Protease encapsulation: effect of particle composition and size on enzymatic
reuse
Encapsulação de protease: efeito da composição e tamanho de partículas na
reutilização enzimática
DOI:10.34117/bjdv6n3-469
Recebimento dos originais: 10/02/2020 Aceitação para publicação: 30/03/2020
Daiane Bortolote Ferreira
Mestranda do Programa de Pós Graduação em Engenharia e Ciência de Alimentos da Universidade Estadual Paulista “Júlio de Mesquita Filho”
Instituição: Universidade Estadual Paulista “Júlio de Mesquita Filho”
Endereço: Rua Monsenhor Braz Baffa,284 – Jardim Nazareth, São José do Rio Preto – SP, Brasil E-mail: daia.bferreira@hotmail.com
Carolina Merheb-Dini
Doutora em Engenharia e Ciência de Alimentos pela Universidade Estadual Paulista “Júlio de Mesquita Filho”
Instituição: Universidade Federal do Triângulo Mineiro
Endereço: Av. Randolfo Borges Júnior, 1400 – Univerdecidade, Uberaba -MG, Brasil E-mail:carolina.dini@uftm.edu.br
ABSTRACT
The increase in the availability of proteases with applied properties for the food industry is of extreme technological and economic importance. Enzymatic immobilization is a device used to reduce costs, since it reuses the enzyme and makes it possible to work with continuous processes. The work evaluated the immobilization efficiency and enzymatic reuse of Corolase in particles with different compositions (alginate; alginate-chitosan and alginate-chitosan-glutaraldehyde) and diameters. The microbial protease was immobilized, weighed and submitted to an enzymatic reaction for 20, 40 and 60 minutes using casein (0.5%) as a substrate. The particle composition and sizes affected significantly (p> 0.05) the recovery of proteolytic activity. Enzymatic reactions carried out for 60 min produced greater activities, for any size and composition, suggesting that a longer time is needed to overcome diffusion restrictions and to provide enzyme-substrate interaction. The enzymatic reuse showed a progressive reduction at each new cycle, regardless of the composition and size of the particles, with the alginate particles having better effects. Immobilization by encapsulation proved to be a good tool for reusing the enzyme.
Keywords: Enzymes. Corolase. Immobilization. Composition. Reuse. RESUMO
O aumento da disponibilidade de proteases com propriedades aplicadas para a indústria de alimentos é de extrema importância tecnológica e econômica. A imobilização enzimática é um dispositivo usado para reduzir custos, pois reutiliza a enzima e possibilita o trabalho com processos contínuos. O trabalho avaliou a eficiência da imobilização e a reutilização enzimática da Corolase em partículas com diferentes composições (alginato; alginato-quitosana e alginato-quitosana-glutaraldeído) e diâmetros. A protease microbiana foi imobilizada, pesada e submetida a uma reação enzimática por 20, 40 e 60 minutos, utilizando caseína (0,5%) como substrato. A composição e o tamanho das partículas afetaram significativamente (p> 0,05) a recuperação da atividade proteolítica. As reações
enzimáticas realizadas por 60 minutos produziram atividades maiores, para qualquer tamanho e composição, sugerindo que é necessário mais tempo para superar as restrições de difusão e proporcionar interação enzima-substrato. A reutilização enzimática mostrou uma redução progressiva a cada novo ciclo, independentemente da composição e tamanho das partículas, com as partículas de alginato apresentando melhores efeitos. A imobilização por encapsulamento provou ser uma boa ferramenta para reutilizar a enzima.
Palavras-chave: Enzimas. Corolase. Imobilização. Composição. Reuso. 1 INTRODUCTION
Most of the products and goods consumed by humans are submitted to industrial processes which ones involve reactions catalyzed by enzymes. The high demand for enzymes in differents áreas like food, textile, pharmaceutical, paper and cellulose industry stands out. The world market for enzymes is divided into three segments with the food industry as the main consumer, which holds 50% of the total enzymes commercialize. In the industrial sector, the most one used are in proteases segment, occupying about 40% of the commercialization, followed by carboidrases and lipases (MENDES et al., 2010). Proteases have wide availability, low cost, easy applicability and frequent use, so immobilization techniques have been used to look for better control and efficiency in industrial processes (SINGH et al., 2011).
The reuse of the biocatalyst is only possible when stability is maintained, which can be achieved by immobilization (ANWAR et al., 2009). Immobilization consists of the confinement of the protein in a solid support, allowing the process to occur in a continuous and controlled way, ensuring the use of the catalytic activity for a longer time and ease in the separation of the final product (MENDES et al., 2010). Encapsulation is a method of enzyme immobilization, widely used, it is characterized by the involvement of membranes or capsules made of polymers, not being chemically bound, which guarantees the structural preservation of the molecules. The enzymes remain free in the solution, but in a restricted space (SILVA et al., 2007).
The greatest contribution to the performance of the immobilized enzyme is given by the support, where inert polymers and inorganic materials are extensively applied. Alginate is a polysaccharide of low cost, easy immobilization and flexible application. It can be used together with chitosan to form complex hydrogels (MENDES et al., 2010). The functional groups presents in its chemical structure, such as hydroxyls and amines. It allows the use of several immobilization methods, while different chitosan conformations can be obtained by the deacetylation of chitin, such as powder, scales, hydrogels, membranes and others (BERGER et al., 2004). The support characterized by alginate and chitosan can be activated with gluteraldehyde, providing a derivative with high immobilization yield and recovered activity (MENDES et al., 2010). Its ability to interact with primary amine groups helps in fixing proteins in the immobilized support, making it more
resistant to extreme pH and temperature values, thus increasing the possibility of enzymatic reuse (MIGNEAULT et al., 2004).
The current work evaluated the composition of these supports in the activity and enzymatic reuse of the immobilized protease in particles of two different diameters.
2 MATERIAL AND METHODS 2.1 ENZYME IMMOBILIZATION
Corolase 7089 was immobilized in alginate according to the procedures described by Anwar et al. (2009). The solution containing the solubilized Corolase was mixed with the sodium alginate solution (2%) in a 1: 1 ratio, and then the enzyme-alginate mixture was added dropwise in a calcium chloride solution (0.2 M) for particle formation. To obtain the alginate-chitosan support, the procedures described by Zhou, Li and Li (2010) were followed by making some modifications. A mixture of solubilized Corolase and sodium alginate was obtained and this was added dropwise in a mixture containing chitosan (0.5%) solubilized in acetic acid (5%) and calcium chloride (2%), where they remained for 20 minutes for the particles to stiffen, followed by washing with distilled water. Seeking the formation of the alginate-chitosan-gluteraldehyde support, the particles were immersed in a gluteraldehyde solution (0.5%) for 20 minutes.
2.2 PARTICLE CHARACTERIZATION
Particles were obtained in two apparently different diameters, the larger one through dripping which was using a Pasteur pipette (1.71 mm opening) and the smaller one using a syringe (0.45 mm opening). The particles were characterized by determination of their average diameters (Ø) using images of the samples recorded in a magnifying glass and analyzed with the Image Pro Plus 6.0 software. The particles had an oval shape, so two measurements were made, one of them referring to the largest axis (a) and the other to the perpendicular axis (b), as shown in Figure 1. An average diameter was calculated between the measurements of the axes a and b through Equation 1.
Ø = ∑(√𝑎. 𝑏) (1)
2.3 DETERMINATION OF PROTEOLYTIC ACTIVITY
Proteolytic activity was determined according to the procedures described by Merheb-Dini et al. (2010), however they had suffer some modifications. A reaction mixture was prepared in 0.1 M phosphate buffer pH 6.5, containing 0.8 mL of substrate, with casein 0.5% (w / v), and the enzyme immobilized. The same was done for calcium chloride and free enzyme by adding 0.2 mL of the substance of interest to be analyzed. A control was prepared, in which there was no presence of immobilized enzyme. All tests were performed in triplicate, incubated at 45ºC and interrupted by the addition of 1 mL of 10% trichloroacetic acid (TCA) in different periods of time, 20, 40 and 60 minutes. Then the samples were centrifuged at 15000 rpm for 15 minutes. The absorbance was read at 280nm in a spectrophotometer. The enzymatic activity was calculated according to Equation 2.
U mL =
∆ Abs 280nm . 10 . dilution factor 𝐸𝑛𝑧𝑦𝑚𝑒 𝑣𝑜𝑙𝑢𝑚𝑒
(2)
Where a unit of enzyme activity (U) can be arbitrarily defined as the amount of enzyme needed to cause an increase in 0.1 in absorbance at 280 nm under test conditions.
2.4 DETERMINATION OF IMMOBILIZATION EFFICIENCY
To determine the efficiency of immobilization, the proteolytic activity of the free enzyme (U / ml), called real free activity, was determined. Then, a known volume of enzyme was immobilized, and all particles formed were weighed. The volume equivalent to a given mass of particles was obtained and the theoretical immobilization activity (ATI) was calculated, corresponding to 1 mL of the real free activity. This known mass of particles was used to determine proteolytic activity (AP). The immobilization efficiency was then calculated according to Equation 3:
EI (%) = 𝐴𝑇𝐼𝐴𝑃 . 100 (3)
2.5 ENZYME REUSE
The immobilized enzyme was submitted to a series of consecutive reactions, in which the activity of the first reaction was considered to be 100%. After a cycle, these particles were removed from the reaction medium, then washed with distilled water and finally reused. This procedure was repeated three more times. Enzymatic reuse was performed only for the best reaction time.
2.6 STATISTICAL ANALYSIS
The results obtained were analyzed statistically with the aid of the Statistica 7.0 software. Analysis of Variance (ANOVA) was used to evaluate the effect of treatments in immobilization efficiency. Tukey's test was used for comparisons of means (p <0.05).
3 RESULTS AND DISCUSSION 3.1 PARTICLE CHARACTERIZATION
The average diameter of the largest and smallest particles (Figure 2) were determined using the Image Pro Plus 6.0 software, where at least 100 particles of each size were measured. An average diameter of 1.41 ± 0.06mm and 3.26 ± 0.25mm was observed for the smallest and largest particles, respectively. The size of the largest particles were approximately 2.5 times greater than the smaller ones.
Figure 2 - Illustration of particles of different diameters (A) obtained with a Pasteur pipette and (B) obtained with a
syringe
3.2 EFFECT OF PARTICLE COMPOSITION AND SIZE AND REACTION TIME ON IMMOBILIZATION EFFICIENCY
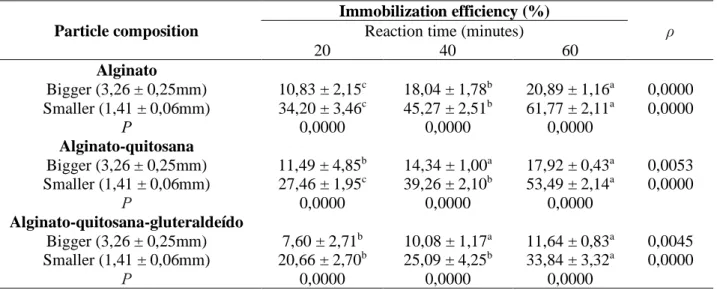
It can be seen through Table 1, that the immobilization efficiency increases while the reaction time goes up, for all the different compositions. It was noticed that the best result is in 60 minutes. This is due to the need for a longer time for the diffusion of casein to occur and also the release of the product into the reaction medium. Regarding the particle size, it can be seen that, for all compositions and times, the 1.41mm particles are the ones that showed the best results. Larger substrates can be associated to difficulties like: control of the pore size of the substrate, desorption of the enzyme and restrictions in mass transfer and diffusion of substrates through the pores (SOUZA et al., 2017).
Table 2 shows that particles composed only of alginate showed better effects, with the exception of the test performed in 20 minutes for larger particles, in which there was no significant
difference in the Tukey test (p <0.05). The addition of chitosan, separately, and gluteraldehyde in the composition of the immobilization supports did not show positive effects. The enzymatic activity of the calcium solution used during the immobilization steps was also analyzed, where no activity was detected. Therefore, it indicates that the entire enzyme had been immobilized. Low efficiencies are justified by the difficulty of the substrate.
Table 1 - Effect of composition, particle size and reaction time in immobilization efficiency (n = 2).
Particle composition
Immobilization efficiency (%)
ρ
Reaction time (minutes)
20 40 60 Alginato Bigger (3,26 ± 0,25mm) 10,83 ± 2,15c 18,04 ± 1,78b 20,89 ± 1,16a 0,0000 Smaller (1,41 ± 0,06mm) 34,20 ± 3,46c 45,27 ± 2,51b 61,77 ± 2,11a 0,0000 Ρ 0,0000 0,0000 0,0000 Alginato-quitosana Bigger (3,26 ± 0,25mm) 11,49 ± 4,85b 14,34 ± 1,00a 17,92 ± 0,43a 0,0053 Smaller (1,41 ± 0,06mm) 27,46 ± 1,95c 39,26 ± 2,10b 53,49 ± 2,14a 0,0000 Ρ 0,0000 0,0000 0,0000 Alginato-quitosana-gluteraldeído Bigger (3,26 ± 0,25mm) 7,60 ± 2,71b 10,08 ± 1,17a 11,64 ± 0,83a 0,0045 Smaller (1,41 ± 0,06mm) 20,66 ± 2,70b 25,09 ± 4,25b 33,84 ± 3,32a 0,0000 Ρ 0,0000 0,0000 0,0000
a,b,c Means with the same letters, in the same line, do not present significant difference (p < 0,05)
Table 2 - Comparison of the effect of time and composition for the two particle diameters on Immobilization Efficiency
(n = 2)
Particle composition
Immobilization efficiency (%)
Reaction time (minutes)
20 40 60 Bigger (3,26 ± 0,25mm) Alginato 10,83 ± 2,15a 18,04 ± 1,78a 20,89 ± 1,16a Alginato-quitosana 11,49 ± 4,85a 14,34 ± 1,00b 17,92 ± 0,43b Alginato-quitosana-gluteraldeído 7,60 ± 2,71a 10,08 ± 1,17c 11,64 ± 0,83c Ρ 0,1460 0,0000 0,0000 Smaller (1,41 ± 0,06mm) Alginato 34,20 ± 3,46a 45,27 ± 2,51a 61,77 ± 2,11a Alginato-quitosana 27,46 ± 1,95b 39,26 ± 2,10b 53,49 ± 2,14b Alginato-quitosana-gluteraldeído 20,66 ± 2,70c 25,09 ± 4,25c 33,84 ± 3,32c Ρ 0,0000 0,0000 0,0000
a,b,c Means with the same letters, in the same line, do not present significant difference (p < 0,05)
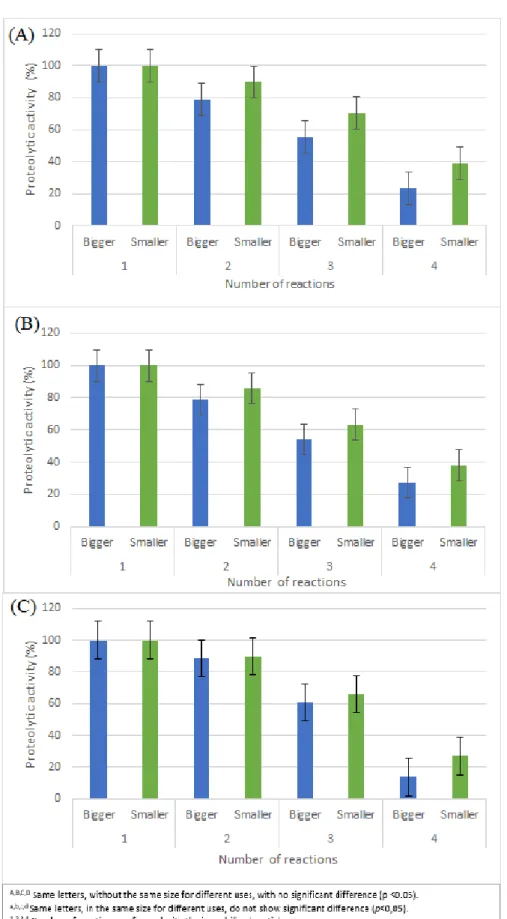
3.3 ENZYMATIC REUSE
Table 3 shows that particles composed only of alginate showed better effects, with the exception of the fourth use for larger particles composed of alginate and alginate-chitosan, where no significant difference was observed in the analysis of variance (ANOVA). The addition of chitosan
and gluteraldehyde in the composition of the particles did not help to maintain the enzymatic activity during the four uses.
Immobilization was feasible, since the first test showed that the enzyme maintained 79% and 90% of its proteolytic capacity for the larger and smaller size, respectively. The fourth trial was not so advantageous, however it still showed proteolytic activity.
Table 3 – Effect of particle composition in each reuse
Particle composition Immobilization efficiency (%) Number of uses 1 2 3 4 Bigger (3,26 ± 0,25mm) Alginato 20,89 ± 1,16a 16,53 ± 0,51a 11,59 ± 0,91a 4,91 ± 1,80a Alginato-quitosana 17,92 ± 0,43b 14,10 ± 0,99b 9,66 ± 0,80b 4,90 ± 0,50a Alginato-quitosana-gluteraldeído 11,64 ± 0,83c 10,29 ± 0,75c 7,09 ± 0,80c 1,60 ± 0,45b Ρ 0,0000 0,0000 0,0000 0,0001 Smaller (1,41 ± 0,06mm) Alginato 61,77 ± 2,11a 55,52 ± 1,36a 43,43 ± 2,62a 24,07 ± 2,97a Alginato-quitosana 53,49 ± 2,14b 45,92 ± 1,66b 33,89 ± 1,59b 20,29 ± 2,05b Alginato-quitosana-gluteraldeído 33,84 ± 3,32c 30,41 ± 2,14c 22,31 ± 1,40c 9,10 ± 1,14c Ρ 0,0000 0,0000 0,0000 0,0000
a,b,c Means with the same letters, in the same line, do not present significant difference (p < 0,05)
Analyzes of protease immobilization in sodium alginate carried out by Anwar et al. (2009) showed 80% of activity during the second use, 35% during the third and in activity in the fourth cycle. This proved to be similar to the result found in the second use and inferior to those found in the third and fourth cycle for both diameters.
Zhou, Li and Li (2010) immobilized the dehydrogenase in support of alginate-chitosan-gluteraldehyde, seeking to evaluate the enzymatic reuse of the particles in six tests. The proteolytic activity of the first utilization was considered to be 100%, and 78.25%, 67.78%, 62.86%, 42.65%, 39.63% for the others ones, what indicate a progressive reduction. It is possible to notice a small progressive reduction in each cycle, different from the results found in this work, where, in the fourth cycle, an efficiency of 13.79% and 26.89% was obtained for the largest and smallest sizes, respectively. The alginate-chitosan-gluteraldehyde particles showed better recovery of proteolytic activity in the second and third use when compared to the other particles.
Figure 3 - Effect of the number of uses of (A) alginate, (B) alginate-chitosan and (C) alginate-chitosan-gluteraldehyde
According to Anwar et al. (2009) the decrease in activity in each new reuse is due to the leakage of particles, since they are washed with distilled water at the end of each cycle.
4 CONCLUSIONS
The set of data obtained showed that it was possible to immobilize the enzyme Corolase 7089 in the three types of support, alginate, alginate-chitosan and alginatoquitosan-gluteraldehyde. The immobilized Corolase showed better immobilization efficiency in the smaller particles diameters, with a support composed only for alginate and a reaction time of 60 minutes.
Immobilization by encapsulation proved to be a good tool for reusing the enzyme for the three different support compositions. However the addition of chitosan and gluteraldehyde did not contribute to maintain the efficiency over the uses of particles.
REFERENCES
ANWAR, A. et al. Calcium Alginate: A Support Material for Immobilization of Proteases from Newly Isolated Strain of Bacillus subtilis KIBGE-HAS. World Applied Sciences Journal, Pakistan, v. 7, n.10, p. 1281-1286, 2009.
BERGER J. et al. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. European Journal Pharm Biopharm, Switzerland, v.57, n. 1, p. 19-34, 2004.
MENDES, A., A. et al. Aplicação de quitosana como suporte para imobilização de enzimas de interesse industrial. Química Nova, São Paulo, v. 34, n. 5, p. 831-840, 2010.
MERHEB-DINI, C. et al. Production and characterization of a milk clotting protease in the crude enzymatic extract from the newly isolated Thermomucor indicae-seudaticae N31 (Milk clotting protease from the newly isolated Thermomucor indicae-seudaticae N31). Food Chemistry, São José do Rio Preto, v. 120, n. 1, p. 87-93, 2010.
MIGNEAULT, I. et al. Comparison of two glutaraldehyde immobilization techniques for solid-phase tryptic peptide mapping of human hemoglobin by capillary zone electrophoresis and mass spectrometry. Electrophoresis, Paris, v. 25, p. 1367-1378, 2004.
SILVA, R. L. F. O. B. et al. Imobilização de enzimas de milho maltado em gel. Ciência e Tecnologia de Alimentos, Campinas, v.28, n. 3, p. 642-648, 2007.
SINGH, A. N. et al. Glutaraldehyde-Activated Chitosan Matrix for Immobilization of a Novel Cysteine Protease, Procerain B. Journal of Agricultural and Food Chemistry, Assam, v. 59, n.11, p. 6256-6262, 2011.
SOUZA, L. T. A. et al. Imobilização enzimática: princípios fundamentais e tipos de suporte. Agro&Indústria, São Paulo, v.4, n. 8, p. 529 -568, 2017.
ZHOU, Z.; LI, G.; LI, Y. Immobilization of Saccharomyces cerevisiae alcohol dehydrogenase on hybrid alginate-chitosan beads. Biological Macromolecules, Guangxi, v. 47, n.1, p. 21-26, 2010.