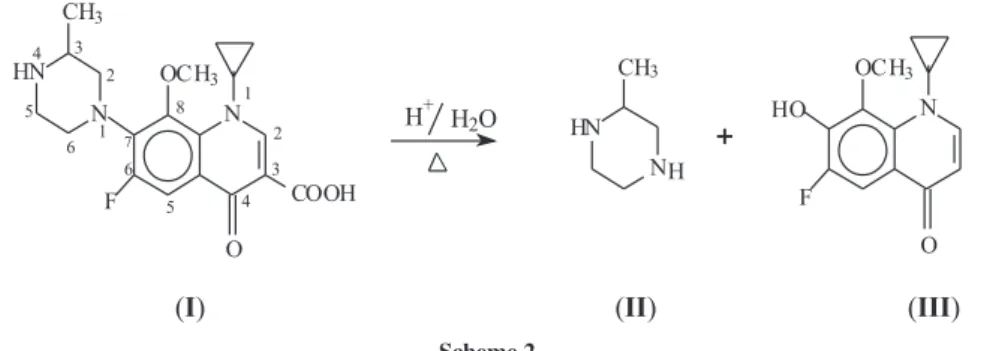
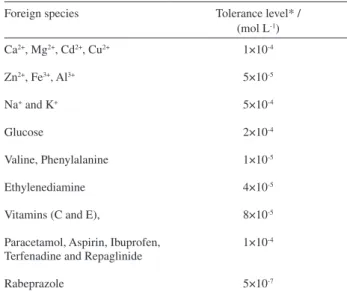
J. Braz. Chem. Soc. vol.20 número10
Texto
Imagem




Documentos relacionados
This work describes a photo-reactor to perform in line degradation of organic compounds by photo-Fenton reaction using Sequential Injection Analysis (SIA) system.. A
This work reports the composition of the volatile oils from aerial parts of ive specimens and of the essential oil from the fruits of one specimen of Poiretia bahiana that were
The source apportionment results of winter 2005 and 2006 indicate that the major contributors of PM 2.5 during winter in Lahore are: secondary aerosols, industrial
The effect of different parameters like pH, concentration of malachite green, reductants, amount of semiconductor and light intensity on the rate of
A convenient procedure by means of oscillating chemical system as an analytical tool to determine the L-aspartic acid (L-Asp) is proposed.. The system involves
Figure 4. Electrophoretic separation of the 1kbp DNA ladder fragments intercalated with TOTO -1 iodide in PDMA column with different HEC concentrations, as indicated. Close
From the results obtained here, the deterioration of copper in presence of propionic acid vapors can be explained through the following mechanism: The irst
Based on the adsorption behavior of nitazoxanide onto the mercury electrode surface, simple and highly sensitive linear sweep, differential pulse and square wave adsorptive