AgeingResearchReviews35(2017)74–86
ContentslistsavailableatScienceDirect
Ageing
Research
Reviews
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / a r r
Pain
perception
in
Parkinson’s
disease:
A
systematic
review
and
meta-analysis
of
experimental
studies
Trevor
Thompson
a,∗,
Katy
Gallop
b,
Christoph
U.
Correll
c,d,
Andre
F.
Carvalho
e,
Nicola
Veronese
f,
Ellen
Wright
g,
Brendon
Stubbs
h,iaFacultyofEducationandHealth,UniversityofGreenwich,LondonSE92UG,UK bAcasterConsulting,LondonSE37HU,UK
cepartmentofPsychiatry,TheZuckerHillsideHospital,NorthwellHealth,GlenOaks,NY11004,USA
dDepartmentofPsychiatryandMolecularMedicine,HofstraNorthwellSchoolofMedicine,Hempstead,NY11549,USA
eDepartmentofClinicalMedicineandTranslationalPsychiatryResearchGroup,FacultyofMedicine,FederalUniversityofCeará,Fortaleza,CE,Brazil fInstituteforClinicalResearchandEducationinMedicine,I.R.E.M.,Padova,Italy
gDepartmentofPrimaryCareandPublicHealthSciences,King’sCollegeLondon,LondonSE13QD,UK hPhysiotherapyDepartment,SouthLondonandMaudsleyNHSFoundationTrust,LondonSE58AZ,UK iHealthServiceandPopulationResearchDepartment,King’sCollegeLondon,LondonSE58AF,UK
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19December2016
Receivedinrevisedform25January2017 Accepted25January2017
Availableonline4February2017
Keywords:
Parkinson’sdisease Dopamine Pain Meta-analysis Systematicreview
a
b
s
t
r
a
c
t
Whilehyperalgesia(increasedpainsensitivity)hasbeensuggestedtocontributetotheincreased
preva-lenceofclinicalpaininParkinson’sdisease(PD),experimentalresearchisequivocalandmechanisms
arepoorlyunderstood.Weconductedameta-analysisofstudiescomparingPDpatientstohealthy
con-trols(HCs)intheirresponsetoexperimentalpainstimuli.Articleswereacquiredthroughsystematic
searchesofmajordatabasesfrominceptionuntil10/2016.Twenty-sixstudiesmetinclusioncriteria,
comprising1292participants(PD=739,HCs=553).Randomeffectsmeta-analysisofstandardizedmean
differences(SMD)revealedlowerpainthreshold(indicatinghyperalgesia)inPDpatientsduring
unmedi-catedOFFstates(SMD=0.51)whichwasattenuatedduringdopamine-medicatedONstates(SMD=0.23),
butunaffectedbyage,PDdurationorPDseverity.Analysisof6studiesemployingsuprathreshold
stim-ulationparadigmsindicatedgreaterpaininPDpatients,justfailingtoreachsignificance(SMD=0.30,
p=0.06).Thesefindings(a)supporttheexistenceofhyperalgesiainPD,whichcouldcontributetothe
onset/intensityofclinicalpain,and(b)implicatedopaminedeficiencyasapotentialunderlying
mecha-nism,whichmaypresentopportunitiesforthedevelopmentofnovelanalgesicstrategies.
©2017ElsevierB.V.Allrightsreserved.
Contents
1. Introduction...75
2. Method...75
2.1. Eligibilitycriteria...76
2.2. Searchstrategy...76
2.3. Studyselection...76
2.4. Painoutcomevariables ... 76
2.5. Dataextraction...76
2.6. Studyvaliditycriteria...76
2.7. Statisticalanalysis...76
2.7.1. Effectsize...76
2.7.2. Meta-analysis...76
2.7.3. Meta-regressionanalyses...80
∗Correspondingauthor.
E-mailaddress:t.thompson@gre.ac.uk(T.Thompson).
T.Thompsonetal./AgeingResearchReviews35(2017)74–86 75
2.8. Publicationbias ... 80
3. Results ... 80
3.1. Studyselection...80
3.2. Participantcharacteristics ... 80
3.3. Studycharacteristics...80
3.4. Studyvaliditycriteria...81
3.5. Meta-analysisresults ... 81
3.5.1. Painthreshold...81
3.5.2. Suprathresholdpainresponse...83
3.5.3. Otheroutcomes:painratingsandsensorythreshold...83
3.6. Meta-regressionanalyses:painthreshold...83
3.6.1. PDseverity...83
3.6.2. Methodofassessment...83
3.6.3. Secondarymoderators...83
3.6.4. Studyvaliditycriteria...83
3.7. Repeated-measuresstudiescomparingONvs.OFFstates...83
4. Discussion...83
4.1. PainhypersensitivityinPDandclinicalimplications...84
4.2. Roleofdopamine...84
4.3. Partialpainthresholdnormalisation...84
4.4. Independenceofmotorimpairmentandpain...84
4.5. Limitations...85
4.6. Futureresearchdirections...85
4.7. Conclusions...85
Disclosures...85
Acknowledgements ... 85
AppendixA.Supplementarydata...85
References...85
1. Introduction
Chronic pain is a common non-motor symptom of Parkin-son’sdisease(PD).Arecentsystematicreviewindicatedamean painprevalenceof 68%inPDpatients(Broenetal.,2012), with anotherstudyfindingthatchronicpaincomplaints,especially mus-culoskeletalpain,weretwiceaslikelyand reportedastwiceas intensein PD patientscompared toage-matchedcontrols with other chronic disorders (Nègre-Pagès et al., 2008). Pain often appearsearlyinthedevelopmentofPDandmaybepresentyears beforeclinicaldiagnosis(Schragetal.,2015).Painhasbeenratedas themostburdensomenon-motorsymptom(ChaudhuriandOdin, 2010),andcontributestoPD-relateddisability,sleepdisturbance, and impairedquality oflife (Chaudhuriand Schapira, 2009;Fil etal.,2013;QuittenbaumandGrahn,2004).Non-motorsymptoms includingpainarealsoafrequentcauseofhospitalisationand insti-tutionalisationofPDpatientsandcanincreasehealthcarecostsby uptofourtimes(ChaudhuriandSchapira,2009).Nevertheless,pain isafrequentlyoverlookedsymptomofPD,oftenunreportedby patientsunawarethatpainfulsymptomsarelinkedtothedisease (Mitraetal.,2008),andconsequentlyunder-treated(Broenetal., 2012)whichcanincreasetheoverallburdenofPD.Thisisespecially unfortunategiventhatpainrepresentsanon-motorsymptomthat iseminentlytreatable(Chaudhurietal.,2010).
While pain in PD is often precipitated by muscular rigidity and/or postural abnormalities (Ford, 2010), neurodegenerative processes couldpotentially affect not only motor function, but alsoperipheral(Nolano etal., 2008)and brain(Filetal., 2013) pathwaysinvolvedinpainprocessing.Forexample,degradationof dopamine-producingcellsinthesubstantianigramayimpair nat-uralanalgesiabydisruptingthedopamine-mediateddescending pathwaysthatblocktransmissionofascendingnociceptivesignals fromthespinalcord(Filetal.,2013).Aroleofdopamineinpain isconsistentwithreducedpainsensitivityseeninschizophrenia (Stubbsetal.,2015),adisorderlinkedtodopaminedysregulation, andthepossiblepartialrestorationofnormalpainthresholdsinPD
duringfunctionalONstatesfollowingtreatmentwith dopaminer-gicagents(Curyetal.,2016).
IfpainprocessingisaffectedcentrallyinPD,ashypothesised, thiscouldresultinageneralisedhypersensitivitytonoxious sen-sations (Cury et al., 2016), which may influence the onset of and/orexacerbate painful symptomsin PD(Broenet al.,2012). Evidenceforthishypersensitivityis,however,inconsistent.While severalstudieshavefoundincreasedpainsensitivityinPDpatients comparedtohealthycontrols(HCs)inresponsetonoxious exper-imentalstimulation (Chen etal.,2015; Limet al.,2008; Mylius etal.,2009),othershavefailedtofindsuchaneffect(Granovsky etal.,2013;Massetanietal.,1989;Velaetal.,2007).This incon-sistencymaybeinfluencedbymethodologicaldifferencesacross studies,includingvariationinsamplesize,dopaminergicand anal-gesic medications, disease duration and symptom severity (Fil etal.,2013;Priebeetal.,2016).Nevertheless,toourknowledge, there hasbeennosystematic efforttosynthesizeavailable evi-dencefromexperimentalstudiesandtoexplorepotentialsources ofstudyheterogeneityusingmeta-analytictechniques.Examining theinfluenceofdopaminemedicationmaybeespeciallyrevealing, bothtoprovideevidenceforpossiblemechanismsofactionandfor informingpotentialanalgesictreatment.
Wethereforeconductedasystematicreviewandmeta-analysis ofstudiescomparingPDpatientsandHCsintheirresponseto nox-iousexperimentalstimulito:(1)examinewhetherPDpatientsand HCsdifferintheirresponsetoexperimentally-inducedpain;(2) quantifythemagnitudeofthisdifference;and(3)explore poten-tialmoderatorsofthisassociationincludingdopaminergicagents, diseaseduration,andsymptomseverity.
2. Method
76 T.Thompsonetal./AgeingResearchReviews35(2017)74–86
guidelines(Stroupetal.,2000)forobservationalstudies.Anapriori
establishedbutunpublishedprotocolwasfollowed.
2.1. Eligibilitycriteria
Thefollowinginclusioncriteriawereapplied:(1)useofagroup withprimary(idiopathic)Parkinson’sdisease(PD),basedon stan-dardizeddiagnosticcriteria(e.g.UKBrainBank);(2)inclusionof acomparativehealthycontrol(HC)groupwithoutPD;(3) appli-cationof anexperimentalpainstimulus;and (4)a quantitative assessmentofpain.Weexcludedstudiesusingparticipantswith secondaryParkinsonismonly(e.g.fromtoxinexposure)andthose publishedinlanguagesotherthanEnglish.
2.2. Searchstrategy
EMBASE,MEDLINEandPsycINFOdatabaseswereindependently searchedbytworeviewers(KG,TT)withthefinalsearchperformed on10th October 2016.The following search terms were used: (Parkinson’sdisease(MeSH)ORParkinson’s)AND(pain(MeSH)OR painORnociception)toidentifythelargestpossiblepoolof poten-tiallyeligiblestudies.Thesearchresultswereaposteriorirefined usinglimitsof‘humanstudies’and‘Englishlanguage’.Thissearch strategywasaugmentedthroughhandsearchingreferencelistsof includedarticlesandrelevantreviews.
2.3. Studyselection
After removal of duplicates, two reviewers (KG, TT) inde-pendently screened titles/abstracts for eligibility, and resolved disagreementsthroughconsensus.Thefull-textofpotentially eli-giblearticleswasthenindependentlyscrutinizedbytwoauthors (TT, BS).Followingconsensus,a full-list ofeligible articleswas defined.Whenastudyprovidedinsufficientdataforinclusion, cor-respondingauthorswerecontactedupto3timesoveran8-week periodtorequestadditionaldata.Of8authorgroupscontacted,6 (Aschermannetal.,2015;Granovskyetal.,2013;Haraetal.,2013; Nandhagopaletal.,2010;Nolanoetal.,2008;Takedaetal.,2013) provideddatasufficienttopermitstudyinclusion.
2.4. Painoutcomevariables
Thefollowingpainoutcomeswereused:(1)painthreshold(the pointatwhichpainisfirstreported),(2)paintolerance(thepoint atwhichpainisreportedasnolongertolerable),and(3)self-report ratingsofpainintensity/affect.Weusedthesemultipleoutcomes toassesswhetherdifferentaspectsofthepainexperiencewere selectivelyaffectedin PD.Thresholdinvolveslow-intensitypain andisinfluencedprimarilybysensoryprocesses(e.g.,localization andinitialdetection),whereastoleranceisasuprathreshold mea-suremorestronglyinfluencedbyaffectivemechanisms(Apkarian etal.,2005).Painratingscalesprovideaneasilyinterpretableindex ofsubjectivepainandtypicallyassesssensory(e.g.,intensity)or affective(e.g.,discomfort)dimensionsofpainonaVASornumerical ratingscale.
Sensorythreshold(thepointatwhichsensationisfirstreported) wasincludedasasecondarymeasuretoexaminewhetherPDwas alsoassociatedwithnon-painfulsensoryimpairment.Asweonly wishedtoexaminedirectmeasuresofpain,wedidnotexamine supplementaryphysiologicaldata.
2.5. Dataextraction
Extractionandcoding ofstudydatawereperformedby two authors(TT,KG),onastandardizedformadaptedfromourprevious
studies(Stubbsetal.,2015;Thompsonetal.,2016).The follow-ingdata wereextracted where available: (1)sociodemographic variables;(2)ForPDgroups:meandiseaseduration(years), symp-tom severityscore, functional state (ON/OFF), body side tested (least/mostaffected), diagnosticcriteria used, cognitive impair-ment,usualtreatment,%ofsamplewithPD-basedclinicalpain; (3)painoutcomesandpaininductionmethod.Meansand stan-dard deviationsfor each painoutcome wererecorded, andany otheravailableinformationthatallowedeffectsizecomputation (LipseyandWilson,2001).Toreducereportingbias,authorswere contactedforstatisticaldetailswhenfindingsweresimplyreported as‘non-significant’.
Anumberofdecisionsweremadewhencomputingeffectsizes from extracted data. First, a few studies (k=3) provided data frommultipleindependentparticipantsamples(Aschermannetal., 2015;Schestatskyetal.,2007;Velaetal.,2007),e.g.with/without dyskinesia,andweretreated asseparatestudiesin theanalysis (Borensteinetal.,2009).Second,foronestudythatusedawide rangeoftemperatures(Nandhagopaletal.,2010),onlythose elicit-ingaself-reportofpain(47.5◦Cand49.5◦C)wereincluded.Third,
forstudies(k=2)thatreportedtheuseofdifferentnoxious elec-tricalfrequencies(Aschermannetal.,2015;Chenetal.,2015),the lowestfrequencywasarbitrarilyselected,asthereappearstobe nopersuasiveevidenceforafrequencyeffect(Chenetal.,2015). Fourth,where studiesperformedrepeatedpainassessments on thesamesetofparticipants(e.g.acrossdifferentstimuli),multiple effectsizeswerecomputedforeachassessmentwithany depen-dencyacrosseffectsizesmodeledusingrobustvarianceestimation (RVE)(seeSection2.7.2).
2.6. Studyvaliditycriteria
Twoauthors(KG,TT)independentlyratedeachstudyonseveral dichotomousvaliditycriteria,withathirdauthor(BS)availablefor mediationintheeventofdisagreement.Weassessedcase/control comparability and participant selection with 5 items from the NewcastleOttawaScale(Wellsetal.,2008),andmethodological soundnesswith14itemsbasedonCochraneCollaboration prin-ciplesasreviewed byDeeks et al.(2003)(see Table1).Overall scoreswerenotcomputedduetoconcernsovertheir interpretabil-ity(Deeksetal.,2003),buttheimpactofpoorlyendorsedvalidity criteriawasexaminedinmoderatoranalysis.
2.7. Statisticalanalysis
2.7.1. Effectsize
Astandardizedmeandifference(SMD)forPDvs.controlgroups wascomputedforeachstudyusingHedges’gformula(Borenstein etal.,2009).Hedges’gisequivalenttoCohen’sd,butcorrectsfor biasinsmallsamples.Effectsizescanbeinterpretedas0.20,0.50 and0.80correspondingtosmall,mediumandlargeeffects respec-tively(Cohen,1988).
Effect size (ES) was coded so that positive values indicated higherpaininthePDcomparedtotheHCgroup.
2.7.2. Meta-analysis
T.
Thompson
et
al.
/
Ageing
Research
Reviews
35
(2017)
74–86
77
Table1
Summaryofincludedstudies.
Study N-PD Duration
years
UPDRS-III ON/OFFstate
duringpain testing
UsualMedication N-CON Modality TestingSite PainMeasure
Priebeetal.(2016) 23 8.1 18.4(OFF) OFF
ON
Mixed(L-DOPA=1;DA agonists=5; L-DOPA+DA agonists=17;MAO inhibitors=9;COMT inhibitors=2;NMDA blockers=5)
23 Heat
Electrical
Forearm Leg
Pain Threshold Pain Threshold (NFR) Pain Intensity EMG response
Myliusetal.(2016) 14 1.8 22.8(ON) OFF NSbutinc.L-DOPA 27 Heat
Electrical
Forearm Leg
Pain Threshold Pain Threshold (NFR)
Allenetal.(2016) 26 – 27.3b(ON) ON NS 11 Pressure(type
notstated)
UpperArmLeg PainThreshold
Aschermannetal. (2015)
6 6.20 11.2(ON) ON Mixed(levodopa,
dopamineagonists, MAOand COMT-inhibitors)
6 Heat Forearm PainThreshold(for
moderatepain) PainIntensity
Aschermannetal. (2015)
6 5.50 15.8(ON) ON Mixed(asabove) 6a Heat Forearm PainThreshold(for
moderatepain) PainIntensity Chenetal.(2015) 72 4.90 29.5(OFF)
23.1(ON)
OFF ON
L-DOPA 35 Electrical Hand Sensory
Threshold Pain Tolerance Grashornetal.(2015) 25 3.70 24.1(OFF)
20.7(ON)
OFF ON
Mixed(L-DOPA=4, L-DOPA+MAO inhibitors=2,L-DOPA+ DAagonists=1;DA agonists=7;DA agonists+MAO Inhibitors=9;MAO inhibitors=2)
30 Heat
Cold pressor
Forearm Leg
PainThreshold(for moderatepain) PainIntensity
Tanetal.(2015) 14 2.50 21.8(OFF) OFF None 17 Heat Forearm SensoryThreshold
PainThreshold Pain
Intensity
Takedaetal.(2014) 23 5.60 27.0(ON) ON Mixed(L-DOPA=4,
L-DOPA+DA agonists=11,MAO inhibitors=8)
12 Electrical Hand
Leg
78
T.
Thompson
et
al.
/
Ageing
Research
Reviews
35
(2017)
74–86
Table1(Continued)
Study N-PD Duration
years
UPDRS-III ON/OFFstate
duringpain testing
UsualMedication N-CON Modality TestingSite PainMeasure
Haraetal.(2013) 42 6.50 21.6(ON) ON Mixed(L-DOPA,DA
agonists,MAO inhibitors, catechol-Omethyl transferaseand amantadine)
17 Electrical Face PainThreshold
Granovskyetal.(2013) 23 6.30 23.6(ON) OFF ON
Mixed(L-DOPA=11, DAagonists=17,MAO inhibitors=19, Anticholinergics=8, Amantadine=14)
19 Heat
Pressure (vonFrey filaments)
Hand Forearm
Pain Threshold Pain Intensity
Velaetal.(2012) 18 11.60 34.5(OFF) 22.1(ON)
OFF ON
Mixed(L-DOPA,DA agonists)
18 Pressure
(algometer) Heat Cold
Neck Head Hand Leg
PainThreshold
CiampideAndrade etal.(2012)
25 15.10 42.7(OFF)
25.8(ON
-viaDBS)
OFF ON
L-DOPA 35 Heat
Cold Pressure (vonFrey filaments)
Hand Sensory
Threshold PainThreshold PainIntensity
Stamelouetal.(2012) 19 6.70 22.4(ON) OFF L-DOPA(nodetailson
othermedications)
17 Heat
Electrical
Forearm Leg
PainThreshold (NFR) PainThreshold
Myliusetal.(2011) 29 7.40 25.6(ON) OFF L-DOPA(nodetailson
othermedications)
27 Electrical
Heat
Forearm Leg
PainThreshold (NFR)
Maruoetal.(2011) 17 15.50 36.3(ON) ON L-DOPA,DAagonists
(nodetailsonother medications)
14 Cold
Heat
Hand Sensory
Threshold PainThreshold
Zambitoetal.(2011) 106 5.70 23.5(OFF) OFF Mixed(L-DOPA=38;
DAagonists=19; L-DOPA+DA agonists=49)
51 Electrical Hand
Foot
T.
Thompson
et
al.
/
Ageing
Research
Reviews
35
(2017)
74–86
79
Nandhagopaletal. (2010)
12 9.40 28.8(OFF)
15.0(ON)
OFF ON
Mixed(L-DOPA with/without other medica-tion=12)
13 Heat Forearm PainIntensity
Pain
Unpleasantness
Myliusetal.(2009) 15 11.00 28.3(OFF) OFF Mixed 18 Heat
Electrical
Forearm Leg
PainThreshold PainThreshold (NFR)
Nolanoetal.(2008) 18 7.60 26.6(ON) ON Mixed(L-DOPA
with/without other medica-tion=14, None=4)
54 Cold
Heat Pressure(nylon monofilament)
Hand Foot
SensoryThreshold PainThreshold
Limetal.(2008) 50 4.38 29.6(OFF) 21.3(ON)
OFF ON
L-DOPA(no detailsonother medications)
20 Coldpressor Hand PainThreshold
PainTolerance
Gerdelat-Masetal. (2007)
13 7.30 21.2(OFF)
10.8(ON)
OFF ON
Mixed(L-DOPA and/orDA agonists)
10 Electrical Leg PainThreshold
(NFR)
Schestatskyetal. (2007)
9 5.40 17.1(NS) OFF
ON
NSbutinc. L-DOPA
9 Heat
Laser
Hand SensoryThreshold
PainThreshold Schestatskyetal.
(2007)
9 6.00 19.1(NS) OFF
ON
NSbutinc. L-DOPA
9a Heat
Laser
Hand SensoryThreshold
PainThreshold
Velaetal.(2007) 25 11.38 24.6(ON) ON NS 25 Pressure
(algometer)
Hand PainThreshold
Velaetal.(2007) 25 4.72 19.9(ON) ON NS 25a Pressure
(algometer)
Hand PainThreshold
Brefel-Courbonetal. (2005)
9 9.60 25.0(OFF)
15.0(ON)
OFF ON
Mixed(L-DOPA and/orDA agonists)
9 Coldpressor Hand PainThreshold
Djaldettietal.(2004) 51 5.30 24.0(OFF) OFF Mixed(L-DOPA
and/orDA agonists)
28 Heat Hand PainThreshold
Massetanietal.(1989) 15 4.10 NA ON Mixed(L-DOPA
withor without peripheral decarboxylase inhibitor=10, drugfree=5)
8 Electrical Face SensoryThreshold
PainThreshold PainThreshold (blinkreflex)
TOTAL for26studies
739 M=7.10years ON:
M=22.2yrs OFF: M=27.0yrs
ON=19; OFF=18
L-DOPA with/without others=23 NotStated=2 None=1
553 Heat=16;
Electrical=1; Cold=7; Pressure=6; Laser=1
Hand=13; Forearm=10; Leg=10;Face=2; Foot=2; Neck/Head/Upper Arm=1
Pain Threshold=21; Sensory Threshold=9; Intensity=7; Suprathreshold (moderate pain/tolerance)=6; Unpleasantness=1
Key:NS=NotStated,NFR=NociceptiveFlexionReflex,DA=Dopamine,MAO=Monoamineoxidase,COMT=Catechol-O-methyltransferase,NMDA=N-methyl-d-aspartate. aSamecontrolgroupusedfor‘-a’and‘-b’studies.
80 T.Thompsonetal./AgeingResearchReviews35(2017)74–86
whichadjustsindividualESweightsbasedonthedegreeoftheir dependency.WeemployedarelativelynewversionofRVEfor cor-relatedeffectsizes,whichprovidesreliableestimatesevenwhen relativelylownumbersofstudiesareavailable.Simulationstudies havedemonstratedaccurateestimation,providedthattheadjusted RVEdegreesof freedom(df)>4(Tipton,2015),and this criterion isusedhere(dfislargerwhen studynumber ishighand when multipleeffectsizesfromastudyarerelativelyindependent).RVE estimatesdependencyfromthedataitself,andthereforeisa supe-riorapproachtoaveragingacrossconditions,whichresultsinboth informationlossandreliesonaknowledgeofthecorrelationsof outcomesacrossconditionswhicharerarelyreported(Fisherand Tipton,2014).
SeparateanalyseswereconductedforOFF/ONfunctionalstates, toexaminewhetherpainsensitivityisalteredbytreatment (typ-icallydopaminergicmedication).Forthespecificoutcomeofpain ratings,weonlyexaminedk=5outof7studieswhere stimula-tionintensitywasidenticalforboththePDandcontrolgroups(i.e., whereafixed-intensity/fixed-timeparadigmwasused),toavoid anyconfoundingofgroupdifferencesinpainratingswithgroup differencesinstimulationintensity.
2.7.3. Meta-regressionanalyses
Ifeffectsizesshowedmoderateorgreaterinconsistencyacross studiesasassessedbyHiggin’sI2(Higginsetal.,2003),with
val-uesof25%,50%and75%correspondingtolow,moderateandhigh inconsistency,meta-regressionwasconductedtoidentifypossible underlyingsourcesofvariation.
First, we examined symptom severity (as measured by the UPDRS-III),diseaseduration(years)and,for painthreshold,the assessmentmethod(limits vs.constant/adjustedstimuli)as pri-marymoderators,basedona rationaledefinedapriori.Greater symptomseverity and longer disease duration likely toreflect increasedneurodegenerationandthereforemaybeassociatedwith agreaterdegreeofabnormalpainperception.Inaddition,the meth-odsof limits (whereintensity increasesto a pre-defined limit) involvesareactiontimeartefact(e.g.frompressingabutton)which couldartificiallyinflatethresholdtimesand underestimatetrue levelsof pain selectively for PD patientsdue to motor impair-ment (Cury et al., 2016). Conversely, the methods of constant stimuli/adjustment(where constant temperaturesaregradually adjusted)(seeYarnitskyandPud,2004)containsnoreactiontime component.
Secondary moderators were study gender composition, age, stimulus modality, PD side subject to pain testing (least/most affected),anatomicsitesubjecttopaintesting,andsample per-centageexperiencingPD-basedpaincomplaints. Thesevariables wereexaminedtoprovidepreliminarydataonanypotential mod-eratinginfluence,asallhavebeensuggestedaspossibleinfluences (Filetal.,2013).Additionally,meaningful studyvalidity criteria wereassessedasmoderators,suchascaseandcontrolselection, criteria-basedPDdiagnosis,explicitmentioningofdisallowingpain medications.Finally,wheretheendorsementofimportant valid-itycriteriavariedacrossstudies,theinfluenceofthesecriteriaas potentialmoderatorsofeffectsizewasalsoassessed.
2.8. Publicationbias
Publicationbiaswasexaminedthroughvisualinspectionof fun-nelplotsof meanstudyESsagainststandard errors. Anyvisual asymmetryresultingfromtheabsenceofsmallsamplestudieswith smallESscansuggestpossiblepublicationbias.Asymmetrywas alsotestedstatisticallywithEgger’sbiastest(Eggeretal.,1997) withp<0.05indicatingasymmetry.Ifpresent,arevisedeffectsize assumingthepresenceofpublicationbiaswascomputedusingthe trimandfillmethod(DuvalandTweedie,2000).
Asthecitationreferstoapackage(robumeta)withina com-puterprogram(R):allanalyseswereperformedusingtherobumeta
package(FisherandTipton,2014)inR(RCoreTeam,2014).
3. Results
3.1. Studyselection
3047uniquehits wereidentifiedthroughdatabasesearches, with6additionalrecordsidentifiedthroughmanualsearchingof referencelists.Followingtheinitialscreeningofabstracts,47 arti-cleswereretainedforfulltextreviewofwhich21wereexcluded, resultinginatotalof26studiesretainedforanalysis(Fig.1).
3.2. Participantcharacteristics
The26retainedstudiesprovidedaggregateddataforN=1292 participants,consistingof739patientsand 553HCs. Themean study age (k=26 studies) was 63.8 years (SD=3.0, range of means=58.8–69.9years)fortheaggregatedPDsample,and62.4 years(SD=3.9,rangeofmeans=54.8–71.4years)fortheaggregated HCsample.ThePDsampleconsistedof35.7%femalesandtheHC sampleconsistedof42.7%offemales(k=26).
ForthePDsample,meandiseaseduration(k=25)was7.1years (SD=3.4, range of means=1.8–15.5). Symptom severity(k=25) wasmostcommonlymeasuredwiththeoriginalUPDRS-IIIMotor Examinationscale(FahnandElton,1987)andwasassessed dur-ingbothON(k=19;UPDRS-IIIM=21.8)andOFF(k=13;UPDRS-III
M=27.0) functional states. ON states were achievedwith anti-Parkinsonmedication(k=18)ordeepbrainstimulation(k=1).
A diagnosisofPD wasbased onUKBBC (k=18),ICD-10:G20 (k=1),GelbNINDSguidelines(k=1)orwassimplyreportedasa clinicaldiagnosisofPD(k=6).Medicationdata(k=24)includeda singlestudywhichusedamedication-naïvepatientsample(Tan et al., 2015), while 23 studies reported regular usage of anti-Parkinsonmedication(L-DOPAwith/withoutothermedication)by someor allenrolled patients(M=95%, study range=67–100%). Most studies (k=22) specified a minimum level of cognitive functioning for inclusion in the study, withscores ≥25 onthe
Mini-MentalStateExaminationbeingthemostextensivelyused inclusioncriterion(k=15).
Presenceorabsenceofaprimarychronicpainconditionwas reported by 13 studies. Of these, 12 reported noco-occurring chronicpainconditionsandasinglestudyreportedthat2patients mayhaveexhibitedchroniclowbackpain.Themeanstudy percent-ageofPDpatientsreportingsecondarypaincomplaintsattributable toPD(k=18)was48%.
3.3. Studycharacteristics
T.Thompsonetal./AgeingResearchReviews35(2017)74–86 81
Records idenfied through database searching
(n = 4212)
Screenin
g
Included
Eligibility
Idenficao
n Addional records idenfied
through other sources (n = 6)
Records aer duplicates removed (n = 3047)
Records screened (n = 3047)
Records excluded (n =3000)
Full-text arcles assessed for eligibility
(n = 47)
Full-text arcles excluded, with reasons (n = 21)
duplicated data (n=9) no healthy control group (n=4) no experimental pain smulus (n=3)
no response to data request (n=2) authors (conference papers) not
locatable (n=2) no direct pain assessment (n=1)
Studies included in qualitave synthesis
(n = 26)
Studies included in quantave synthesis
(meta-analysis) (n = 26)
Fig.1.PRISMAflowdiagram.
The majority of studies used pain threshold (k=21) as the methodofpainassessment(k=11methodoflimits,k=7methodof levels/adjustment,k=3notstated),withotheroutcomesincluding painintensityratings(k=7),paintolerance(k=3)andthreshold formoderatepain(k=3),withseveralindividualstudies employ-ingmultipleinductionandassessmentmethods.Ninestudiesalso assessedsensorythreshold.Aside fromonestudythatincluded onlydrug-naïvepatients(Tanetal.,2015),OFFstateswereachieved bythewithdrawalofdopaminergicmedication,withpaintesting usuallytakingplaceaftera≥12-hwashoutperiod.
Asummaryofthekeycharacteristicsofthe26individualstudies ispresentedinTable1.
3.4. Studyvaliditycriteria
Acceptable agreement across the two raters was found for mostitems(Kappa=0.77–1.00)exceptfor thosewithhighrates ofendorsement(minKappa=0.25).Highendorsementratescan resultinlowkappavaluesduetomarginalhomogeneity(Gwet, 2002)andthuspercentageagreementacrossraterswascomputed, withresultantpercentagesindicatinggoodagreement(>87%)for thoseitems.Completeconsensuswasreachedwheneverany dis-agreementoccurred(seeAppendixS1forallitemratings).
Ratings indicated methodological soundness (e.g., reporting offunctionalstateduringtesting,completedataprovided,clear descriptionofprocedures)forthemajorityofstudies(>85%formost criteria).Moststudiesexcludedpatientswithcomorbiddepression (77%)orsomatosensorydisorder(73%),with42%explicitly specify-ingthatuseofpainmedication(<24h)wasanexclusioncriterion. Lowendorsementof‘selectionofPDcases(23%)andcontrols(27%) validitycriterionreflectedlimiteddetailonrecruitmentmethods;
althoughstudiesdidgenerallystatethenameoftherecruiting hos-pitalandprovidegooddescriptionsofcharacteristicsofcontrols, usuallymatchingcasesandcontrolseitherexperimentallyorby statisticallycontrollingforage/gender(77%).
Somestatisticalinconsistencieswerenoted.Onestudy(Nolano etal.,2008)reportedimplausiblepainthresholds(9.1–12.4◦C)for
heatstimulation(valuesforotherstimuliwereplausible),anda furtherstudy(Schestatskyetal.,2007)reportedmeansandSDsfor painthresholdhighlyinconsistentwithreportedp-values(even whenSDsweretreatedasSEMs),andsothisspecificdatawere excluded.
3.5. Meta-analysisresults
3.5.1. Painthreshold
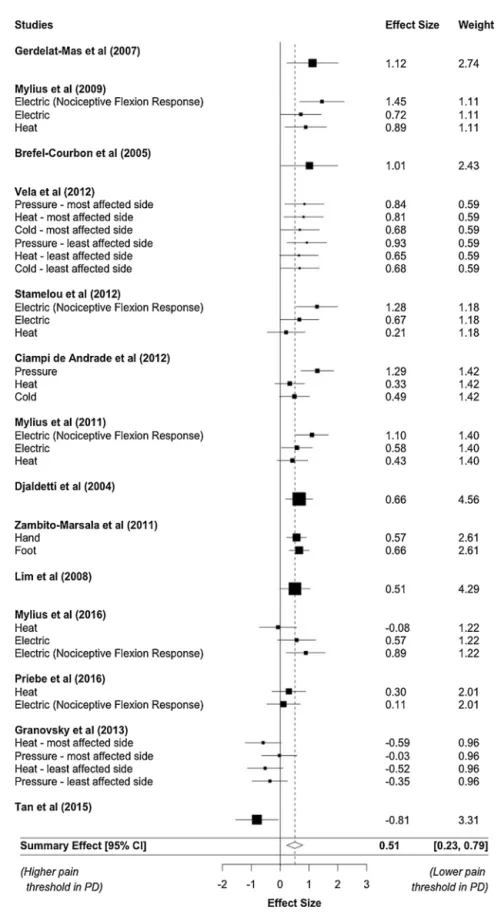
Painthresholddatawasexaminedfork=20studies compris-inga total sampleofN=926(n=577PD,n=473HCs),withPD patents assessed during both OFF (k=14; N=728) and/or ON (k=13;N=617)states.Sevenrepeated-measuresstudiesprovided painthresholddataforbothONandOFFstates.
Meta-analysis ofpain threshold,aggregating data fromboth ONandOFFstates,foundsignificantlyloweroverallpain thresh-old(i.e.,greaterpainsensitivity)inPDpatientscomparedtoHCs (SMD=0.37,CI95[0.16,0.57],p=0.001).Moderatetohigh
hetero-geneity(I2=63%)wasobservedandk=16of20studiesreported
lowerpainthresholdsinPD.
Whenseparatemeta-analyseswereperformedforONandOFF statedata,significantlylowerpainthresholdsinPDpatients com-pared toHCs werefound for the OFFstate (k=14;SMD=0.51, CI95[0.23,0.79],p=0.002),alongwithmoderate-high
82 T.Thompsonetal./AgeingResearchReviews35(2017)74–86
Fig.2.Forestplotofpainthreshold,withboxsizesproportionaltostudyweights(forstudieswithmultipleoutcomes,weightsaredividedevenlyacrossoutcomes).
wasalsofoundbutwithareducedeffectsize(k=13;SMD=0.23, CI95[0.02,0.45],p=0.04)andmoderateheterogeneity(I2=49%).A
forestplotofpainthresholddatafortheOFFstateisprovidedin
Fig.2.
T.Thompsonetal./AgeingResearchReviews35(2017)74–86 83
3.5.2. Suprathresholdpainresponse
Threestudiesreportedpaintolerancedata,and3other stud-ies reported ‘threshold to moderate pain’ (where participants weretold towithdraw fromnoxious stimulation when experi-encingmoderatepain).Bothmeasureswerecollapsedtoasingle suprathresholdpaincategorytomaximisepower(threestudiesis insufficientforRVE analysis).The 6combined studiesprovided aggregatedatafor442participants(n=288PDpatients,n=154 HCs).Painassessment occurredduring bothON (k=5)and OFF states(k=4),with3studiesprovidingdataforbothstates.
Meta-analysisofoverallsuprathresholddatafoundlower tol-erancetosuprathresholdpain(suggestinggreatersensitivity)in PDpatients,butthisjustfailedtoachievestatisticalsignificance (SMD=0.30,CI95[−0.01,0.61],p=0.06).Analysisofdifferent
func-tional states indicated lower tolerance to suprathreshold pain duringOFF(k=4;SMD=0.44,p=0.04)statesbut notduringON (k=5;SMD=0.15,p=0.28)states.ForanalysisofOFFstatedata, populationconfidenceintervals(andthusp-values)maybewider ornarrower(Tipton,2015)astheadjusteddf<4.Lowheterogeneity wasobservedinallinstances(I2=4–27%).
Afunnelplotofoverallsuprathresholddatasuggested asymme-tryduetothepresenceofasmallsamplestudywithalargenegative
effectsize.Someindicationofpossibleasymmetryemergedfrom Egger’stest,whichfailedtoachievesignificance(butisalsolikelyto beunderpoweredwithonly6studies),z=1.90,p=0.054,Arevised estimateusing trimandfillmethodssuggested aslightly larger effectsize(SMD=0.36,p=0.01)ifpublicationbiasisassumed.
3.5.3. Otheroutcomes:painratingsandsensorythreshold
TheadjusteddffromRVEmeta-analysisofpainratingsand sen-sorythresholdwere<4(reflectingbothlimitednumberofstudies usingtheseassessmentmethodsanddatadependency),which pre-cludedreliableestimationofESs(Tipton,2015)fortheseoutcomes.
3.6. Meta-regressionanalyses:painthreshold
Meta-regressionanalyseswereconductedtoidentify underly-ingsourcesofheterogeneityinESsacrosspainthresholdstudies (other pain outcomes were not examined due to limited data not meetingRVE requirements).Functional state (ON/OFF)was includedasanadditionalvariablein eachanalysistomaximise powerbyallowinguseofallpainthresholddata(i.e.fromboth ONandOFFstates),andgiventhatfunctionalstateduringtesting islikelytorepresentasubstantialsourceofvariationinESacross studiesthatshouldbecontrolledforasapossibleconfounder.In addition,includingfunctionalstateasamoderatorallowsthedirect assessmentofwhetherdifferencesacrossONvs.OFFstates identi-fiedinthemeta-analysis(Section3.5.1)weresignificant.
3.6.1. PDseverity
We examinedwhetherreduced pain threshold(greaterpain sensitivity)inPDwasexacerbatedinpatientswithmoreadvanced diseasestates,byincludingdiseasedurationandUPDRS-IIIscores as moderators in separate analyses. Results indicated that dif-ferences between PD and controls in pain threshold were not significantly influenced by disease duration (k=19; B=0.035,
p=0.24)orUPDRS-IIIsymptomseverity(k=19;B=0.006,p=0.71). Resultsalsoindicatedthat effectsizewassignificantlylarger (k=19;SMD=0.33,CI95[0.02,0.64],p=0.039)forOFF(B=0.58)
thanON(B=0.25)states.Thispatternofresultswasreproduced (k=19;SMD=0.33,CI95[0.02,0.66],p=0.041),whenanalysiswas
restrictedtoONstatesachievedthroughdopaminemedicationonly (i.e.thesingledeepbrainstimulationstudywasexcluded).
3.6.2. Methodofassessment
Enteringassessmentmethod(limitsvs.levels/adjustment)asa moderatorinmeta-regressionofpainthresholdrevealedthatthe differenceinpainthresholdbetweenPDpatientsandcontrolswas largerwhenthemethodoflevels/adjustmentwasused,although this failedto reach statistical significance (k=18; SMD=0.34,
p=0.061).
3.6.3. Secondarymoderators
Separatemeta-regressionanalyseswereconductedtoexplore othermoderatorsspecifiedinSection2.7.3.As15painthreshold studiesexplicitlystatedtheuseofestablisheddiagnosticcriteria and5studiesdidnotprovideinformation,wealsoexaminedthis variableasapotentialmoderator.Givenlimiteddataforcertain anatomiccategories,wecollapseddatatoformtwobroadanatomic categoriesofarm(forearm,upperarm,hand)andleg(leg,foot). Forthemultiplecategoricalvariableofstimulusmodality,a no-interceptmodelwasanalysedsothateachcoefficientrepresented theabsoluteESforeachstimulusmodality(ratherthanthe dif-ference inESbetweenthat modalityand anarbitraryreference modality).
No evidence was found for moderating effects of studies’ gender composition (k=20; p=0.38), mean study age (k=20;
p=0.62), affected vs. non-affected side tested (k=9; p=0.91), anatomic site tested (k=17;p=0.43)or diagnosticcriteria pro-vided(k=20;p=0.55).Forstimulusmodality(k=20),however,PD patientsdemonstratedlowerpainthresholdthanHCsinresponse to cold (SMD=0.62, p=0.011), electrical (SMD=0.53, p<0.001) and pressure (SMD=0.41, p=0.055) stimuli, but not heat stim-uli(SMD=−0.05,p=0.79).PD-relatedpaincouldnotbereliably
assessedasamoderator,astheadjusteddfwas<4(Tipton,2015).
3.6.4. Studyvaliditycriteria
Given low endorsement for validity criteria of selection of cases/controlsandreportingofpainmedicationuse(Section3.4), these variables were entered as moderators in separate meta-regression analyses of pain threshold. Neither case selection (p=0.79)orcontrolselection(p=0.68)significantlymoderatedthe ES.DifferencesinpainthresholdbetweenPDpatientsandcontrols wereamplifiedinstudiesexplicitlystatingthatparticipantswere notusingpainmedication,althoughthisdidnotachievestatistical significance(SMD=0.40,p=0.052).
3.7. Repeated-measuresstudiescomparingONvs.OFFstates
Aspreviousanalyses(Section3.6.1)revealedthatdifferences betweenPDsandcontrolswerereducedduringtheONstate,we conductedastricterevaluationbyrepeatingtheanalysisincluding onlythe7studiesthatdirectlycomparedONvs.OFFina repeated-measuresdesignstoensuresuperiorcontrolofconfounds.Results wereinlinewithpreviousfindings(Section3.6.1),withdifferences inpainthresholdbetweenPDpatientsandHCsattenuated dur-ingONstates(k=7;SMD=−0.25,CI95[−0.11,−0.38],p=0.004).
Removalof two studiesthat didnotrandomize/counterbalance ON/OFFstateorfailedtotestHCsatequivalentintervals,hadlittle impactonresults(k=5;SMD=−0.22,p=0.045).Only4studies
providedL-DOPAdosagesduringONstates,whichwasnot suffi-cienttomeetrequirementsforRVEmeta-regression.
4. Discussion
84 T.Thompsonetal./AgeingResearchReviews35(2017)74–86
severalkeyfindings:(1)OverallpainthresholdwaslowerinPD patients(indicatinggreaterpainsensitivity)comparedtoHCs;(2) SuprathresholdpainwaslowerinPDpatients,althoughthis nar-rowlyfailedtoreachsignificance;(3)Abnormalpainthresholds in peoplewith PD duringOFF states (SMD=0.51) were signifi-cantlydiminished,butnotcompletelynormalizedduringONstates (SMD=0.23) produced bydopaminergic medication; (4) Abnor-malpainthresholdswerenotsignificantlyinfluencedbysymptom severity,diseaseduration,sexorage;(5)Whilemost(16/20) indi-vidualstudiesindicatedlowerpainthresholdinPD,moderateto highvariationineffectsizessuggeststhatother,unidentified vari-ablescouldinfluenceabnormalpainresponsesinPD.
4.1. PainhypersensitivityinPDandclinicalimplications
Resultsfromourmeta-analysisprovideevidencethatpatients withPDdemonstrate greatersensitivity tonoxious stimulation comparedtoHCs,whichseemtobeindependentofageandsex,and whichoccurformosttypesofaversivestimuli.Althoughnooverall differenceswerefoundforheatstimulation,thepost-hocnature ofthisresultmeansanyconclusionsofmodality-specific nocicep-tiveprocessing abnormalities in PDshould bemade extremely cautiouslyandwouldrequireindependentreplication.
Takentogether,theseresultssuggestthattheincreased preva-lence and intensity of clinicalpain complaints in PD couldbe, atleastin part,influenced byabnormal nociceptiveprocessing. In particular, these results could explain the increased preva-lenceof non-musculoskeletalpain complaints(e.g., neuropathic pain),whichdonotobviouslydirectlyresultfrommotor dysfunc-tion.Althoughcaremustbetakenintranslatingeffectsizesfrom experimentally-inducedpaintoreal-lifepainexperiences,thefact thatanSMD=0.51(inmedication-freeOFFstates)canbe classi-fiedasamoderateeffect(Cohen,1988),providessomepreliminary indicationthattheimpactofPDonpaincomplaintsoutsideofthe laboratorymaynotbetrivial.
Thereisalsosometentativeevidencetosuggestthatthetrue extentofincreasedpainsensitivitycouldbeunderestimatedbythe moderateeffectsizeweobserved.Studiesthatexplicitlystatedthat participantswerenotreceivingpainmedication(withPDpatients generallybeingmorelikelytoregularlyusepainkillers)werelinked toalargereffectsize(anSMDincreaseby0.40).Inaddition, stud-iesemployingthemethodsoflevels/adjustment,whichhasbeen arguedtogiveamoreaccurateestimateof painthresholdthan themethodoflimitsduetoareducedreactiontimeartefact,also demonstratedalargereffectsize(anSMDincreaseby0.34).Itis importantthatthesefindingsaretreatedcautiously,asalthough suggestive,neithersubgroupanalysis reachedstatistical signifi-cance(p=0.052–0.061).
4.2. Roleofdopamine
ThepartialnormalisationoftheatypicalpainthresholdsinPD resultingfromdopaminergicmedicationappearstobea robust finding, which was observed when all studies were examined andwhenonlyrepeated-measuresstudies,whichprovidesuperior controlof potentialconfounds,wereincluded.Thispartial nor-malisationofpainthresholdhasanumbertheoreticalandclinical implicationswhichwarrantfurtherelaboration.
First, this finding offers indirect evidence for an underly-ing role of dopamine depletion in pain hypersensitivity in PD. Although identifying exact underlying mechanisms is difficult fromtheavailabledata,dopaminecouldelicitpain hypersensitiv-ityeitherindirectlythroughmodulatoryeffectsonaffectivepain processingand/or directlyby affecting neuronal activityat key pain-modulatingareas inthe brainsuchasthethalamus, basal ganglia,insula,anteriorcingulatecortexandperiaqueductalgrey
(Filetal.,2013;Jarchoetal.,2012).Reduceddopaminergic neu-rotransmissionmayimpairnaturalanalgesiathroughadecreased activationofdopamine-mediatedpaininhibitorypathways.These descendfromthesubstantianigratothesubstantiagelatinosain thespinalcordandinhibittransmissionofascendingnociceptive signals(Filetal.,2013).Thisdirectroleofdopamineisconsistent withPET studies in healthy participantsthat show an associa-tion between greater subjective pain and decreased dopamine activity(Curyetal.,2016),thediminishedpainresponseseenin schizophrenia(Stubbsetal.,2015)adisorderlinkedtoaberrant dopaminergicneurotransmission,andevidencefromanimal mod-elssuggestingaroleofdopamineinchronicregionalpainsyndrome (Wood,2008).ExperimentalresearchonpaininPDpatients assess-ingtheeffectofpro-dopaminergicandantidopaminergicstatesor medicationscouldhelpfurtherelucidatetheroleofdopaminein painperception.
Second,ifattenuationofpaincanbeachievedthrough dopamin-ergicmedication,thissuggeststhatthedevelopmentofclassesof novelcompoundsthatefficientlytargetdopaminepain-inhibitory pathwaysmayhavepotentialaseffectiveanalgesics.Such com-poundsmaybeeffectiveanalgesicsbothforPDandforotherpainful disorderslinkedtodisruptedendogenousdopamineactivitysuch as fibromyalgia, burning mouth syndrome and painful diabetic neuropathy(Jarchoetal.,2012;Wood,2008).Furthermore,ifthe suggestionthat the underuseof conventional painkillers in PD patientsisattributabletopoorefficacyinthisgroup(Perez-Lloret etal.,2012),suchmedicationscouldprovidepotentiallysuperior alternatives.Theutilityofsuchanalgesicscouldevenextendto conventional treatmentin healthyindividuals withpain. While theroleofopioidsandnon-dopaminergic (e.g.noradrenergicor serotoninergic)pathwaysiswellrecognised,thecurrentfindings furthersuggestameaningfulrolefordopamine-mediatedanalgesia andmayprovidetheimpetusforfurtherstudyinvolvingpreclinical modelsandneuroimagingtechniquesinhumans.
4.3. Partialpainthresholdnormalisation
The fact that dopamine medication diminished but did not appeartocompletelynormalisepainthreshold,suggeststhat addi-tionalmechanismsarelikelytocontributetopainhypersensitivity inPD.Althoughitisdifficulttoidentifysuchmechanismsfrom thecurrentavailabledata,thesecouldincludealossofepidermal nerve fibres(Nolano etal., 2008) ordeficiencies in other, non-dopaminergicpain pathways.Analternativeexplanationis that painhypersensitivityisentirelymediatedbydopaminedeficiency inPDbutthatstudymedicationdosageswereinsufficienttorestore dopamineneurotransmissiontonormalphysiologicallevels.Due toinsufficientavailabledata,wewerenotabletoassesswhether higherL-DOPAdosageswereassociatedwithgreaternormalisation ofpainthreshold.
4.4. Independenceofmotorimpairmentandpain
T.Thompsonetal./AgeingResearchReviews35(2017)74–86 85
4.5. Limitations
Whilstthesedataprovidenovelinsightsintoalteredpain per-ceptioninPD,somelimitationsshouldbenoted.Firstly,findings arebasedprimarilyonpainthresholdwhichwasthemost com-monlystudiedpainoutcome.Althoughanestablishedmeasureof painsensitivity,painthresholdrepresentspainatthelowerendof theintensitycontinuumandcannotbeautomaticallygeneralised tomoreintenselevelsofclinicalpain.Second,althoughadequate datawasavailableformeta-regression,genuinemoderatingeffects ofvariables(e.g.diseaseduration)thatwerenon-significant can-notbedismissed,andmaybedetectablewithmoreavailabledata; especiallyifthemagnitudeoftheseeffectsissmall.Third,although exclusion of PDpatientswithcognitiveimpairment in primary studies wasnecessary tomaximise adherence to experimental requirements, current findings should be necessarily restricted toPD patientswho have relativelypreserved overall cognition (Folsteinetal.,1975).
4.6. Futureresearchdirections
Despitetheselimitations,thecurrentfindingsprovidestrong supportthatindividualswithPDexhibitgreatersensitivityto nox-iousstimulithan HCs,basedonlaboratorystudiesthatprovide acontrolofpotentialconfoundersnoteasilyachievablein clini-calsettings.Futurestudiesareneededtohelpestablishwhether pain hypersensitivityin PDextends tosuprathreshold levelsof pain,andinsightsmayalsobegainedfromtheuseof ischemic anddermalcapsaicinexperimentalpainmodelsthatevoke sev-eralaspectsofchronicpainwhilstpreservingstrictexperimental control(Staahletal.,2009).Moreresearchinvolvingpreclinical modelsandneuroimagingtechniquesinhumanswouldalsohelpto elucidatepotentialmechanisticpathwaysunderlyingalteredpain perceptioninPD.
4.7. Conclusions
Totheauthors’knowledge, this istheonly published meta-analysisofstudiescomparingPDpatientswithhealthycontrolsin theirresponsetocontrolledexperimentalpainstimulation.Results indicatesignificantlylowerpainthresholdinPDpatients (indica-tiveofgreaterpain)intheOFFstatecomparedtocontrols.Thiswas partially(butnotcompletely)normalizedbydopaminergic medi-cation,suggestingthatdisruptionofdopaminepainpathwaysmay contributeto abnormalpain processing.These findings suggest thatPDmayconferahypersensitivitytonociceptiveinformation thatcouldbothexacerbatethemusculoskeletalpainresultingfrom motorrigidityandabnormalposturecontrol,andcontributetothe lesscommonbutneverthelesstroublingnon-musculoskeletalpain complaintsthatoccurinPD.
Disclosures
TT,KG,NV,EWandBShavenoconflictsofinterest.AFCis sup-portedbyaresearchfellowshipawardfromtheConselhoNacional deDesenvolvimentoCientíficoeTecnológico(CNPq;Brazil).CUC has been a consultant and/or advisor to or has received hon-oraria from: Alkermes, Allergan, Bristol-Myers Squibb, Forum, GersonLehrman Group, IntraCellular Therapies, Janssen/J&J,LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer,ProPhase,Sunovion,Supernus,Takeda,and Teva.He has providedexperttestimonyforBristol-MyersSquibb,Janssen,and Otsuka.HeservedonaDataSafetyMonitoringBoardforLundbeck andPfizer.HereceivedgrantsupportfromTakeda.
Acknowledgements
WeareespeciallygratefultoDrsGergelyOrsi,YelenaGranovsky, JonStoessl,MariaNolano,TomohikoNakamura,MasanakaTakeda, JanoschPriebefortheirrapidandexceptionallyhelpfulresponses torequestsforadditionalstudydata.Wearealsoextremelygrateful toProfessorJohnLeesandDrAlastairNoyceofUniversityCollege Londonfortheirinvaluableadviceandextremelyhelpful commen-taryonthemanuscript.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.arr.2017.01.005.
References
Allen,N.E.,Moloney,N.,Hassett,L.M.,Canning,C.G.,Lewis,S.J.G.,Cruz-Mavignier, K.,Barry,B.K.,2016.Exerciseinducedanalgesiaispresentinpeoplewith Parkinson’sdisease.Mov.Disord.31.
Apkarian,A.V.,Bushnell,M.C.,Treede,R.D.,Zubieta,J.K.,2005.Humanbrain mechanismsofpainperceptionandregulationinhealthanddisease.Eur.J. Pain9,463–484.
Aschermann,Z.,Nagy,F.,Perlaki,G.,Janszky,J.,Schwarcz,A.,Kovacs,N.,Bogner,P., Komoly,S.,Orsi,G.,2015.Wind-up’inParkinson’sdisease:afunctional magneticresonanceimagingstudy.Eur.J.Pain19,1288–1297. Borenstein,M.,Hedges,L.,Higgins,J.,Rothstein,H.R.,2009.Introductionto
Meta-Analysis.Wiley,WestSussex.
Brefel-Courbon,C.,Payoux,P.,Thalamas,C.,Ory,F.,Quelven,I.,Chollet,F., Montastruc,J.L.,Rascol,O.,2005.Effectoflevodopaonpainthresholdin Parkinson’sdisease:aclinicalandpositronemissiontomographystudy.Mov. Disord.20,1557–1563.
Broen,M.P.,Braaksma,M.M.,Patijn,J.,Weber,W.E.,2012.Prevalenceofpainin Parkinson’sdisease:asystematicreviewusingthemodifiedQUADAStool. Mov.Disord.27,480–484.
Chaudhuri,K.R.,Odin,P.,2010.Thechallengeofnon-motorsymptomsin Parkinson’sdisease.Prog.BrainRes.184,325–341.
Chaudhuri,K.R.,Schapira,A.H.,2009.Non-motorsymptomsofParkinson’sdisease: dopaminergicpathophysiologyandtreatment.LancetNeurol.8,464–474. Chaudhuri,K.R.,Prieto-Jurcynska,C.,Naidu,Y.,Mitra,T.,Frades-Payo,B.,Tluk,S.,
Ruessmann,A.,Odin,P.,Macphee,G.,Stocchi,F.,Ondo,W.,Sethi,K.,Schapira, A.H.,MartinezCastrillo,J.C.,Martinez-Martin,P.,2010.Thenondeclarationof nonmotorsymptomsofParkinson’sdiseasetohealthcareprofessionals:an internationalstudyusingthenonmotorsymptomsquestionnaire.Mov.Disord. 25,704–709.
Chen,Y.,Mao,C.J.,Li,S.J.,Wang,F.,Chen,J.,Zhang,H.J.,Li,L.,Guo,S.S.,Yang,Y.P., Liu,C.F.,2015.Quantitativeandfiber-selectiveevaluationofpainandsensory dysfunctioninpatientswithParkinson’sdisease.ParkinsonismRelat.Disord. 21,361–365.
CiampideAndrade,D.,Lefaucheur,J.P.,Galhardoni,R.,Ferreira,K.S.,BrandãoPaiva, A.R.,Bor-Seng-Shu,E.,Alvarenga,L.,Myczkowski,M.L.,Marcolin,M.A.,de Siqueira,S.R.,Fonoff,E.,Barbosa,E.R.,Teixeira,M.J.,2012.Subthalamicdeep brainstimulationmodulatessmallfiber-dependentsensorythresholdsin Parkinson’sdisease.Pain153,1107–1113.
Cohen,J.,1988.StatisticalPowerAnalysisfortheBehavioralSciences.Lawrence EarlbaumAssociates,Hillsdale,NJ.
Cury,R.G.,Galhardoni,R.,Fonoff,E.T.,PerezLloret,S.,DosSantosGhilardi,M.G., Barbosa,E.R.,Teixeira,M.J.,CiampideAndrade,D.,2016.Sensory
abnormalitiesandpaininParkinsondiseaseanditsmodulationbytreatment ofmotorsymptoms.Eur.J.Pain20,151–165.
Deeks,J.J.,Dinnes,J.,D’Amico,R.,Sowden,A.J.,Sakarovitch,C.,Song,F.,Petticrew, M.,Altman,D.G.,International,S.T.C.G.,European,C.S.T.C.G.,2003.Evaluating non-randomisedinterventionstudies.HealthTechnol.Assess.7,iii–x,1. Djaldetti,R.,Shifrin,A.,Rogowski,Z.,Sprecher,E.,Melamed,E.,Yarnitsky,D.,2004.
QuantitativemeasurementofpainsensationinpatientswithParkinson disease.Neurology62,2171–2175.
Duval,S.,Tweedie,R.,2000.Trimandfill:asimplefunnel-plot-basedmethodof testingandadjustingforpublicationbiasinmeta-analysis.Biometrics56, 455–463.
Egger,M.,DaveySmith,G.,Schneider,M.,Minder,C.,1997.Biasinmeta-analysis detectedbyasimple,graphicaltest.BMJ315,629–634.
Fahn,S.,Elton,R.L.,1987.In:Fahn,S.,Marsden,C.D.,Calne,D.B.,Goldstein,M. (Eds.),RecentDevelopmentsinParkinson’sDiseaseII.MacMillanHealthCare Information,NewJersey,pp.153–163.
Fil,A.,Cano-de-la-Cuerda,R.,Mu ˜noz-Hellín,E.,Vela,L.,Ramiro-González,M., Fernández-de-Las-Pe ˜nas,C.,2013.PaininParkinsondisease:areviewofthe literature.ParkinsonismRelat.Disord.19,285–294,discussion285. Fisher,Z.,Tipton,E.,2014.Robumeta:Robustvariancemeta-regression.Rpackage
86 T.Thompsonetal./AgeingResearchReviews35(2017)74–86
Folstein,M.F.,Folstein,S.E.,McHugh,P.R.,1975.Mini-mentalstate:apractical methodforgradingthecognitivestateofpatientsfortheclinician.J.Psychiatr. Res.12,189–198.
Ford,B.,2010.Paininparkinson’sdisease.Mov.Disord.25(Suppl.1),S98–103. Gerdelat-Mas,A.,Simonetta-Moreau,M.,Thalamas,C.,Ory-Magne,F.,Slaoui,T.,
Rascol,O.,Brefel-Courbon,C.,2007.Levodoparaisesobjectivepainthreshold inParkinson’sdisease:aRIIIreflexstudy.J.Neurol.Neurosurg.Psychiatry78, 1140–1142.
Goetz,C.G.,Stebbins,G.T.,Tilley,B.C.,2012.CalibrationofunifiedParkinson’s diseaseratingscalescorestoMovementDisorderSociety-unifiedParkinson’s diseaseratingscalescores.Mov.Disord.27,1239–1242.
Granovsky,Y.,Schlesinger,I.,Fadel,S.,Erikh,I.,Sprecher,E.,Yarnitsky,D.,2013. AsymmetricpainprocessinginParkinson’sdisease.Eur.J.Neurol.20, 1375–1382.
Grashorn,W.,Schunke,O.,Buhmann,C.,Forkmann,K.,Diedrich,S.,Wesemann,K., Bingel,U.,2015.Influenceofdopaminergicmedicationonconditionedpain modulationinparkinson’sdiseasepatients.PLoSOne10,e0135287. Gwet,K.,2002.Inter-raterreliability:dependencyontraitprevalenceand
marginalhomogeneity.Stat.MethodsInter-RaterReliab.Assessment2,1–9. Hara,T.,Hirayama,M.,Mizutani,Y.,Hama,T.,Hori,N.,Nakamura,T.,Kato,S.,
Watanabe,H.,Sobue,G.,2013.ImpairedpainprocessinginParkinson’sdisease anditsrelativeassociationwiththesenseofsmell.ParkinsonismRelat.Disord. 19,43–46.
Hedges,L.V.,Tipton,E.,Johnson,M.C.,2010.Robustvarianceestimationin meta-regressionwithdependenteffectsizeestimates.Res.Synth.Methods1, 39–65.
Higgins,J.P.,Thompson,S.G.,Deeks,J.J.,Altman,D.G.,2003.Measuring inconsistencyinmeta-analyses.BMJ327,557–560.
Jarcho,J.M.,Mayer,E.A.,Jiang,Z.K.,Feier,N.A.,London,E.D.,2012.Pain,affective symptoms,andcognitivedeficitsinpatientswithcerebraldopamine dysfunction.Pain153,744–754.
Lim,S.-Y.,Farrell,M.J.,Gibson,S.J.,Helme,R.D.,Lang,A.E.,Evans,A.H.,2008.Do dyskinesiaandpainsharecommonpathophysiologicalmechanismsin Parkinson’sdisease.Mov.Disord.23,1689–1695.
Lipsey,M.,Wilson,D.,2001.PracticalMeta-Analysis.Sage,London.
Maruo,T.,Saitoh,Y.,Hosomi,K.,Kishima,H.,Shimokawa,T.,Hirata,M.,Goto,T., Morris,S.,Harada,Y.,Yanagisawa,T.,Aly,M.M.,Yoshimine,T.,2011.Deep brainstimulationofthesubthalamicnucleusimprovestemperaturesensation inpatientswithParkinson’sdisease.Pain152,860–865.
Massetani,R.,Lucchetti,R.,Vignocchi,G.,Siciliano,G.,Rossi,B.,1989.Pain thresholdandpolysynapticcomponentsoftheblinkreflexinParkinson’s disease.Funct.Neurol.4,199–202.
Mitra,T.,Naidu,Y.,Martinez-Martin,P.,2008.Thenondeclarationofnonmotor symptomsofParkinson’sdiseasetohealthcareprofessionals.Aninternational surveyusingtheNMSQuest.In:6thInternationalCongressonMental DysfunctionsandOtherNon-motorFeaturesinParkinson’sDiseaseand RelatedDisordersDresdenOctober,2008ParkRelatedDisorders,P0II:161. Moher,D.,Liberati,A.,Tetzlaff,J.,Altman,D.G.,PRISMA,G.,2009.Preferred
reportingitemsforsystematicreviewsandmeta-analyses:thePRISMA statement.BMJ339,b2535.
Mylius,V.,Engau,I.,Teepker,M.,Stiasny-Kolster,K.,Schepelmann,K.,Oertel,W.H., Lautenbacher,S.,Möller,J.C.,2009.Painsensitivityanddescendinginhibition ofpaininParkinson’sdisease.J.Neurol.Neurosurg.Psychiatry80,24–28. Mylius,V.,Brebbermann,J.,Dohmann,H.,Engau,I.,Oertel,W.H.,Möller,J.C.,2011.
PainsensitivityandclinicalprogressioninParkinson’sdisease.Mov.Disord. 26,2220–2225.
Mylius,V.,Pee,S.,Pape,H.,Teepker,M.,Stamelou,M.,Eggert,K.,Lefaucheur,J.P., Oertel,W.H.,Möller,J.C.,2016.Experimentalpainsensitivityinmultiple systematrophyandParkinson’sdiseaseatanearlystage.Eur.J.Pain20, 1223–1228.
Nègre-Pagès,L.,Regragui,W.,Bouhassira,D.,Grandjean,H.,Rascol,O.,DoPaMiP, S.G.,2008.ChronicpaininParkinson’sdisease:thecross-sectionalFrench DoPaMiPsurvey.Mov.Disord.23,1361–1369.
Nandhagopal,R.,Troiano,A.R.,Mak,E.,Schulzer,M.,Bushnell,M.C.,Stoessl,A.J., 2010.ResponsetoheatpainstimulationinidiopathicParkinson’sdisease.Pain Med.11,834–840.
Nolano,M.,Provitera,V.,Estraneo,A.,Selim,M.M.,Caporaso,G.,Stancanelli,A., Saltalamacchia,A.M.,Lanzillo,B.,Santoro,L.,2008.Sensorydeficitin Parkinson’sdisease:evidenceofacutaneousdenervation.Brain131, 1903–1911.
Perez-Lloret,S.,Rey,M.V.,Dellapina,E.,Pellaprat,J.,Brefel-Courbon,C.,Rascol,O., 2012.EmerginganalgesicdrugsforParkinson’sdisease.ExpertOpin.Emerg. Drugs17,157–171.
Priebe,J.A.,Kunz,M.,Morcinek,C.,Rieckmann,P.,Lautenbacher,S.,2016. ElectrophysiologicalassessmentofnociceptioninpatientswithParkinson’s disease:amulti-methodsapproach.J.Neurol.Sci.368,59–69.
Quittenbaum,B.H.,Grahn,B.,2004.QualityoflifeandpaininParkinson’sdisease: acontrolledcross-sectionalstudy.ParkinsonismRelat.Disord.10,129–136. RCoreTeam,2014.R:ALanguageandEnvironmentforStatisticalComputing.R
FoundationforStatisticalComputing,Vienna,Austria.
Schestatsky,P.,Kumru,H.,Valls-Sole,J.,Valldeoriola,F.,Marti,M.J.,Tolosa,E., Chaves,M.L.,2007.Neurophysiologicstudyofcentralpaininpatientswith Parkinsondisease.Neurology69,2162–2169.
Schrag,A.,Horsfall,L.,Walters,K.,Noyce,A.,Petersen,I.,2015.Prediagnostic presentationsofParkinson’sdiseaseinprimarycare:acase-controlstudy. LancetNeurol.14,57–64.
Staahl,C.,Olesen,A.E.,Andresen,T.,Arendt-Nielsen,L.,Drewes,A.M.,2009. Assessinganalgesicactionsofopioidsbyexperimentalpainmodelsinhealthy volunteers–anupdatedreview.Br.J.Clin.Pharmacol.68,149–168. Stamelou,M.,Dohmann,H.,Brebermann,J.,Boura,E.,Oertel,W.H.,Höglinger,G.,
Möller,J.C.,Mylius,V.,2012.Clinicalpainandexperimentalpainsensitivityin progressivesupranuclearpalsy.ParkinsonismRelat.Disord.18,606–608. Stroup,D.F.,Berlin,J.A.,Morton,S.C.,Olkin,I.,Williamson,G.D.,Rennie,D.,Moher,
D.,Becker,B.J.,Sipe,T.A.,Thacker,S.B.,2000.Meta-analysisofobservational studiesinepidemiology:aproposalforreporting.Meta-analysisOf
ObservationalStudiesinEpidemiology(MOOSE)group.JAMA283,2008–2012. Stubbs,B.,Thompson,T.,Acaster,S.,Vancampfort,D.,Gaughran,F.,Correll,C.U.,
2015.Decreasedpainsensitivityamongpeoplewithschizophrenia:a meta-analysisofexperimentalpaininductionstudies.Pain156,2121–2131. Takeda,M.,Okada,F.,Tachibana,H.,Kajiyama,K.,Yoshikawa,H.,2013.
NeurophysiologicstudyofpaininParkinson’sdisease:astudywith pain-relatedevokedpotentials.Mov.Disord.28,S79.
Takeda,M.,Tachibana,H.,Okada,F.,Kasama,S.,Yoshikawa,H.,2014.Painin patientswithparkinson’sdisease;apain-relatedevokedpotentialstudy. Neurosci.Biomed.Eng.2,36–40.
Tan,Y.,Tan,J.,Luo,C.,Cui,W.,He,H.,Bin,Y.,Deng,J.,Tan,R.,Tan,W.,Liu,T.,Zeng, N.,Xiao,R.,Yao,D.,Wang,X.,2015.Alteredbrainactivationinearlydrug-naive Parkinson’sdiseaseduringheatpainstimuli:anfMRIstudy.Parkinson’sDis. 2015,273019.
Thompson,T.,Correll,C.U.,Gallop,K.,Vancampfort,D.,Stubbs,B.,2016.Ispain perceptionalteredinpeoplewithdepression?Asystematicreviewand meta-analysisofexperimentalpainresearch.J.Pain17,1257–1272. Tipton,E.,2015.Smallsampleadjustmentsforrobustvarianceestimationwith
meta-regression.Psychol.Methods20,375–393.
Valkovic,P.,Minar,M.,Singliarova,H.,Harsany,J.,Hanakova,M.,Martinkova,J., Benetin,J.,2015.Paininparkinson’sdisease:across-sectionalstudyofits prevalence,types,andrelationshiptodepressionandqualityoflife.PLoSOne 10,e0136541.
Vela,L.,Lyons,K.E.,Singer,C.,Lieberman,A.N.,2007.Pain-pressurethresholdin patientswithParkinson’sdiseasewithandwithoutdyskinesia.Parkinsonism Relat.Disord.13,189–192.
Vela,L.,Cano-de-la-Cuerda,R.,Fil,A.,Mu ˜noz-Hellín,E.,Ortíz-Gutiérrez,R., Macías-Macías,Y.,Fernández-de-Las-Pe ˜nas,C.,2012.Thermalandmechanical painthresholdsinpatientswithfluctuatingParkinson’sdisease.Parkinsonism Relat.Disord.18,953–957.
Wells,G.A.,Shea,B.,O’Connell,D.,Peterson,J.,Welch,V.,Losos,M.,Tugwell,P., 2008.TheNewcastle-OttawaScale(NOS)forAssessingtheQualityof NonrandomisedStudiesinMeta-Analyses(Accessed8May2016).Availableat: http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp.
Wood,P.B.,2008.Roleofcentraldopamineinpainandanalgesia.ExpertRev. Neurother.8,781–797.
Yarnitsky,D.,Pud,D.,2004.In:Binnie,C.D.,Cooper,R.,Mauguiere,F.,Osselton, J.W.,Prior,P.F.,Tedman,B.M.(Eds.),ClinicalNeurophysiology:EMG,Nerve ConductionandEvokedPotentials,vol.1.Elsevier,Amsterdam,p.305. Zambito,M.S.,Tinazzi,M.,Vitaliani,R.,Recchia,S.,Fabris,F.,Marchini,C.,Fiaschi,