolatileconstituents wereobtainedbypeak-areanormalizationusingaFOCUSGC/FID operatedundersimilarconditionsfortheGC–MS,exceptthecarrier gaswhichwasnitrogen.Theretentionindexwascalculatedforall thevolatilesconstituentsusingahomologousseriesofn-alkanes (C8–C32,Sigma–Aldrich),accordingVandenDoolandKratz(1963).
2.4. Antioxidantassay
TheantioxidantactivityoftheoilsofPiperwasdeterminedby theDPPHradical-scavengingassay.DPPHisastabledarkvioletfree radicalwithamaximumabsorptionat517nm,whichisreduced inthepresenceofantioxidants.Eachsample(10L)wasmixed with900Lof100mMTris–HClbuffer(pH7.4),40Lofethanol, and50Lof0.5%(w/w)ofTween20solutionandthenaddedto 1mLof0.5mMDPPHinethanol.Thestandardcurvewasprepared tousingTrolox,anantioxidantderivedfromwater-soluble vita-minE,expressedasmilligramsofTroloxequivalentpermilliliter ofsample(Choietal.,2000).
2.5. Antifungalassay
Thebioautographicmethodwasappliedtothemicroorganisms thatmaygrowdirectlyonTLCplates(Rahalisonetal.,1994).Ten Loftheoilsolution(correspondingto0.1,0.5,1.0,10.0,50.0,and 100.0g)wereappliedtopre-coatedTLCplates,developedonn -hexane/ethylacetate(8:2)solutionanddriedforcompleteremoval ofthesolvent.Theplatesweresprayedwithsporesuspensionof
CladosporiumsphaerospermumandCladosporiumcladosporioidesin glucoseandsaltsolutionand,then,incubatedfor48hinadark andmoistenedchamber,at22◦C.Aclearinhibitionzoneappeared againstadarkbackgroundindicatingtheminimalamountofthe usedoils.Miconazolewasusedasapositivecontrol(Silvaetal., 2011).
2.6. Acetylcholinesteraseassay
The enzyme acetylcholinesterase (500U) was dissolved in tris–hydrochloricacidbuffer(pH7.8)andstabilizedbytheaddition ofbovineserumalbumin–fractionV(0.1%).TLCplateswere spot-tedwiththeessentialsoilsinrangingfrom0.01to1000ng/spot. Thealkaloidphysostigmine(eserine)wasusedasthepositive con-trol.Theplatesweresprayedwiththeenzymesolution(3.33U/mL) and thendried and incubatedat37◦C for20min(moist atmo-sphere).Enzymeactivitywasdetectedbysprayingwithasolution of0.25%of1-naphtylacetateinEtOH(5mL)plus0.25%aqueous solutionofFastBlueBsalt(20mL).Potentialacetylcholinesterase inhibitorshaveappearedasclearzonesonapurplecolored back-ground(Marstonetal.,2002).
2.7. Cytotoxicityassay(againstcancercelllines)
TheMTTcolorimetricassaywasusedtomeasuretheactivityof cellularenzymes,byindicatingthecellviability(Mosmann,1983). Theoils(0.2–25g/mL)weretestedforcytotoxicactivityagainst three cancercell lines: HCT-116(colon), SKMEL19(melanoma), ACP-03(gastric).AllcelllinesweremaintainedinDMEMmedium supplemented with 10% fetal bovine serum, 2mM glutamine, 100U/mLpenicillin,100g/mLstreptomycinat37◦Cwith5%CO
2.
Eachoilwasdissolvedtoobtainaconcentrationof10mg/mLwith DMSO.ThefinalconcentrationofDMSOintheculturemediumwas keptconstant,below0.1%(v/v).Essentialoils(25g)were incu-batedwiththecellsfor72h.Thenegativecontrolreceivedthesame amountofDMSO(0.001%inthehighestconcentration).Thecell viabilitywasdeterminedbyreduction oftheyellowdye 3-(4,5-dimethyl-2-thiazol)-2,5-diphenyl-2H-tetrazoliumbromide(MTT) toablueformazanproduct.TheIC50’swerecalculatedbynonlinear
regressionusingGraphPadprogram(IntuitiveSoftwareforScience, SanDiego,CA).
2.8. Cellmembranedisruption
Thepotentialofthecellmembranelysesisevaluatedbythe releaseoftheerythrocytesinthemedium.Thetestwasperformed in96-wellplatesusinga2%mouseerythrocytesuspensionin0.85% NaClsolution,containingCaCl2(10mM).Theoils,dilutedas
2.9. Statisticalanalysis
Sampleswereassayedintriplicate,andtheresultsareshownas means±standarddeviation.Analysisofvariancewasconducted, andthedifferencesbetweenvariablesweretestedforsignificance byTukeytest,usingGraphPadPrism5.0software.Differencesat
p<0.05wereconsideredstatisticallysignificant.
3. Resultsanddiscussion
TheaerialpartsofP.aleyreanum,P.anonifolium,andP.hispidum
providedoilyieldsof0.8%,0.6%,and1.0%,respectively.Individual componentswereidentifiedbycomparisonofbothmassspectrum andGCretentiondatawithauthenticcompounds,whichwere pre-viouslyanalyzedandstoredinthedatasystem,aswellaswiththe aidofcommerciallibrariescontainingretentionindicesandmass spectraofvolatilecompounds,commonlyfoundinessentialoils (NIST,2005;Adams,2007).
In total, 87 volatilecomponents wereidentified, comprising approximately90%ofthetotalcompositionoftheoils(Table1). Sesquiterpenoidswerethemosthighlyrepresentedclass,asmany as hydrocarbons and oxygenated compounds. In the oil of P. aleyreanum,the principalconstituentswere -elemene (16.3%), bicyclogermacrene(9.2%),␦-elemene(8.2%),germacreneD(6.9%), -caryophyllene(6.2%),andspathulenol(5.2%).TheoilofP. anon-ifoliumwasdominatedbyselin-11-en-4-␣-ol(20.0%),-selinene (12.7%), ␣-selinene (11.9%), and ␣-pinene (8.8%). The oil of P. hispidumshowed-caryophyllene(10.5%),␣-humulene(9.5%),␦ -3-carene(9.1%),␣-copaene(7.3%),limonene(6.9%),caryophyllene oxide(5.9%),and-selinene(5.1%)aschiefcompounds.
ThepresenceofsesquiterpenescompoundsinPiperoilshasbeen previouslyreported (Parmaret al.,1997; Andradeetal., 2011). The oil composition of the present specimen of P. aleyreanum
differsfromanotherreportedtoRondôniastate,Brazil(Facundo and Moraes, 2003; Facundo et al., 2007), whose oil showed -caryophyllene (18.6%) and isocaryophyllene (17.5%) as main sesquiterpenehydrocarbons.Moreover,thedataoftheoils compo-sitionofeightspecimensofP.anonifolium,collectedinParástate, Brazil,weregroupedbytheHierarchicalClustersAnalysismethod (HCA). It resulted in two differentchemotypes(Andradeet al., 2009):onewiththepredominanceof␣-pinene(40.9–53.1%)and -pinene(17.2–22.9%)andtheotherwith␣-eudesmol(27.0–33.5%) andgermacreneD(8.0–9.6%).Wehaveinmindthatfortheoilof thisspecimenshouldbeassignedanotherchemotype,nowwith theselinane-structuresesquiterpenesinitscomposition.Oilsof differentspecimensofP.hispidum,whichwerecollectedat sev-eralareasofParástate,Brazil(Andradeetal.,2009),haverevealed thepresenceofgermacreneD(34.0–42.5%)and-caryophyllene (10.6–14.3%).However,theoilofaspecimencollectedinRondônia state,Brazil,wasdominatedby mono-andsesquiterpenes con-stituents,mainly␥-cadinene(25.1%),camphene(15.6%),␣-guaiene (11.5%),and␥-elemene(10.9%)(Machadoetal.,1994).
TheadditionofthePiperoilstotheDPPHsolutioncausedacolor reductioninitsopticaldensityat517nm.Theinhibitionpercentage variedfrom13%to36%,andtheresultsfortheantioxidantactivity oftheoilsareshowninTable2.Thehighestactivitywasobserved fortheP.aleyreanumoil(TEAC=412.2mgTE/mL).Usually,theoils richinsesquiterpenehydrocarbonsexhibitantioxidantproperties. AgoodexampleistheoilofEupatoriumpolystachyum,dominated by-caryophyllene(15.4%),germacreneD(9.4%),and bicycloger-macrene(19.2%),whichshowedsignificantantioxidantactivityby DPPHassay(Souzaetal.,2007).Furthermore,thesesquiterpenes -caryophyllene(13.2%),-selinene(10.6%),and␣-selinene(9.7%) werealsofoundintheextractofAlpiniagalanga,whichshowed
antioxidantactivitybythe-carotene/linoleicacidassay,withan inhibitionof70%(MayachiewandDevahastin,2008).
Manyevidenceshavebeenreportedfromwhichtheoxidative stress is intimately involved in theage-related neurodegenera-tivediseases,aswellasaboutthepositivebenefitsofantioxidants to reduce or to block the neuronal death occurring in the physiopathologyofthesedisorders(Ramassamy,2006).Inthe anti-cholinesteraseassay,theoilsofP.anonifoliumandP.hispidum,both showingadetectionlimit(DL)equalto0.01ng,haveprovedtobe hundredtimesmoreactivethanthephysostigmine(DL=1.0ng),as anticholinesteraseinhibitors.TheoilofP.aleyreanum(DL=10.0ng) showed lower effect to anticholinesterase inhibition, with a detection limit ten timeslower than physostigmine. The oilof
Cymbopogon schoenanthus, which is rich in hydrocarbons and oxygenatedsesquiterpenes,showedananticholinesterase inhibi-tionthreetimeshigherthangalantamine,whichisusedforthe treatmentofAlzheimer’sdiseaseandothermemoryimpairments (Khadrietal.,2008).Thus,thesignificantanticholinesterase activ-ityobservedforthePiperspeciesmaybeduetothelargecontent ofsesquiterpenecompoundsfoundintheiroils.Furthermore,itis assumedthatthedifferentstructuraltypesofsesquiterpenes iden-tifiedinthesePiperspeciescancontributetoagreaterorlesser activityoftheiroils.
Intheantifungalassay,thePiperoilsshoweddetectionlimit (DL)equal toorlowerthan1.0gagainsttheC.cladosporioides
and C. sphaerospermum fungi, values which are comparable to the standard miconazole (DL=0.5g). The oil of P.aleyreanum
(DL<0.1g) wasaboutfivetimesmoreactivethanmiconazole. TheoilofP.hispidumshoweddetectionlimitbetween0.1gand 1.0gandthevalueoftheoilofP.anonifolium(DL=0.5g)was equivalenttomiconazole(Table3).Previously,otherPiperoilsrich insesquiterpenes,showedantifungalactivitywiththedetection limitbetween10and50g(Navickieneetal.,2006).
ThePiperoilsweretestedinvitroagainstthreecancercelllines (HCT-116,colon;ACP03,gastric;SKMEL19,melanoma)and com-pared withdoxorubicin, a well-known anticancerdrug usedin medicalclinics.OnlyoilswithIC50 valueslower25g/mLwere
consideredactive,inatleastonecellline(Table4).P.aleyreanum
oilshowedhighcytotoxicityandselectivitytomelanomacellline (IC50=7.4g/mL), whereastheothertwoPiper oilsdidnot
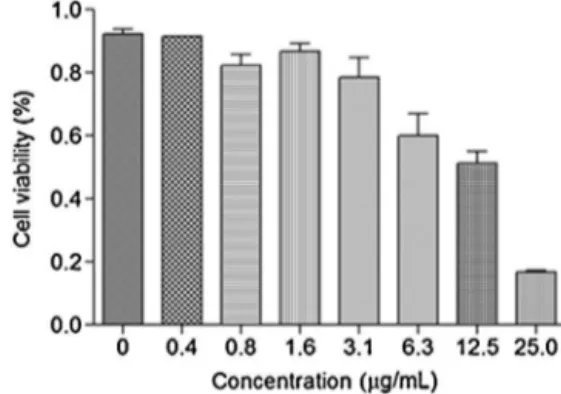
dis-playeffectsagainstthecancercellstreated,whencomparedwith thedoxorubicin.ThegraphofFig.1showsdose–responsedataof
P.aleyreanumoil,whichinduced50%inhibitioningrowthofthe cellsandtheirlyticpotential,whichwascalculatedbynon-linear regression.
Inprinciple,thesignificantactivityattributedtoP.aleyreanum
oilmaybeaccountedbythepresenceoftheexpressivecontentof -and␦-elemene(24.5%intotal)initscomposition(seeTable1).
Table1
CompositionoftheoilsofP.aleyreanum,P.anonifolium,andP.hispidum.
Constituents RIa P.aleyreanum P.anonifolium P.hispidum
Calc.b Lit.c Oil%
␣-Pinene 937 932 0.3 8.8 0.2
Sabinene 973 969 0.1 0.1
-Pinene 980 974 0.4 0.2 0.2
myrcene 990 988 0.4 0.2 0.3
␣-Phellandrene 1010 1002 0.3
␦-3-Carene 1011 1008 9.1
␣-Terpinene 1018 1014 0.7
p-Cymene 1025 1020 2.0 0.9
o-Cymene 1030 1022 3.7
Limonene 1031 1024 1.1 6.9
(E)--Ocimene 1046 1044 0.9 0.1
␥-Terpinene 1059 1054 2.0 0.1
p-Mentha-2,4(8)-diene 1086 1085 0.5
Terpinolene 1087 1086 0.7
n-Nonanal 1106 1100 0.1
trans-p-Mentha-2,8-dien-1-ol 1122 1119 0.1
allo-Ocimene 1129 1128 0.1 0.1
cis-Limoneneoxide 1137 1132 0.2
trans-Carveol 1218 1215 0.2
cis-Carveol 1230 1226 0.1
Carvone 1243 1239 0.3
Thymol 1289 1289 0.1
␦-Elemene 1333 1335 8.2 0.3 0.8
␣-Cubebene 1345 1345 0.2 0.1
␣-Ylangene 1365 1373 0.2 0.1 0.4
␣-Copaene 1373 1374 1.0 0.4 7.3
-Bourbonene 1380 1387 0.3
-Elemene 1380 1389 16.3 2.0 1.8
␣-Gurjunene 1400 1409 0.1 0.6
Siberene 1404 1400 0.1
-Caryophyllene 1415 1417 6.2 3.7 10.5
␥-Elemene 1426 1434 0.9 1.5
trans-␣-Bergamotene 1431 1432 0.2
␣-Guaiene 1432 1437 0.4
Aromadendrene 1434 1439 0.1 0.1
6,9-Guaiadiene 1438 1442 0.4 0.1 0.1
Geranylacetone 1446 1453
␣-Humulene 1452 1452 0.9 0.7 9.5
allo-Aromadendrene 1456 1458 0.6 0.2 0.1
Ishwarane 1464 1465 3.6
4,5-di-epi-Aristolochene 1466 1471 0.3
␥-Muurolene 1471 1478 0.9 0.5
GermacreneD 1477 1484 6.9 2.4 3.4
-Selinene 1485 1489 1.1 12.7 5.1
Viridiflorene 1487 1496 0.8
Bicyclogermacrene 1491 1500 9.2 2.3
␣-Selinene 1494 1498 11.9
␣-Muurolene 1495 1500 0.6
␥-Cadinene 1509 1513 0.4 0.3 0.3
Cubebol 1511 1514 1.2 0.6
7-epi-␣-Selinene 1515 1520 2.2
␦-Cadinene 1515 1522 2.6 1.4
trans-Calamenene 1517 1521 0.3
10-epi-Cubebol 1526 1533 0.2
trans-Cadina-1,4-diene 1529 1533 0.1 0.1
␣-Cadinene 1532 1537 0.1 0.1
␣-Calacorene 1537 1544 0.1 0.6 0.4
Elemol 1545 1548 0.3 0.3
-Calacorene 1558 1564 0.2 0.2
E-Nerolidol 1559 1561 3.0 0.5
Palustrol 1565 1567 0.3 0.4
Spathulenol 1572 1577 5.2 0.2 1.9
Caryophylleneoxide 1576 1582 1.0 0.5 5.9
Globulol 1580 1590 1.4 0.4 0.6
Viridiflorol 1589 1592 0.7 3.5
Guaiol 1592 1600 0.6 0.5
Ledol 1598 1602 0.3 1.6 0.3
5-epi-7-epi-␣-eudesmol 1602 1607 0.7
HumuleneepoxideII 1603 1608 2.9
1,10-di-epi-Cubenol 1608 1618 0.2 0.3
Junenol 1616 1618 0.6
CompositionoftheoilsofP.aleyreanum,P.anonifolium,andP.hispidum.
Constituents RIa P.aleyreanum P.anonifolium P.hispidum
Calc.b Lit.c Oil%
1-epi-Cubenol 1623 1627 0.7 1.7
␣-Acorenol 1627 1632 1.0
allo-Aromadendreneepoxide 1627 1639 0.1 0.1
epi-␣-Cadinol 1638 1638 0.5 0.2
Cubenol 1638 1639 0.6
epi-␣-Muurolol 1639 1640 0.7 0.5 0.3
␣-Muurolol 1642 1644 0.6 0.9 0.5
␣-Cadinol 1650 1652 1.6 0.6
␣-Eudesmol 1651 1652 1.9
Selin-11-en-4-␣-ol 1651 1658 20.0 0.8
neo-Intermedeol 1653 1658 2.6 0.8 0.3
Bulnesol 1660 1670 0.5
14-Hydroxy-(Z)-caryophyllene 1665 1666 0.2
Germacra-4(15),5,10(14)-trien-1-␣-ol 1677 1685 0.3 0.2
Eudesm-7(11)-en-4-ol 1692 1700 0.6
Monoterpeneshydrocarbons 10.1 11.7 18.5
Oxygenatedmonoterpenes 1.0
Sesquiterpeneshydrocarbons 56.7 38.6 52.2
Oxygenatedsesquiterpenes 23.1 38.9 16.6
Other 0.1
Total 89.9 89.2 88.4
aRetentiontime(onDB-5mscolumn). bCalculatedvalues.
c Literaturevalues(Adams,2007).
Table2
RadicalscavengingactivityandantioxidantcapacityofthePiperoils.
Oils DPPHinhibition(%)A TEAC(mgET/mL)A
P.aleyreanum 36.0±0.9 412.2±9.5a
P.anonifolium 13.0±2.3 148.6±26.9b
P.hispidum 26.4±4.2 303.1±49.2c
a,b,cValueswithdifferentlettersarestatisticallydifferentatthep<0.05level(Tukey’s
test).
AMean
±standarddeviation(n=3).
Elemeneisamixtureofsesquiterpenehydrocarbonscomposedby -,␦-,and␥-elemene,where-elemeneisaccountedfor60–72% ofthethreeisoformsintheoilsofCurcumalongaL.andCurcuma zedoaria (Christm.)Roscoe (Zhang et al., 2011). Previously,the elemenesesquiterpeneswerereportedintheproliferation inhi-bition,apoptosisstimulationandinductionofcellcyclearrestin malignantcell(Liuetal.,2011;Wangetal.,2005;Yaoetal.,2008).It hasbeenfoundthat-elemeneexertsanti-canceractivityofbrain, laryngeal,lung,breast,prostate,cervical,colonandovarian(Lietal., 2005,2010;Wangetal.,2005).
TheseresultsshowedthattheP.aleyreanumoilhasselectivity tomelanomacellsand,seemstousthatthisactivityisprobably relatedwithamostglobaleffectof-elemeneanditsderivatives sinceitwascytotoxictoothercancercelltypes.Toverifywhether thecytotoxicityoftheoilswaslinkedtothemembranedisruption, theabilitytoinducelysesofmouseerythrocyteswasinvestigated, andnomembranedamagewasfoundforalltestedoils(seeTable4). Atthepresenttime,someessentialoilsoftheBrazilianAmazon with-elemenecontentabove30%arebeingsubjectedtotesting theircytotoxicactivities.Thus,wewillhavemorecertaintyabout thebiologicalactivityobservedfortheoilofP.aleyreanumandits mainconstituent,-elemene.Atthesametime,someoftheseoils willbefractionatedinordertoobtainthissesquiterpene hydro-carbonwithsuitablepurityforfurthertests.Unfortunately, this compoundisnotavailablecommercially.
4. Conclusions
Inconclusion, mostof thevolatileconstituentsoftheoilsof
P. aleyreanum, P. anonifolium and, P. hispidum were identified,
Table3
Detectionlimit(DL)fortheantifungalandanticholinesteraseactivitiesofthePiper
oils.
Oils Antifungalactivity
(DL,g)
Anticholinesterase activity(DL,g)
Cladosporium cladosporioides
Cladosporium sphareospermum
P.aleyreanum <0.1 <0.1 10.00
P.anonifolium 0.5 0.5 0.01
P.hispidum 0.1 1.0 0.01
Miconazole 0.5 0.5 –
Physostigmine – 1.00
Table4
CytotoxicactivityofthePiperoilsoncancercelllines.a
Oil HCT-116
(Colon) IC50,g/mL
SKMEL19 (Melanoma) IC50,g/mL
ACP03(Gastric) IC50,g/mL
Hemolysis (g/mL)
P.aleyreanum >25 7.4(5.5–9.9) >25 >200
P.anonifolium >25 >25 >25 >200
P.hispidum >25 >25 >25 >200
Doxorubicin 0.05 0.20 0.15 >200
aDataarepresentedasIC
50valuesand95%confidenceintervalsobtainedby
non-linearregressionforallcelllinesfromthreeindependentexperiments.Onlyoilswith IC50valueslower25g/mLwereconsideredactive,inatleastonecellline.
Doxorubicin(DOX)wasusedaspositivecontrol.
Acknowledgements
WearegratefulforCNPQ/Bionorte,CAPES/Pró-Amazôniaand
FAPESPA/PAfortheirfinancialsupport.
References
Adams,R.P.,2007.IdentificationofEssentialOilComponentsbyGas Chromatog-raphy/MassSpectrometry,4thed.AlluredPublishingCorp.,CarolStream,IL, USA.
Andrade,E.H.A.,Ribeiro,A.F.,Guimarães,E.F.,Maia,J.G.S.,2005.Essentialoil compo-sitionofPiperanonifolium(Kunth)C.DC.J.Essent.OilBear.Plants8,289–294.
Andrade,E.H.A.,Guimarães,E.F.,Maia,J.G.S.,2009.Variabilidadequímicaemóleos essenciaisdeespéciesdePiperdaAmazônia.FaculdadedeEngenhariaQuímica, UniversidadeFederaldoPará,Belém,Brazil.
Andrade,E.H.A.,Alves,C.N.,Guimarães,E.F.,Carreira,L.M.M.,Maia,J.G.S.,2011. Vari-abilityinessentialoilcompositionofPiperdilatatumL.C.Rich.Biochem.Syst. Ecol.39,669–675.
Choi,H.-S.,Sun Song,H., Ukeda, H.,Sawamura, M.,2000. Radical-scavenging activitiesofcitrusessentialoilsandtheircomponents:detectionusing 1,1-diphenyl-2-picrylhydrazyl.J.Agric.FoodChem.48,4156–4161.
Christophe,W.,2006.MedicinalPlantsofAsiaandPacific.CRCPress,USA.
Facundo,V.A.,Moraes,S.M.,2003.ConstituentsofPiperaleyreanum(Piperaceae). Biochem.Syst.Ecol.31,111–113.
Facundo,V.A.,Ferreira,S.A.,Morais,S.M.,2007.EssentialoilsofPiperdumosumRudge andPiperaleyreanumC.DC.(Piperaceae)fromBrazilianAmazonianforest.J. Essent.OilRes.19,165–166.
Jaramillo,M.A.,Manos,P.S.,2001.Phylogenyandpatternsoffloraldiversityinthe genusPiper(Piperaceae).Am.J.Bot.88,706–716.
Khadri,A.,Serralheiro,M.L.M.,Nogueira,J.M.F.,Neffati,M.,Smiti,S.,Araújo,M.E.M., 2008.Antioxidantandantiacetylcholinestaraseactivitiesofessentialoilsfrom CymbopogonschoenanthusL.Spreng:dccompositionbyGC–massspectrometry and13CNMR.FoodChem.109,630–637.
Lago,J.H.G.,Chen,A.,Young,M.C.M.,Guimarães,E.F.,Oliveira,A.,Kato,M.J.,2009.
PrenylatedbenzoicacidderivativesfromPiperaduncumL.andP.hostmannianum C.DC.(Piperaceae).Phytochemistry24,96–98.
Li,X.,Wang,G.,Zhao,J.,Ding,H.,Cunningham,C.,Chen,F.,Flynn,D.C.,Reed,E.,Li, Q.Q.,2005.Antiproliferativeeffectofbeta-elemeneinchemoresistantovarian carcinomacellsismediatedthrougharrestofthecellcycleattheG2-Mphase. Cell.Mol.LifeSci.62,894–904.
Li,Q.Q.,Wang,G.D.,Huang,F.R.,Banda,M.,Reed,E.,2010.Antineoplasticeffectof beta-elemeneonprostatecancercellsandothertypesofsolidtumourcells.J. Pharm.Pharmacol.62,1018–1027.
Liu,J.,Zhang,Y.,Qu,J.,Xu,L.,Hou,K.,Zhang,J.,Qu,X.,Liu,Y.,2011. -Elemene-inducedautophagyhumangastriccancercellsfromundergoingapoptosis.BMC Cancer11,183–193.
Lima,D.K.,Ballico,L.J.,Rocha,L.F.,Gonc¸alves,H.P.,deSouza,L.M.,Iacomini,M., Werner,M.F.,Baggio,C.H.,Pereira,I.T.,daSilva,L.M.,Facundo,V.A.,Santos,A.R., 2012.Evaluationoftheantinociceptive,anti-inflammatoryandgastric antiul-ceractivitiesoftheessentialoilfromPiperaleyreanumC.DC.inrodents.J. Ethnopharmacol.142,274–282.
Machado,S.M.F.,Militão,J.S.L.T.,Facundo,V.A.,Ribeiro,A.,Morais,S.M.,Machado, M.I.L.,1994.LeafoilsoftwoBrazilianPiperspecies:PiperarboretumAubletvar. latifolium(C.DC.)YunckerandPiperhispidumSw.J.Essent.OilRes.6,643–644.
Marston,A.,Kissling,J.,Hostettmann,K.,2002.ArapidTLCbioautographicmethod forthedetectionofacetylcholinesteraseandbutyrylcholinesteraseinhibitorsin plants.Phytochem.Anal.13,51–54.
Mayachiew,P.,Devahastin,S.,2008.Antimicrobialandantioxidantactivitiesof Indiangooseberryandgalangalextracts.LWT-FoodSci.Technol.41,1153–1159.
Morales,A.,Rojas,J.,Moujir,L.M.,Araujo,L.,Rondón,M.,2013.Chemical compo-sition,antimicrobialandcytotoxicactivitiesofPiperhispidumSw.essentialoil collectedinVenezuela.J.Appl.Pharm.Sci.3(6),16–20.
Mosmann,T.,1983.Rapidcolorimetricassayforcellulargrowthandsurvival: appli-cationtoproliferationandcytotoxicityassays.J.Immunol.Methods65,55–63.
NIST(NationalInstituteofStandardsandTechnology),2005.MassSpectralLibrary (NIST/EPA/NIH,V.2.0d).TheNISTMassSpectrometryDataCenter,Gaithersburg, USA.
Navickiene,H.M.D.,Alécio,A.C.,Kato,M.J.,Bolzani,V.S.,Young,M.C.M.,Cavalheiro, A.J.,Furlan,M.,2000.AntifungalamidesfromPiperhispidumandPiper tubercu-latum.Phytochemistry55,621–626.
Navickiene,H.M.D.,Morandim,A.A.,Alécio,A.C.,Regasini,L.O.,Bergamo,D.C.B., Telascrea,M.,Cavaleiro,A.J.,Lopes,M.N.,Bolzani,V.S.,Furlan,M.,Marques, M.O.M.,Young,M.C.M.,Kato,M.J.,2006.Compositionandantifungalactivityof essentialoilsfromPiperaduncum,PiperarboreumandPipertuberculatum.Quim. Nova29,467–470.
Oliveira,A.C.,Hillard,E.A.,Pigeon,P.,Rocha,D.D.,Rodrigues,F.A.R.,Montenegro, R.C.,Costa-Lotufo,L.V.,Goulart,M.O.F.,Jaouen,G.,2011.Biologicalevaluationof twenty-eightferrocenyltetrasubstituedolefins:cancercellgrowthinhibition, ROSproductionandhemolyticactivity.Eur.J.Med.Chem.46,3778–3787.
Parmar,V.S.,Jain,S.C.,Bisht,K.S.,Jain,R.,Taneja,P.,Jha,A.,Tyagi,O.D.,Prasad, A.K.,Wengel,J.,Olsen,C.E.,Boll,P.M.,1997.PhytochemistryofthegenusPiper. Phytochemistry46,591–673.
Rahalison,L.,Hamburger,M.,Monod,M.,Frenk,E.,Hostettmann,K.,1994.Antifungal testsinphytochemicalinvestigations:comparisonofbioautographicmethods usingphytopathogenicandhumanpathogenicfungi.PlantaMed.60,41–44.
Ramassamy,C.,2006.Emergingroleofpolyphenoliccompoundsinthetreatment ofneurodegenerativediseases:areviewoftheirintracellulartargets.Eur.J. Pharmacol.545,51–64.
Santos,M.R.A.,Silva,A.G.,Lima,R.A.,Lima,D.K.S.,Sallet,L.A.P.,Teixeira,C.A.D.,Polli, A.R.,Facundo,V.A.,2010.AtividadeinseticidadoextratodasfolhasdePiper hispidum(Piperaceae)sobreabroca-do-café(Hypothenemushampei).Rev.Bras. Bot.33,319–324.
Silva,J.K.R.,Andrade,E.H.A.,Kato,M.J.,Carreira,L.M.M.,Guimarães,E.F.,Maia,J.G.S., 2011.Antioxidantcapacityandlarvicidalandantifungalactivitiesofessential oilsandextractsfromPiperkrukoffii.Nat.Prod.Commun.6,1361–1366.
Souza,T.J.T.,Apel,M.A.,Bordignon,S.,Matzenbacher,N.I.,Zuanazzi,J.A.S.,Henriques, A.T.,2007.Composic¸ãoquímicaeatividadeantioxidantedoóleovolátilde Eupa-toriumpolystachyumDC.Rev.Bras.Pharmacogn.17,368–372.
VandenDool,H.,Kratz,P.D.J.A.,1963.Generalizationoftheretentionindexsystem includinglineartemperatureprogrammedgas–liquidpartition chromatogra-phy.J.Chromatogr.A11,463–471.
Wang,G.,Li,X.,Huang,F.,Zhao,J.,Ding,H.,2005.Antitumoreffectofbeta-elemene innon-small-celllungcancercellsismediatedviainductionofcellcyclearrest andapoptoticcelldeath.Cell.Mol.LifeSci62,881–893.
Yao,Y.Q.,Ding,X.,Jia,Y.C.,Huang,C.X.,Wang,Y.Z.,Xu,Y.H.,2008.Anti-tumoreffect ofbeta-elemeneinglioblastomacellsdependsonp38MAPKactivation.Cancer Lett.264,127–134.