Miocene Fossils Reveal Ancient Roots for New
Zealand
’
s Endemic
Mystacina
(Chiroptera)
and Its Rainforest Habitat
Suzanne J. Hand1*, Daphne E. Lee2, Trevor H. Worthy3, Michael Archer1, Jennifer P. Worthy3, Alan J. D. Tennyson4, Steven W. Salisbury5, R. Paul Scofield6, Dallas C. Mildenhall7, Elizabeth M. Kennedy7, Jon K. Lindqvist2
1School of Biological, Environmental and Earth Sciences, University of New South Wales, Sydney, New South Wales, Australia,2Department of Geology, University of Otago, Dunedin, New Zealand,3School of Biological Sciences, Flinders University, Adelaide, South Australia, Australia,4Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand,5School of Biological Sciences, The University of Queensland, Brisbane, Queensland, Australia,6Canterbury Museum, Rolleston Avenue, Christchurch, New Zealand,
7GNS Science, Lower Hutt, New Zealand
*s.hand@unsw.edu.au
Abstract
The New Zealand endemic bat family Mystacinidae comprises just two Recent species re-ferred to a single genus,Mystacina. The family was once more diverse and widespread, with an additional six extinct taxa recorded from Australia and New Zealand. Here, a new mystacinid is described from the early Miocene (19–16 Ma) St Bathans Fauna of Central Otago, South Island, New Zealand. It is the first pre-Pleistocene record of the modern genus and it extends the evolutionary history ofMystacinaback at least 16 million years. Ex-tantMystacinaspecies occupy old-growth rainforest and are semi-terrestrial with an excep-tionally broad omnivorous diet. The majority of the plants inhabited, pollinated, dispersed or eaten by modernMystacinawere well-established in southern New Zealand in the early Miocene, based on the fossil record from sites at or near where the bat fossils are found. Similarly, many of the arthropod prey of livingMystacinaare recorded as fossils in the same area. Although none of the Miocene plant and arthropod species is extant, most are closely related to modern taxa, demonstrating potentially long-standing ecological associations withMystacina.
Introduction
New Zealand’s only native terrestrial mammals are three bat species: the insectivorous Chalino-lobus tuberculatus(Forster, 1844) and the omnivorousMystacina tuberculataGray, 1843 and
M.robustaDwyer, 1962.Chalinolobus tuberculatusbelongs to the cosmopolitan bat family Vespertilionidae. It has close living relatives in Australia and the southwest Pacific (e.g., Chali-nolobus gouldiiandC.neocaledonicus), and molecular data suggest its ancestor dispersed to New Zealand during the last 2 million years [1]. In contrast, the twoMystacinaspecies belong
a11111
OPEN ACCESS
Citation:Hand SJ, Lee DE, Worthy TH, Archer M, Worthy JP, Tennyson AJD, et al. (2015) Miocene Fossils Reveal Ancient Roots for New Zealand’s EndemicMystacina(Chiroptera) and Its Rainforest Habitat. PLoS ONE 10(6): e0128871. doi:10.1371/ journal.pone.0128871
Academic Editor:Thierry Smith, Royal Belgian Institute of Natural Sciences, BELGIUM
Received:February 9, 2015
Accepted:May 1, 2015
Published:June 17, 2015
Copyright:© 2015 Hand et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement:All relevant data are within the paper.
Funding:This research has been supported by Australian Research Council grants DP120100486 and DP130100197 and a Marsden Grant from the Royal Society of New Zealand.
to the endemic family Mystacinidae and are thought to have had a much longer history in New Zealand, but until now a pre-Pleistocene record for the genus was lacking.
Mystacinidae is one of eight families in the Gondwanan bat superfamily Noctilionoidea, to-gether with five extant South American families (Noctilionidae, Phyllostomidae, Mormoopi-dae, ThyropteriMormoopi-dae, Furipteridae) [2], one in Madagascar (Myzopodidae, whose fossil record includes North Africa) [3] and one extinct family in southern North America (Speonycteridae) [4]. The distribution of Mystacinidae once extended beyond New Zealand, with four Oligo– Miocene taxa known from Australia [5,6]. Two small, indeterminate taxa are recorded from the early Miocene of New Zealand [7]. The two RecentMystacinaspecies are well-represented in Quaternary deposits throughout New Zealand [8], the oldest record being 17,340 +/- 140 BP yrs BP (uncalibrated), from Hermit’s Cave, near Charleston, South Island [9]. The threatened
M.tuberculatatoday occupies about a third of its past geographic distribution [10]. The larger
M.robustais critically endangered; it has not been sighted since 1967, on islands off the south-ern coast of South Island [10,11], but may possibly still be extant (C. O’Donnell pers. comm. 2013).
Mystacinids, or burrowing bats, are renowned for their peculiar semi-terrestrial habits, spending up to 30% of their foraging time on the forest floor and tree branches [12]. Studies by Riskin et al. [13] ofMystacina tuberculatashowed that, unlike the vast majority of bats, it uses a true quadrupedal walking gait when manoeuvring terrestrially, and these habits are reflected in numerous specializations of the skeleton and soft tissues in bothMystacinaspecies (summa-rized in [14], and see below). Their omnivorous diet of nectar, pollen, fruit, and flying and ter-restrial arthropods is among the broadest of any bat [15,16] and they are one of the few temperate zone bats known to pollinate plants [17–20].
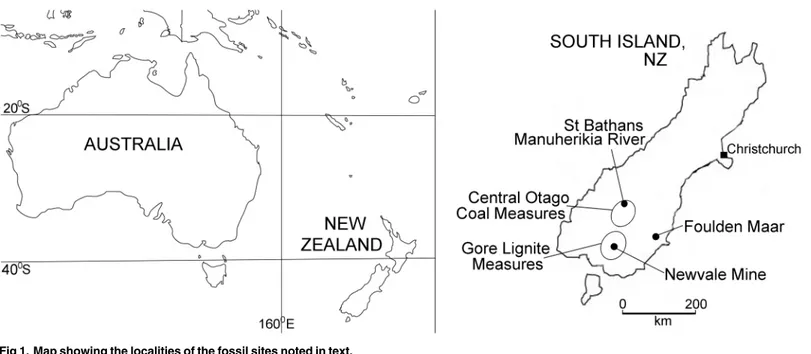
Here, we describe a new species ofMystacinafrom the early Miocene (19–16 Ma) St Bath-ans Fauna of Central Otago, South Island (Fig 1). The St Bathans deposit, which consists of near-shore freshwater lacustrine sediments of Miocene Lake Manuherikia, has also produced New Zealand’s oldest frogs, lizards, sphenodontine, land birds (including kiwi, moa and par-rots), its only crocodilian, terrestrial turtle and a non-volant mammal [21–29]. We also review the fossil record for plants that are pollinated, dispersed, eaten or used as roosts by modern
Mystacina, as well as their arthropod prey, and note potentially deep-rooted ecological associa-tions between New Zealand’s endemic bats, vegetation and arthropod prey.
Materials and Methods
Bat taxonomy follows Simmons (2005) [2]. Dental terminology follows Hand [30], Hand et al. [6] and seeFig 2; M refers to upper molar, and P to upper premolar. All necessary permits were obtained for the described study, which complied with all relevant regulations. Fossil specimens are registered in the Canterbury Museum, Christchurch, New Zealand (prefix CM) and the Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand (prefix NMNZ).
Comparative Fossil and Subfossil Material
Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended Internation-al Code of ZoologicInternation-al Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the no-menclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated in-formation viewed through any standard web browser by appending the LSID to the prefix "http://zoobank.org/". The LSID for this publication is: urn:lsid:zoobank.org:pub:
D40335E8-A564-4E5B-A863-0D593C83DC10. The electronic edition of this work was pub-lished in a journal with an ISSN, and has been archived and is available from the following digi-tal repositories: PubMed Central, LOCKSS.
Systematic Paleontology
Mammalia Linnaeus, 1758 Chiroptera Blumenbach, 1779 Noctilionoidea Gray, 1821 Mystacinidae Dobson, 1875
MystacinaGray, 1843
Type Species:Mystacina tuberculataGray, 1843
Additional Species:Mystacina robustaDwyer, 1962;Mystacina miocenalissp. nov.
Mystacina miocenalissp. nov. Hand, Lee, Worthy & Archer urn:lsid:zoobank.org:act: BFA97159-09A1-4170-AD24-6DBA2269B485 (Fig 2)
Holotype: CM2013.18.381, right M1.
Etymology: The species name is derived from the Miocene age of this species and Latinalis, belonging or pertaining to.
Type Locality and Age: In the lower part of a clay layer enveloping stromatolites, Site FF1 [31], at 44.90359°S, 169.85840°E, Manuherikia River, Central Otago, South Island, New Zea-land. The localities are registered in the New Zealand Fossil Record File (NZ FRF) system
Fig 1. Map showing the localities of the fossil sites noted in text.
administered by the Geoscience Society of New Zealand and GNS Science as H41/f058 (stro-matolites) and H41/f059 (clay draping stro(stro-matolites). Early Miocene (Altonian local stage) 19–16 Ma; St Bathans Fauna [22].
Paratype: NMNZ S.52355, left M2. Locality HH1a, Manuherikia River section, Bannock-burn Formation, Home Hills Station, St Bathans 44.907944° S, 169.858222°E. NZ FRF number H41/f088. Early Miocene (Altonian) 19–16 Ma; St Bathans Fauna.
Species Diagnosis:Mystacina miocenalis(Fig2Aand2B) differs from all other mystacinids in its larger size and from QuaternaryMystacinaspecies (Fig2Cand2E) in having M1 and M2 with a distinct and continuous anterior, lingual and posterior cingulum. It differs fromM.
tuberculatain its M1 lacking a metaloph and its M2 with hypocone shelf (heel) directed more posteriorly than posterolingually and shallower protofossa, and differs fromM.robustain its M2 being square (rather than transversely wide).
Mystacina miocenalisdiffers fromIcaropsspecies and mystacinid indet. [6] (Fig2Fand2G) in M1-2 having a less developed (smaller) hypocone shelf and paraconule, and M1-2 being nearly square, rather than transversely developed (wider than long). It differs additionally from
I.paradoxin M2 having only a faint metaloph (that does not close the protofossa) and fromI.
aenaein M1 having a wider paracingulum.
Description: CM2013.18.381, the holotype ofMystacina miocenalis, represents a right M1 (Fig 2A). This tooth is just longer than it is wide. The metacone is larger and taller than the paracone, which is approximately the same height as the protocone. The apex of the protocone is located posterolingual to the paracone apex. The ectoloph is W-shaped with the centrocrista reaching the buccal margin of the tooth. On the buccal margin, a shallow ectoflexus occurs be-tween the mesostyle and metastyle. The preparacrista is shorter than the postparacrista and premetacrista, which in turn are shorter than the postmetacrista. The curved preparacrista merges with the short, anteriorly directed parastyle in an obtuse angle. The paracone is low, and the paracone basin is very shallow. A discontinuous buccal cingulum occurs between the flattened buccal flanks of the parastyle, mesostyle and metastyle. The pre-and postparacrista meet at an angle of nearly 90° which is slightly greater than that formed between pre-and post-metacristae (80°) and postparacrista and premetacrista (70°). The posteriorly opening proto-fossa is longer than broad and is relatively deep. The base of the paracone flank (lingual crest) bears a wear facette and it is unclear whether a paraloph was present or not. Even if a paraloph were present, it did not extend to meet the preprotocrista. A swelling (?paraconule) is devel-oped independently in the preprotocrista. No metaloph is evident. The preprotocrista contin-ues buccally as the paracingulum to the base of the parastyle. The posterobuccally directed postprotocrista ends well short of the metacone base, terminating in a small metaconule. A nar-row posterior cingulum extends from the metastyle to the posterolingual cingulum surround-ing the small heel, with which it is continuous. The lsurround-ingual csurround-ingulum continues to a point anterior to the base of the paracone. The heel is short, narrow and directed posteriorly. It lacks a hypocone and there is no lingual notch separating it from the protocone base. The heel lacks a basin but its wide but not tall cingulum forms a conspicuous shelf posterolingually. The tooth has three roots; the protocone root is very long and broad, the metacone root large and posteri-orly inclined, and the paracone root straighter and smaller.
Fig 2. Upper teeth of extinct and extant mystacinid species.A–B,Mystacina miocenalissp. nov., St Bathans, Central Otago, New Zealand; Early Miocene. A, holotype, CM2013.18.381, right M1. B, paratype, MNZ S.52355, left M2. C–D,Mystacina tuberculata, Predator Cave, Takaka Hill, Nelson, NZ; Holocene. NMNZ S.32400. C, left M1. D, left M2. E,Mystacina robusta, Exhale Air Cave, Ellis Basin, Mt Arthur, Nelson, NZ; Holocene. NMNZ S.35205, left P4-M3. F,Icarops paradox, Judith’s Horizontalis Site, Riversleigh, Queensland Australia; Early Miocene. QM F30582, left P4-M3. G,Icaropssp., Outasite, Riversleigh; Early Miocene. QM F30586, left M1. Abbreviations: c, cingulum; mcl, metaconule; me, metacone; ml, metaloph; ms, mesostyle; mt, metastyle; pa, paracone; pcl, paraconule; pf, profossa; pl, paraloph; pr, protocone; ps, parastyle. To scale; bar = 2 mm.
NMNZ S.52355 is a left M2 (Fig 2B). It is described in so far as it differs from M1. M2 is just wider than long. The metacone is clearly taller than both the paracone and protocone. In lingual view, the paracone has a much deeper basin than in M1. On the buccal margin two ectoflexae occur, one between the parastyle and mesostyle and the other between the mesos-tyle and metasmesos-tyle. The preparacrista, postparacrista, premetacrista and postmetacrista are of increasing length. The preparacrista is relatively much longer than in M1 and meets the para-style at an angle of approximately 90°. The pre-and postparacristae, pre-and postmetacristae and postparacrista and premetacrista meet at angles of approximately 55–60°. A mesostylar shelf extends from the parastyle to metastyle. A discontinuous, poorly developed buccal cin-gulum occurs between the buccal flanks of the parastyle, mesostyle and metastyle. A well-de-veloped paraloph extends posterolingually from the base of the paracone but does not reach the tip of the protocone. A paraconule is not evident. A very faint metaloph extends lingually from the base of the metacone to meet the posterobuccally directed postprotocrista at the metaconule, thereby closing the protofossa. The hypocone shelf (heel) is shorter but broader than in M1.
Measurements of the new mystacinid specimens are given inTable 1.
Results
As in other mystacinids [6], the St Bathans molars described here exhibit the following combi-nation of features: M1–2 with a low protocone, some hypocone shelf development but hypo-cone absent, metaconule present, large mesostyle, central crests of ectoloph not parallel, paracingulum continuous with preprotocrista, postprotocrista terminates before reaching metacone (and does not continue as metacingulum), and a conspicuously long protocone root; M2 wider than M1; parastyles increasing in size and more lingually directed from M1 to M2.
Table 1. Measurements (mm) of upper teeth (P4-M2) and postcranial remains (humerus and radius) of St Bathans Early Miocene mystacinids (bold) compared with summary statistics for those elements in New Zealand QuaternaryMystacinaspecies and Australian Oligo–MioceneIcarops
species.
Taxon P4L P4W M1L M1W M2L M2W HPW HDW RPW
†Mystacina miocenalis 2.97 2.8 2.78 2.94
†Mystacinid indet. 1 1.55 1.75 4.35 2.15 2.65
†Mystacinid indet. 2 1.25 1.45 2.7
Mystacina tuberculata min. 1.18 1.19 1.65 1.45 1.65 1.6 3.76 3.1 2.46
max. 1.34 1.48 1.9 1.7 1.85 1.85 4.19 3.65 2.8
Mystacina robusta(E) min. 1.69 1.81 1.9 1.9 1.9 2.0 4.65 3.76 3.04
max. 2.37 2.25 2.2 2.4 4.83 4.3 3.22
M.robusta(Waitomo) min. 2.1 2.0 2.1 2.2 4.4 4.1 2.84
max. 2.5 2.6 2.5 2.6 4.8 4.5 3.33
†Icarops paradox min. 0.9 1.1 1.3 1.5 1.3 1.7
max. 1.1 1.2 1.5 1.6 1.5 1.8
†Icarops aenae min. 1.9 1.9 3.15
max. 2.0 2.1
Measurements of New Zealand QuaternaryMystacinaspecies from Worthy et al. [32], Worthy and Scofield [33] and this study; those of Australian Oligo–
MioceneIcaropsspecies are from Hand et al. [6,14].Mystacina robusta(E) is from Stewart Island area [33];M.robusta(Waitomo) is from Waitomo and Hawkes Bay, North Island, where this species is largest [32]. Abbreviations: D, distal; H, humerus; L, length; P, proximal; P4, posterior upper premolar; M1,first upper molar; M2, second upper molar; max., largest specimen in sample; min., smallest specimen in sample; R, radius; W, width;
†
, extinct.
UnlikeIcaropsspecies and as inMystacinaspecies, the M1-2 hypocone shelf (heel) is only moderately developed and the apex of the protocone is located posterolingually to the paracone rather than directly lingual to that cusp. On M1 the paracone basin is shallow and there is no ectoflexus between the parastyle and mesostyle; on M2 the parastyle is shorter and less hooked (less lingually directed) than inIcaropsspecies. InM.tuberculataandM.robusta, an anteriorly directed paraloph on M1 extends to a conspicuous paraconule or swelling on the preproto-crista. In the new species, this feature is possibly absent on M1 (see Description) but present on M2. The presence of an M1-2 paraloph and metaloph may be intraspecifically variable inM.
miocenalis, and possibly other mystacinid taxa: for example, in a sample ofM.tuberculata
from Predator Cave, Nelson (NMNZ S.32400) that we examined, a metaloph on M2 is variably present irrespective of wear.
Mystacina miocenalisshares features with bothM.tuberculataandM.robusta(see Diagno-sis above). No obvious features exclude it from ancestry of either, or both, Quaternary species. The evolutionary relationship between QuaternaryM.tuberculataand the largerM.robustais not anagenetic, with the two species occurring sympatrically for as long as records exist (c. 17,500 yrs BP (uncalibrated) at Hermit’s Cave, South Island) [9]. A gap in the New Zealand fossil bat record from then until the 19–16 Ma St Bathans deposit precludes further insight. Because the MioceneMystacina miocenalisrepresents a species larger thanM.robusta, ex-ceeding even the largest northern specimens of this latitudinally variable taxon [32] as well as
M.tuberculata(Table 1), it would appear that theMystacinalineage did not increase in size over time (contravening Cope’s Rule). The molars ofM.miocenalisare almost twice the size of those of small individuals ofM.tuberculata(e.g. from Codfish Island) [33] and up to 36% larg-er than those of southlarg-ernM.robusta(from Stewart Island area) [33]. In comparison, a size dif-ference of between 11–34% for tooth measurements of the sympatric speciesM.robustaand
M.tuberculatain southern New Zealand was found by Worthy and Scofield [33].
Hand et al. [7] referred previously recovered mystacinid specimens from the St Bathans fos-sil deposit to Mystacinidae indet 1 & 2. These specimens consist of an upper canine, a lower ca-nine, two posterior upper premolars, two distal humeri and two proximal radii. Although their familial identity is clear, they currently cannot be referred confidently to eitherMystacinaor
Icarops. It is possible that these specimens may be referred ultimately toMystacina miocenalis, but they are smaller than the same elements inM.robusta(and mostly even smaller than inM.
tuberculata;Table 1), and simple linear regression analyses confirm that they are unlikely to belong toM.miocenalis(Fig 3).
To estimate body mass in extinct bats, Gunnell et al. [34] developed a set of algorithms based on dental, skeletal and weight measurements in 1,160 extant bats representing eight fam-ilies (but not including Mystacinidae). Using the proxy of upper first molar (M1) area [34;
Table 1], the estimated weight forM.miocenalisis 39.2 g, suggesting a relatively large bat (com-pared with the median value of 13.8 g for 905 extant bat species [35,36]). The body mass esti-mate forM.tuberculatausing this equation [34] and M1 data fromTable 1is 10.6–14.5 g, compared with its measured average weight of 13.6 g [sd 1.3; 16]. Live weights forM.robusta
are not known, but values extrapolated from forearm measurements ofM.tuberculatarange from 15 g [16] to 25–35 g [12,37] forM.robusta, compared with 16.3–30.3 g calculated here based on M1 area [34] from data inTable 1.
Worthy et al. [32] and Worthy & Scofield [33] found that overall size inM.robusta in-creases markedly with decreasing latitude (contravening Bergmann’s Rule), while that ofM.
tuberculatadoes not, with northern populations slightly smaller than southern ones in South Island. Those trends result in character (size) displacement betweenMystacina tuberculataand
climes) may be due to roosting behaviour and hibernation physiology, with smaller bats being better adapted to re-warming after hibernation in higher latitudes than are larger ones. A large size difference, probably greater than that found between the QuaternaryMystacinaspecies, also appears to separateMystacina miocenalisand the smaller mystacinid(s) in early Miocene St Bathans, which at that time had a warmer climate than today, with the estimated mean an-nual temperature of 18°C for southern New Zealand exceeding that of northern-most New Zealand today [38,39]. The presence of the two smaller mystacinids in the warmer-than-now early Miocene precludes explaining the large size ofM.miocenalisas an effect of a decreasing temperature gradient over time and thus it is not a corollary of the latitudinal trend in Quater-nary New Zealand.
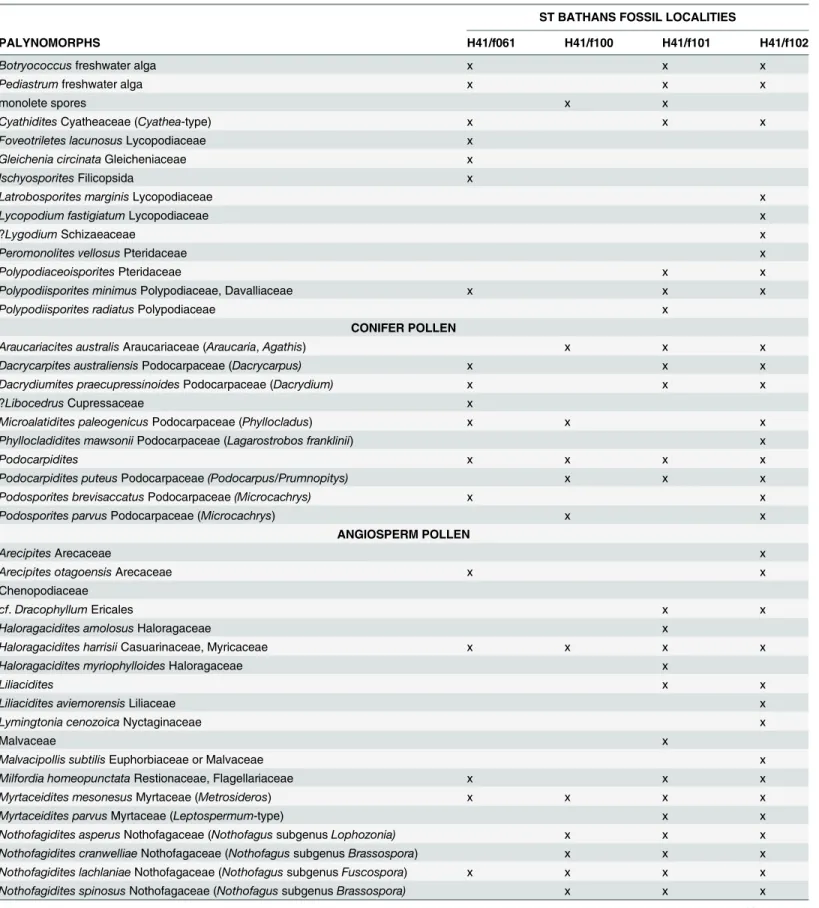
Although no plant fossils are present in the sediment immediately surrounding the Mysta-cinafossils, palynofloras were obtained from the same horizon (upper part of clay draping stro-matolite-encrusted boulders; H41/f061) and from several other samples slightly higher in the section (H41/f100; H41/f101; H41/f102) (Table 2). The palynoflora in the samples associated with stromatolites was dominated by the lacustrine algaePediastrumandBotryococcus[40]. It also contained several ferns, including the tree fernCyatheaand a range of lake margin and forest species which likely represent the rainforest habitat occupied by early Miocene Mysta-cina. All samples contain a range of conifers, including an araucarian (Agathis(or perhaps ex-tinctAraucaria)) and several podocarps including the swamp-forestDacrycarpus(kahikatea),
Dacrydium(rimu),Phyllocladus(celery pine), severalPodocarpus(totara) species, as well as the now locally extinctMicrocachrys(creeping strawberry pine) andLagarostrobos franklinii
(Huon pine). At least one species of palm was present in the vegetation, as were species of
Metrosideros(Myrtaceae). The pollen count is overwhelmingly dominated by the high pollen
Fig 3. Simple linear regression plots (OLS) with 95% confidence limits (blue lines) of dental and postcranial measurements of mystacinids (Table 1).A, Posterior upper premolar width (P4W) against first upper molar width (M1W). B, Distal humerus width (HDW) against first upper molar length (M1L). Square in each graph indicates M1 ofMystacina miocenalisplotted against value for the largest specimens of P4 and HD (respectively) for
mystacinids previously recovered from St Bathans [7].Mystacina tuberculata(filled circle),M.robusta(open circle),Icarops paradox(filled triangle),I.aenae
(open triangle).
Table 2. List of palynomorphs recorded from the St BathansMystacinafossil locality (H41/f061), and from three other sites (H41/f100, H41/f101, H41/f102) stratigraphically higher in the same section.
ST BATHANS FOSSIL LOCALITIES
PALYNOMORPHS H41/f061 H41/f100 H41/f101 H41/f102
Botryococcusfreshwater alga x x x
Pediastrumfreshwater alga x x x
monolete spores x x
CyathiditesCyatheaceae (Cyathea-type) x x x
Foveotriletes lacunosusLycopodiaceae x
Gleichenia circinataGleicheniaceae x
IschyosporitesFilicopsida x
Latrobosporites marginisLycopodiaceae x
Lycopodium fastigiatumLycopodiaceae x
?LygodiumSchizaeaceae x
Peromonolites vellosusPteridaceae x
PolypodiaceoisporitesPteridaceae x x
Polypodiisporites minimusPolypodiaceae, Davalliaceae x x x
Polypodiisporites radiatusPolypodiaceae x
CONIFER POLLEN
Araucariacites australisAraucariaceae (Araucaria,Agathis) x x x
Dacrycarpites australiensisPodocarpaceae (Dacrycarpus) x x x
Dacrydiumites praecupressinoidesPodocarpaceae (Dacrydium) x x x
?LibocedrusCupressaceae x
Microalatidites paleogenicusPodocarpaceae (Phyllocladus) x x x
Phyllocladidites mawsoniiPodocarpaceae (Lagarostrobos franklinii) x
Podocarpidites x x x x
Podocarpidites puteusPodocarpaceae(Podocarpus/Prumnopitys) x x x
Podosporites brevisaccatusPodocarpaceae(Microcachrys) x x
Podosporites parvusPodocarpaceae (Microcachrys) x x
ANGIOSPERM POLLEN
ArecipitesArecaceae x
Arecipites otagoensisArecaceae x x
Chenopodiaceae
cf.DracophyllumEricales x x
Haloragacidites amolosusHaloragaceae x
Haloragacidites harrisiiCasuarinaceae, Myricaceae x x x x
Haloragacidites myriophylloidesHaloragaceae x
Liliacidites x x
Liliacidites aviemorensisLiliaceae x
Lymingtonia cenozoicaNyctaginaceae x
Malvaceae x
Malvacipollis subtilisEuphorbiaceae or Malvaceae x
Milfordia homeopunctataRestionaceae, Flagellariaceae x x x
Myrtaceidites mesonesusMyrtaceae (Metrosideros) x x x x
Myrtaceidites parvusMyrtaceae (Leptospermum-type) x x
Nothofagidites asperusNothofagaceae (NothofagussubgenusLophozonia) x x x
Nothofagidites cranwelliaeNothofagaceae (NothofagussubgenusBrassospora) x x x
Nothofagidites lachlaniaeNothofagaceae (NothofagussubgenusFuscospora) x x x x
Nothofagidites spinosusNothofagaceae (NothofagussubgenusBrassospora) x x x
producers Casuarinaceae (sheoaks) and several species of Nothofagaceae (southern beech); al-though Casuarinaceae and theBrassosporagroup of southern beeches are no longer present in the New Zealand flora, theFuscosporabeeches and Myrtaceae are still major components of the modern vegetation [41].
Discussion
The new large mystacinid from St Bathans, Central Otago is referred toMystacinaand pro-vides the first pre-Pleistocene record for New Zealand’s endemic bat genus. The St Bathans sediments are estimated to be early Miocene in age on the basis of palynological data that con-strain the age of the Bannockburn Formation to the local Altonian Stage, or 19–16 Ma [43–46] (Fig 1). The discovery extends the evolutionary history ofMystacinaback at least 16 million years, a longevity consistent with that for other bat genera, which range from around 40 to less than 2 million years [47]. The presence of a species ofMystacinain the St Bathans Fauna also suggests separation of New Zealand and Australian mystacinid lineages since at least the early Miocene. It confirms thatMystacinahas had a long presence in New Zealand, in common with other genera of terrestrial vertebrates in the St Bathans Fauna including frogs (e.g.Leiopelma), lizards (Hoplodactylus,Oligosoma) and some birds (Aptornis,Pelecanoides,Aegotheles) [25,28,
29,48].
The family Mystacinidae itself is an ancient lineage that molecular data indicate diverged from other bats c.51–41 Ma [49–52]. It is one of eight families in the diverse and speciose su-perfamily Noctilionoidea, whose geographic origins are unclear, but which probably represents a Southern Hemisphere group [51]. Gunnell et al. [3] suggested that noctilionoids originated in Eastern Gondwana with a subsequent dispersal south into Australia (mystacinids) and then westward to South America via Antarctica (this lineage leading to the five Neotropical noctilio-noid families). The presence of what may be plesiomorphic mystacinids (species ofIcarops) in the Australian fossil record (as argued by Hand et al. [5,6,14]) also suggests that Australia was the source of New Zealand’s mystacinids. The oldest fossil record for mystacinids is from c. 26 Ma Etadunna Formation of Lake Palankarinna, South Australia [6,53]. The divergence of mys-tacinids from ancestral noctilionoids post-dates separation of Zealandia from the rest of
Table 2. (Continued)
ST BATHANS FOSSIL LOCALITIES
PALYNOMORPHS H41/f061 H41/f100 H41/f101 H41/f102
Nyssapollenites endobalteusEuphorbiaceae x
Palaeocoprosmadites zelandiaeRubiaceae(Coprosma) x
Ptychotriporines x x
Rhoipites alveolatus? Euphorbiaceae x
Rhoipites hekelii x
Rhoipites rhomboidaliformis x x
Tetracolporites x
?Tricolpites delicatulus x x
Triptyches x x
Tubulifloridites antipodicaAsteraceae x x
Tubulifloridites simplisAsteraceae x x
TyphaTyphaceae x
Full citations and botanical affinities in Raine et al. [42] and Mildenhall et al. [40]
Gondwana, beginning about 81 Ma, with its last connection to Australia, via the Lord Howe Rise, severed at about 52 Ma [54].
Like its congeners,Mystacina miocenaliswas probably semi-terrestrial. All mystacinids for which postcranial elements are known exhibit skeletal specializations for semi-terrestrial loco-motion [14], including smaller mystacinids of unclear generic identity also recovered from the St Bathans deposit [7] and Oligo–Miocene species ofIcaropsfrom Australia [6]. These speciali-zations include derived features of the distal humerus such as development of the lateral supraepicondylar groove and lateral inclination of the humeroradial articulation: study of the myology ofM.tuberculataindicates that these features are functionally correlated with terres-trial locomotion in mystacinids [14].Mystacina tuberculata’s terrestrial habits are reflected in numerous specializations of the wing, foot, leg, spine and pectoral and pelvic girdles [12,55–
57]. When moving on its wrists and backward facing feet, its wings are furled tightly in a pro-tective sheath-like portion of the plagiopatagium [12]. Reduced pro- and uropatagia enable free movement of fore and hindlimbs respectively [58,59], while secondary talons on the thumb and toe claws increase grip on the substrate, as does a system of adhesive grooves in the soles of its feet [60].
Mystacina tuberculatapopulations are restricted today to extensive areas of undisturbed old-growth, temperate, closed evergreen forest types dominated byPodocarpus,Dacrydium,
Agathisand species ofNothofagus[16,61] with large trees (>1 m girth and>25 m high) avail-able for well-insulated colonial roosts, abundant epiphytes and deep leaf-litter: the species also occurs in low numbers in areas adjacent to undamaged old-growth forest [10,62]. The habitat preferences ofM.robustaare not well known, but it is presumed to have had similar require-ments to M.tuberculata[10,16]. All of the tall forest conifers (Agathis,Dacrydium, Dacrycar-pus,Podocarpus,Prumnopitys) and most of the angiosperms (Nothofagus,Metrosideros,
Weinmannia) in which extantMystacinais known to roost have a long history in New Zea-land, dating back at least to the Oligocene to early Miocene (e.g. Gore Lignite Measures, New-vale Mine, Southland [63]; Foulden Maar, Otago [40,41]) (Fig 1).Fig 4provides a schematic reconstruction of the mixed Nothofagaceae—Podocarpaceae forest habitat that would have been occupied byMystacina, based on the palynofloras recorded inTable 2.
Possibly correlated with its semi-terrestrial capabilities, extantMystacina tuberculatais an omnivorous and opportunistic feeder [12,15–17,64] that consumes moths and other flying in-sects and terrestrial arthropods, as well as gleaning wood and moss fragments, eating fruit and sipping nectar and perhaps incidentally ingesting flower fragments from several plant groups (see below). The new fossil speciesM.miocenalisprobably had a similarly broad diet, based on strikingly similar molar morphology to that ofM.tuberculataandM.robustaand little to sug-gest any different specialization. These similarities include features typically found in insectivo-rous as well as omnivoinsectivo-rous bats [65] including unspecialised dilambdodonty with a relatively wide buccal shelf, a deep protofossa and prominent protocone. Differences observed inM. mio-cenalis, such as better developed para- and metaconules (producing more complex blade sys-tems) and a continuous, thickened cingulum (better protecting the gums), may indicate inclusion of harder and perhaps more diverse foods in the diet, as has been proposed for other large extinct bats [e.g.,66]. In contrast, more conspicuous differences betweenMystacinaand
Icaropsspecies in molar morphology, such as much larger hypocone shelves (heels) and longer postmetacristae, have been interpreted to indicate greater reliance on insectivory by some Aus-tralian mystacinids [6].
(Metrosiderosspp.) and rewarewa (Knightia excelsa) (Proteaceae) [10,16], with pollen occur-ring in its guano, stomach and fur [10,12,15–17,19,20]. Of theseMystacina-pollinated plants,
Metrosideroshas a long fossil record in New Zealand (asMyrtaceidites mesonesus)extending back to the Oligocene to early Miocene in Southland and Central Otago [42,44,67]. Leaf fossils and pollen both confirm a long record of Proteaceae that were much more diverse in New Zea-land in the past (e.g. Newvale Mine and Foulden Maar [68]).Knightiahas probably been pres-ent since the Miocene andK.excelsasince the Pliocene [69].
In modern New Zealand forests,Mystacinaappears to be one of the few native pollinators of the unusual obligate root parasite,Dactylanthus taylorii(wood rose) [70]. In general, most flowers visited by bats and non-volant mammals are dull-coloured and odorous, producing co-pious amounts of nectar and pollen, whereas diurnal birds usually visit brightly coloured (often red) and odourless flowers ([71]; but see [72]).Dactylanthus tayloriiis the only fully holopara-sitic plant in the New Zealand flora and the sole New Zealand representative of the family Bala-nophoraceae which is widespread in the tropics and subtropics. It is characterized by ground-flowering, sweetly scented, pollen-filled inflorescences that are extremely attractive to Mysta-cina[64,70].Dactylanthus tayloriihas distinctive periporate pollen grains and a well-con-strained fossil history in New Zealand. Fossil pollen grains ofParsonsidites multiporus
Mildenhall & Crosbie [73] are assigned with confidence toDactylanthus[42] and are recorded back to the Waiauan local stage (12.7–11.0 Ma), and with less certainty back to the Altonian (18.7–15.9 Ma) in the southern South Island (Gore Lignite Measures: D.C. Mildenhall pers. ob-servation). AlthoughDactylanthus tayloriiis currently restricted to the North Island and per-haps the northern South Island [74], pollen grains of this species were found in an early Pleistocene warm interglacial fossil forest site near Colac Bay, on the shore of Foveaux Strait (D.C. Mildenhall, pers. observation), confirming suggestions that during past periods of warm-er climate it was able to migrate much furthwarm-er south [75].
Fruits eaten by modernMystacinainclude those of kiekie, perching lilies, and hinau ( Elaeo-carpus dentatus: Elaeocarpaceae) [12].Mystacinaalso disperses the seeds of kiekie and perch-ing lilies among others [10,16,17]). Daniel [12] reported thatMystacinaate the succulent
Fig 4. Schematic reconstruction of the forest habitat on the shores of paleolake Manuherikia, South Island, New Zealand in the early Miocene.
bracts of kiekie in autumn and winter, as well as berries of two species ofCollospermumand part of the exocarp and mesocarp ofElaeocarpus dentatus[17].Freycinetia(as the pollen type
Lateropora glabra[67]) has a long fossil record in New Zealand, dating back at least to the late Oligocene in Southland [67] and possibly the late Eocene [76]. The epiphytic lilyCollospermum
is closely related to, and possibly nested within, the genusAstelia[77]:Asteliapollen ranges back to the late Eocene and well-preservedAstelialeaves occur in earliest Miocene sediments at Foulden Maar [78] (Fig 1).
As well as eating plant material,Mystacinais noted for consuming large quantities of both flying and terrestrial arthropods. On average,Mystacina tuberculataindividuals consume 5–7 g of arthropods per night or 36–50% of pre-feeding body mass [12,16,79]. Arkins et al. [15] and Lloyd [16] noted that most of the identifiable arthropod taxa observed in the droppings of
Mystacinawere from four insect orders: Coleoptera (Scarabeidae, Curculionidae, Carabidae and Chrysomelidae), Lepidoptera, Diptera (Tipulidae, Muscidae and Psychodidae) and Or-thoptera. Spiders were also present, as were Myriapoda and less commonly Neuroptera, Acar-ina and Hymenoptera (Formicidae) [15]. A high proportion of these families of native
arthropods eaten byMystacinahave been discovered recently as fossils in early Miocene depos-its at Foulden Maar, geographically close to St Bathans [80,81]. Foulden Maar is a paleolake deposit in the Waipiata Volcanic Field of earliest Miocene age that was surrounded by a dense, evergreen rainforest [40] (Fig 1). It preserves New Zealand’s first described pre-Quaternary ter-restrial arthropod fauna, the majority of which are ground-dwelling, forest litter and wood-living taxa with low dispersal ability. The fauna to date includes representatives of the coleop-teran families Curculionidae and Chrysomelidae, the dipcoleop-teran family Tipulidae, hymenopter-ans (Formicidae), and a variety of spiders (Araneae) [81], all of which are included in the prey of livingMystacina.
Within the superfamily Noctilionoidea, nectarivory and bat-plant pollination systems ap-pear to have developed several times: independently at least twice in the Neotropical family Phyllostomidae [82–85] and presumably once in Mystacinidae. Flower-visiting bats are gener-ally found in the tropics and subtropics [86], although some may migrate seasonally into tem-perate regions [18]. In temperate regions, in contrast, bats visiting flowers are rare, seasonal or absent and mammalian pollinators are more typically non-volant (e.g. rodents, primates and marsupials) [71,72]. Perhaps for this reason, the importance of bat pollination in modern New Zealand’s temperate ecosystems by the semi-terrestrialMystacinahas been significantly under-estimated, as noted by Pattemore and Wilcove [19] and Cummings et al. [20].
The presence of at least two, and possibly three, mystacinid species in the 19–16 Ma St Bath-ans deposit of Central Otago signals that members of the family had arrived and diversified in New Zealand by the earliest Miocene. Identification of one of these as a species of the modern genusMystacinaprovides further evidence that the landmass of New Zealand has remained large enough to maintain long-term survival of indigenous vertebrate lineages. Accumulating data indicate that even at maximum marine transgression in the late Oligocene–earliest Mio-cene, emergent New Zealand was at least equivalent in size to modern New Caledonia [87]. The St Bathans mystacinids provide one of the longest fossil records for an endemic lineage of island bats globally [7]. The only other“island”record of similar length is the recently reported, as yet undescribed Miocene hipposiderids from Madagascar [88], another microcontinent rifted from the supercontinent Gondwana.
and terrestrial arthropods are remarkably similar to those in the modern endemic New Zealand biota, and suggest remarkably long-term ecological associations withMystacinain regards to its colonial roosts, arthropod prey, and pollination and seed dispersal services for forest plants. In tropical ecosystems, many bat species provide pollination and seed dispersal services, but in temperate New Zealand only one currently fills this role. Nevertheless, the fossil record sug-gests that this could represent a deep-rooted, mutualistic relationship between a semi-terrestri-al bat lineage and the forest ecosystems in which it has evolved for at least 16 million years.
Acknowledgments
We thank landowners Ann and Euan Johnstone for graciously allowing us access to the bat-bearing fossil deposits and many colleagues for helping in the St Bathans excavations. We also gratefully acknowledge the following for access to and loan of comparative material in their care: R. Coory and G. Stone, Museum of New Zealand Te Papa Tongarewa; S. Ingleby and T. Ennis, Australian Museum, Sydney; E. Westwig and N. Duncan, American Museum of Natural History, New York; H. Kafka, National Museum of Natural History, Smithsonian Institution, Washington D.C.; K. Krohmann, Naturmuseum Senckenberg, Frankfurt; and J.-M. Pons, Mu-séum National d'Histoire Naturelle, Paris. We thank N.B. Simmons, W.G. Lee, J.G. Conran, T. Myers, A. Ravel and an anonymous reviewer for constructive criticism of the drafts of the man-uscript, and T. Reichgelt for preparingFig 4.
Author Contributions
Analyzed the data: SJH DEL THW MA JPW AJDT SWS RPS DCM EMK JKL. Contributed re-agents/materials/analysis tools: SJH DEL THW MA JPW AJDT SWS RPS DCM EMK JKL. Wrote the paper: SJH DEL THW MA JPW AJDT SWS RPS DCM EMK JKL.
Appendix
List of comparative bat material used in this study
Institutional abbreviations: AM, Australian Museum, Sydney; AMNH, American Museum of Natural History, New York; AR, Vertebrate Palaeontology Collection, University of New South Wales, Sydney; CM, Canterbury Museum, Christchurch; FMNH, Field Museum of Natural History, Chicago; MNHN, Muséum national d'Histoire naturelle, Paris; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington; QM, Queensland Museum, Brisbane, Australia; SMF, Naturmuseum Senckenberg, Frankfurt; USNM, National Museum Natural History, Smithsonian Institution, Washington D. C.
Fossil and subfossil specimens: Mystacinidae:Mystacina robustaNMNZ S.35205, Exhale Air Cave, Ellis Basin, Mt Arthur, Nelson, New Zealand;M.tuberculataNMNZ S.32400-parts 1–20, Predator Cave, Takaka Hill, Nelson, NZ; Mystacinidae indet. 1 & 2, NMNZ S.41867, S.42215, S.44260, S.51739, S.51742, S.52083, S.52401, S.52402, S.52920, Manuherikia River sec-tion, Bannockburn Formasec-tion, Home Hills Stasec-tion, St Bathans, Central Otago, New Zealand;
Icarops aenaeQM F30573–4 andI.paradoxQM F30580–2, Riversleigh World Heritage Area, Queensland, Australia.
Modern comparative specimens: Craseonycteridae:Craseonycteris thonglongyaiSMF 54513. Emballonuridae:Emballonura atrataFMNH 176360;Saccolaimus saccolaimusAM M3491;Saccopteryx bilineataAMNH M267842;Taphozous georgianusAR20502. Furipteridae:
Furipteris horrensAMNH M267213. Hipposideridae:Anthops ornatusAM M5831;Aselliscus tricuspidatusSMF 24745;Hipposideros bicolorAM M9231;Hipposideros diademaAR5194;
AR20505. Miniopteridae:Miniopterus orianaeAR1868;M.australisAM M5556. Molossidae:
Cheiromeles parvidensAMNH M241942;Chaerephon plicatusAMNH M107932;Cynomops planirostrisAMNH M234455;Eumops auripendulusAMNH M248212;Molossus molossus
AMNH M267243;Mops brachypterusAMNH M241062;Mormopterus beccariiAM M8509;
Mormopterus planicepsAR20503;Myopterus daubentoniiAMNH M48855;Promops nasutus
AMNH M184648;Tadarida australisAM M7910. Mormoopidae:Mormoops blainvillei
AMNH M238144;Pteronotus davyiAMNH M204961. Mystacinidae:Mystacina tuberculata
NMNZ LM1231. Myzopodidae:Myzopoda auritaUSNM 448932; MNHN 1907.618. Natalidae:
Natalus stramineusUSNM 362103. Noctilionidae:Noctilio albiventrisUSNM 390592. Nycteri-dae:Nycteris capensisAM M6071;Nycteris javanicaAMNH M103269. Phyllostomidae:
Anoura geoffroyiAMNH M264935;Artibeus jamaicensisAR21504;Desmodus rotundus
AMNH M248941;Glossophaga soricinaAMNH M230206;Micronycteris hirsutaAMNH M267860;Phyllostomus hastatusAMNH M267901;Tonatia saurophilaAMNH M267912;
Trachops cirrhosusAMNH M266081. Pteropodidae:Balionycteris maculataAMNH M233970;
Nyctimene robinsoniAR17520;Pteropus scapulatusAR4764;Rousettus amplexicaudatus
USNM 78616. Rhinolophidae:Rhinolophus megaphyllusAR1655. Rhinopomatidae: Rhino-poma hardwickeiAR21506. Thyropteridae:Thyroptera tricolorAMNH M266356. Vespertilio-nidae:Antrozous pallidusUSNM 564036;Chalinolobus morioAR1653;Eptesicus fuscus
AMNH M139513;Falsistrellus tasmaniensisAM M3360;Kerivoula papillosaAMNH M247576;Myotis macropusAM M5453;Nyctophilus gouldiAM M12548;Scoteanax ruepelli
AM M5163;Scotorepens orionAM M5163;Vespadelus vulturnusAM M11413.
References
1. O'Donnell CFJ (2005) New Zealand long-tailed bat. In: King CM, editor. The Handbook of New Zealand Mammals 2nd edition. Melbourne: Oxford University Press. pp. 98–109.
2. Simmons NB (2005) Order Chiroptera. In: Wilson DE, Reeder DM, editors. Mammal Species of the World: A Taxonomic and Geographic Reference. Washington D.C.: Smithsonian Institution Press. pp. 312–529.
3. Gunnell GF, Simmons NB, Seiffert ER (2014) New Myzopodidae (Chiroptera) from the Late Paleogene of Egypt: emended family diagnosis and biogeographic origins of Noctilionoidea. PLoS ONE 9(2): e86712. doi:10.1371/journal.pone.0086712PMID:24504061
4. Czaplewski NJ, Morgan GS (2012) New basal noctilionoid bats (Mammalia: Chiroptera) from the Oligo-cene of subtropical North America. In: Gunnell GF, Simmons NB, editors. Evolutionary History of Bats. Boston: Cambridge University Press. pp. 162–209
5. Hand SJ, Murray PF, Megirian D, Archer M, Godthelp H (1998) Mystacinid bats (Microchiroptera) from the Australian Tertiary. Journal of Paleontology 72: 538–545.
6. Hand SJ, Archer M, Godthelp H (2005) Australian Oligo-Miocene mystacinids (Microchiroptera): upper dentition, new taxa and divergence of New Zealand species. Geobios 38: 339–352.
7. Hand SJ, Worthy TH, Archer M, Worthy JP, Tennyson AJD, Scofield RP (2013) Miocene mystacinids (Chiroptera: Noctilionoidea) indicate a long history for endemic bats in New Zealand. Journal of Verte-brate Paleontology 33: 1442–1448.
8. Worthy TH, Holdaway RN (2002) The Lost World of the Moa: Prehistoric Life of New Zealand. Indiana: Indiana University Press. Xxxiii + 718 pp.
9. Worthy TH, Holdaway RN (1994) Scraps from an owl’s table—predator activity as a significant tapho-nomic process recognised from New Zealand Quaternary deposits. Alcheringa 18: 229–245.
10. Lloyd BD (2005) Lesser short-tailed bat. In: King CM, editor. The Handbook of New Zealand Mammals 2nd edition. Melbourne: Oxford University Press. pp. 111–126.
11. O’Donnell CFJ (1999) Search for Pekapeka (Bats) on Putauhina Island, Southern Titi Islands, 6–9 No-vember 1999. Christchurch, New Zealand: Department of Conservation.
13. Riskin DK, Parsons S, Schutt WA, Carter GG, Hermanson JW (2006) Terrestrial locomotion of the New Zealand short-tailed batMystacina tuberculataand the common vampire batDesmodus rotundus. Journal of Experimental Biology 209: 1725–1736. PMID:16621953
14. Hand SJ, Weisbecker V, Beck RMD, Archer M, Godthelp H, Tennyson AJD et al. (2009) Bats that walk: a new evolutionary hypothesis for the terrestrial behaviour of New Zealand’s endemic mystacinids. BMC Evolutionary Biology 9: 169 (13 p.). doi:10.1186/1471-2148-9-169PMID:19615105
15. Arkins AM, Winnington AP, Anderson S, Clout MN (1999) Diet and nectarivorous foraging behaviour of the short-tailed bat (Mystacina tuberculata). Journal of Zoology (London) 247: 183–187.
16. Lloyd BD (2001) Advances in New Zealand mammalogy 1990–2000: short-tailed bats. Journal of the Royal Society of New Zealand 31: 59–81.
17. Daniel MJ (1976) Feeding by the short-tailed batMystacina tuberculataon fruit and possibly nectar. New Zealand Journal of Zoology 3: 391–398.
18. Rojas-Martinez A, Valiente-Banuet A, Arizmendi MC, Alcantara-Eguren A, Arita HT (1999) Distribution of the long-nosed bat (Leptonycteris curasoae) in North America: does a generalized migration pattern really exist? Journal of Biogeography 26: 1065–1077.
19. Pattemore DE, Wilcove DS (2012) Invasive rats and recent colonist birds partially compensate for the loss of endemic New Zealand pollinators. Proceedings of the Royal Society of London, Series B 279: 1597–1605. doi:10.1098/rspb.2011.2036PMID:22090388
20. Cummings G, Anderson S, Dennis T, Toth C, Parsons S (2014) Competition for pollination by the lesser short-tailed bat and its influence on the flowering phenology of some New Zealand endemics. Journal of Zoology 293: 281–288.
21. Worthy TH, Tennyson AJD, Archer M, Musser AM, Hand SJ, Jones C et al. (2006) Miocene mammal re-veals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proceedings of the Nation-al Academy of Sciences USA 103: 19419–19423. PMID:17159151
22. Worthy TH, Tennyson AJD, Jones C, McNamara JA, Douglas BJ (2007) Miocene waterfowl and other birds from Central Otago, New Zealand. Journal of Systematic Palaeontology 5: 1–39.
23. Jones MEH, Tennyson AJD, Worthy JP, Evans SE, Worthy TH (2009) A sphenodontine (Rhynchoce-phalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon). Pro-ceedings of the Royal Society of London B 276: 1385–1390.
24. Tennyson AJD, Worthy TH, Jones CM, Scofield RP, Hand SJ (2010) Moa’s Ark: Miocene fossils reveal the great antiquity of moa (Aves: Dinornithiformes) in Zealandia. Records of the Australian Museum 62: 105–114.
25. Lee MSY, Hutchinson MN, Worthy TH, Archer M, Tennyson AJD, Worthy JP et al. (2009) Miocene skinks and geckos reveal long-term conservatism of New Zealand's lizard fauna. Biology Letters doi: 10.1098/rsbl.2009.0440
26. Worthy TH, Tennyson AJD, Scofield RP (2011) An Early Miocene diversity of parrots (Aves, Strigopi-dae, Nestorinae) from New Zealand. Journal of Vertebrate Paleontology 31: 1102–1116.
27. Schwarzhans W, Scofield RP, Tennyson AJD, Worthy JP, Worthy TH (2012) Fish remains, mostly oto-liths, from the non-marine Early Miocene of Otago, New Zealand. Acta Palaeontologica Polonica 57: 319–350.
28. Worthy TH, Tennyson AJD, Scofield RP, Hand SJ (2013a) Early Miocene fossil frogs (Anura: Leiopel-matidae) from New Zealand. Journal of the Royal Society of New Zealand 4: 211–230.
29. Worthy TH, Worthy JP, Tennyson AJD, Salisbury SW, Hand SJ, Scofield RP (2013b) Miocene fossils show that kiwi (Apteryx, Apterygidae) are probably not phyletic dwarves. In: Göhlich UB, Kroh A, edi-tors. Paleornithological Research 2013—Proceedings of the 8thInternational Meeting of the Society of Avian Paleontology and Evolution. Vienna: Natural History Museum Vienna. pp. 63–80. doi:10.1371/ journal.pone.0082000PMID:24312392
30. Hand SJ (1990) First Tertiary molossid (Microchiroptera: Molossidae) from Australia: its phylogenetic and biogeographic implications. Memoirs of the Queensland Museum 28: 175–192.
31. Lindqvist J (1994) Lacustrine stromatolites and oncoids: Manuherikia Group (Miocene), New Zealand. In: Bertrand-Safati J, Monty C, editors. Phanerozoic Stromatolites II. Netherlands: Kluwer Academic Publishers. pp. 227–254
32. Worthy TH, Daniel MJ, Hill JE (1996) An analysis of skeletal size variation inMystacina robustaDwyer, 1962 (Chiroptera: Mystacinidae). New Zealand Journal of Zoology 23: 99–110.
33. Worthy TH, Scofield RP (2004) Skeletal and dental variation within and betweenMystacinaspecies in southern New Zealand. New Zealand Journal of Zoology 31: 351–361.
35. Smith FA, Lyons SK, Morgan EK, Jones KE, Kaufman DM, Dayan T et al. (2003) Body mass of Late Quaternary mammals. Ecological archives E084-094. Ecology 84: 3403.
36. Giannini NP, Gunnell GF, Habersetzer J, Simmons NB (2012) Early evolution of body size. In: Gunnell GF, Simmons NB, editors. Evolutionary History of Bats. Boston: Cambridge University Press. pp. 530–555
37. Daniel MJ (1990) Lesser short-tailed bat. In: King CM, editor. The Handbook of New Zealand Mammals. Auckland: Oxford University Press. pp. 123–130
38. Pole M (2014) The Miocene climate in New Zealand: estimates from paleobotanical data. Palaeontolo-gia Electronica 17: 2; 27A; 79 pp.
39. Reichgelt T, Kennedy EM, Conran JG, Mildenhall DC, Lee DE (2015) The early Miocene paleolake Manuherikia: vegetation heterogeneity and warm-temperate to subtropical climate in southern New Zealand. Journal of Paleolimnology doi:10.1007/s10933-015-9827-5
40. Mildenhall DC, Kennedy EM, Lee DE, Kaulfuss U, Bannister JM, Fox B et al. (2014) Palynology of the early Miocene Foulden Maar, Otago, New Zealand: Diversity following destruction. Review of Palaeo-botany and Palynology 204: 27–42.
41. Lee DE, Conran JG, Lindqvist JK, Bannister JM, Mildenhall DC (2012) New Zealand Eocene, Oligo-cene and MioOligo-cene macrofossil and pollen records and modern plant distributions in the Southern Hemi-sphere. The Botanical Review 78: 235–260.
42. Raine JI, Mildenhall DC, Kennedy EM (2011) New Zealand fossil spores and pollen: an illustrated cata-logue. 4th edition. GNS Science Miscellaneous Series 4.http://data.gns.cri.nz/sporepollen/index.htm 43. Mildenhall DC (1989) Summary of the age and paleoecology of the Miocene Manuherikia Group,
Cen-tral Otago, New Zealand. Journal of the Royal Society of New Zealand 19: 19–29.
44. Mildenhall DC, Pocknall DT, (1989) Miocene-Pleistocene spores and pollen from Central Otago, South Island, New Zealand. New Zealand Geological Survey Palaeontological Bulletin 59: 1–128.
45. Pole M, Douglas BJ (1998) A quantitative palynostratigraphy of the Miocene Manuherikia Group, New Zealand. Journal of the Royal Society of New Zealand 28: 405–420.
46. Pole M, Douglas BJ, Mason G (2003) The terrestrial Miocene biota of southern New Zealand. Journal of the Royal Society of New Zealand 33: 415–426.
47. Eiting TP, Gunnell GF (2009) Global completeness of the bat fossil record. Journal of Mammalian Evo-lution 16: 151–173.
48. Worthy TH, Worthy JP, Archer M, Hand SJ, Scofield RP, Marshall BA et al. (2011) A decade on, what the St Bathans Fauna reveals about the Early Miocene terrestrial biota of Zealandia. In: Litchfield NJ, Clark K, editors. Abstract volume, Geosciences 2011 Conference, Nelson, New Zealand. Geoscience Society of New Zealand Miscellaneous Publication 130A: 120.
49. Kennedy M, Paterson AM, Morales JC, Parsons S, Winnington AP, Spencer HG (1999) The long and short of it: branch lengths and the problem of placing the New Zealand short-tailed bat,Mystacina. Mo-lecular Phylogenetics and Evolution 13: 405–416. PMID:10603267
50. Van Den Bussche RA, Hoofer SR (2000) Further evidence for inclusion of the New Zealand short-tailed bat (Mystacina tuberculata) within Noctilionoidea. Journal of Mammalogy 81: 865–874.
51. Teeling EC, Springer MS, Madsen O, Bates P, O'Brien SJ, Murphy WJ (2005) A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307: 580–584. PMID:15681385 52. Miller-Butterworth CM, Murphy WJ, O'Brien SJ, Jacobs DS, Springer MS, Teeling EC (2007) A family
matter: conclusive resolution of the taxonomic position of the long-fingered bats,Miniopterus. Molecu-lar and Biological Evolution 24: 1553–1561.
53. Archer M (1978) Australia’s oldest bat, a possible rhinolophid. Proceedings of the Royal Society of Queensland 89: 23.
54. Gaina C, Müller DR, Royer J- Y, Stock J, Hardebeck J, Symonds P (1998) The tectonic history of the Tasman Sea: a puzzle with 13 pieces. Journal of Geophysical Research 103: 12413–12433.
55. Dobson GE (1884) On some peculiarities in the geographical distribution and in the habits of certain mammals inhabiting continental and oceanic islands. Annals and Magazine of Natural History 1884, 14 (5th ser.): 153–159.
56. Miller GS (1907) The families and genera of bats. Bulletin of the United States National Museum 57: 1–282.
57. Walton DW, Walton GM (1970) Post-cranial osteology of bats. In: Slaughter BH, Walton DW, editors. About Bats: a Chiropteran Symposium. Dallas: Southern Methodist University Press. pp. 93–126.
58. Dwyer PD (1960) New Zealand bats. Tuatara 8: 61–71.
60. Carter GG, Riskin DK (2006) Mystacina tuberculata. Mammalian Species 790: 1–8.
61. Sedgeley JA (2003) Roost site selection and roosting behaviour in lesser short-tailed bats (Mystacina tuberculata) in comparison with long-tailed bats (Chalinolobus tuberculatus) inNothofagusforest, Fiordland. New Zealand Journal of Zoology 30: 227–241.
62. O’Donnell CFJ (2009) The ecology and conservation of New Zealand bats. In: Fleming TH, Racey PA, editors. Island Bats. Evolution, Ecology and Conservation. Chicago: University of Chicago Press. pp. 460–495.
63. Jordan GJ, Carpenter RJ, Bannister JM, Lee DE, Mildenhall DC, Hill RS (2011) High conifer diversity in Oligo-Miocene New Zealand. Australian Systematic Botany 24: 121–136.
64. McCartney J, Stringer IAN, Potter MA (2007) Feeding activity in captive New Zealand lesser short-tailed bats (Mystacina tuberculata). New Zealand Journal of Zoology 34: 227–238.
65. Freeman PW (1988) Frugivorous and animalivorous bats (Microchiroptera): dental and cranial adapta-tions. Biological Journal of the Linnean Society 33: 249–272.
66. Ravel A, Adaci M, Bensalah M, Mahboubi M, Mebrouk F, Essid EM et al. (2014) New philisids (Mamma-lia, Chiroptera) from the Early–Middle Eocene of Algeria and Tunisia: new insight into the phylogeny, palaeobiogeography and palaeoecology of the Philisidae. Journal of Systematic Palaeontology doi:10. 1080/14772019.2014.941422
67. Pocknall DT, Mildenhall DC (1984) Late Oligocene–early Miocene spores and pollen from Southland, New Zealand. New Zealand Geological Survey Paleontological Bulletin 51: 66 pp.
68. Carpenter RJ, Bannister JM, Lee DE, Jordan GJ (2012) Proteaceae leaf fossils from the Oligo-Miocene of New Zealand: new species and evidence of biome and trait conservatism. Australian Systematic Bot-any 25: 375–389.
69. Pole M (2007) Plant-macrofossil assemblages during Pliocene uplift, South Island, New Zealand. Aus-tralian Journal of Botany 55: 118–142.
70. Ecroyd CE (1995)Dactylanthusand bats: the link between two unique endangered New Zealand spe-cies and the role of the community in their survival. In: Saunders AD, Craig JL, Mattiske EM, editors. Nature Conservation 4: the Role of Networks. Chipping Norton, New South Wales: Surrey Beatty and Sons. pp. 78–87
71. Sussman RW, Raven PH (1978) Pollination by lemurs and marsupials: an archaic coevolutionary sys-tem. Science 200: 731–736. PMID:17743224
72. Carthew SM RL Goldingay RL (1997) Non-flying mammals as pollinators. Trends in Ecology and Evolu-tion 12: 104–108. PMID:21237993
73. Mildenhall DC, Crosbie YM (1980) Some porate pollen from the upper Tertiary of New Zealand. New Zealand Journal of Geology and Geophysics 22: 499–508.
74. Macphail MK, Mildenhall DC (1980)Dactylanthus taylori: in North-West Nelson, New Zealand? New Zealand Journal of Botany 18: 148–152.
75. Wood JR, Wilmshurst JM, Worthy TH, Holzapfel AS, Cooper A (2012) A lost link between a flightless parrot and a parasitic plant and the potential role of coprolites in conservation paleobiology. Conserva-tion Biology 26: 1091–1099. doi:10.1111/j.1523-1739.2012.01931.xPMID:23025275
76. Pocknall DT, Turnbull IM (1989) Palaeoenvironmental and stratigraphic significance of palynomorphs from Upper Eocene (Kaiatan) Beaumont Measures and Orauea Mudstone, Waiau Basin, western Southland, New Zealand. New Zealand Journal of Geology and Geophysics 32: 371–378.
77. Birch J, Keeley SC, Morden CM (2012) Molecular phylogeny and dating of the Asteliaceae (Aspara-gales):Astelia s.l. evolution provides insight into the Oligocene history of New Zealand. Molecular Phy-logenetics and Evolution 65: 102–115. doi:10.1016/j.ympev.2012.05.031PMID:22664642
78. Maciunas E, Conran JG, Bannister JM, Paull R, Lee DE (2011) MioceneAstelia(Asparagales: Astelia-ceae) macrofossils from southern New Zealand. Australian Systematic Botany 24: 19–31.
79. Lloyd BD, McQueen SM (2002) Measuring mortality in short-tailed bats (Mystacina tuberculata) as they return from foraging after an aerial 1080 possum control operation. New Zealand Journal of Ecology 26: 53–59.
80. Kaulfuss U, Harris AC, Lee DE (2010) A new fossil termite (Isoptera, Stolotermitidae,Stolotermes) from the early Miocene of Otago, New Zealand. Acta Geologica Sinica 84: 705–709.
81. Kaulfuss U, Lee DE, Barratt BIP, Leschen RAB, Larivière M-C, Dlussky GM et al. (2014) A diverse fossil
terrestrial arthropod fauna from New Zealand: evidence from the early Miocene Foulden Maar fossil
lagerstätte. Lethaia doi:10.1111/let.12106
83. Rojas D, Vale A, Ferrero V, Navarro L (2011) When did plants become important to leaf-nosed bats? Di-versification of feeding habits in the family Phyllostomidae. Molecular Ecology 20: 2217–2228. doi:10. 1111/j.1365-294X.2011.05082.xPMID:21481051
84. Monteiro LR, Nogueira MR (2011) Evolutionary patterns and processes in the radiation of phyllostomid bats. BMC Evolutionary Biology 11: 137 (23 p.). doi:10.1186/1471-2148-11-137PMID:21605452 85. Dávalos LM, Cirranello AL, Geisler JH, Simmons NB (2012) Understanding phylogenetic incongru-ence: lessons from phyllostomid bats. Biological Reviews 87: 991–1024. doi:10.1111/j.1469-185X. 2012.00240.xPMID:22891620
86. Fleming TH, Geiselman C, Kress WJ (2009) The evolution of bat pollination: a phylogenetic perspec-tive. Annals of Botany (London) 104: 1017–1043. doi:10.1093/aob/mcp197PMID:19789175 87. Lee DE, Lindqvist JK, Beu AG, Robinson JH, Ayress MA, Morgans HEG et al. (2014) Geological setting
and diverse fauna of a Late Oligocene rocky shore ecosystem, Cosy Dell, Southland. New Zealand Journal of Geology and Geophysics 57: 195–208.