The
Brazilian
Journal
of
INFECTIOUS
DISEASES
w w w .e l s e v i e r . c o m / l o c a t e / b j i d
Review
article
Challenges
in
the
development
of
drugs
for
the
treatment
of
tuberculosis
Adeeb
Shehzad
a,
Gauhar
Rehman
a,
Mazhar
Ul-Islam
b,
Waleed
Ahmad
Khattak
b,
Young
Sup
Lee
a,∗aSchoolofLifeSciences,CollegeofNaturalSciences,KyungpookNationalUniversity,Daegu,RepublicofKorea
bDepartmentofChemicalEngineering,KyungpookNationalUniversity,Daegu,RepublicofKorea
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received13June2012 Accepted3October2012 Availableonline1January2013
Keywords:
Antibiotics Drugresistance Tuberculosis Pathogen
a
b
s
t
r
a
c
t
Tuberculosisinfectionisaserioushumanhealththreatandtheearly21stcenturyhasseen aremarkableincreaseinglobaltuberculosisactivity.Thepathogenresponsiblefor tuber-culosisisMycobacteriumtuberculosis,whichadoptsdiversestrategiesinordertosurvivein avarietyofhostlesions.Thesesurvivalmechanismsmakethepathogenresistantto cur-rentlyavailabledrugs,amajorcontributingfactorinthefailuretocontrolthespreadof tuberculosis.Multipledrugsareavailableforclinicaluseandseveralpotentialcompounds arebeingscreened,synthesized,orevaluatedinpreclinicalorclinicalstudies.Lastingand effectiveachievementsinthedevelopmentofanti-tuberculosisdrugswilldependlargelyon theproperunderstandingofthecomplexinteractionsbetweenthepathogenanditshuman host.Ampleevidenceexiststoexplainthecharacteristicsoftuberculosis.Inthisstudy, wehighlightedthechallengesforthedevelopmentofnoveldrugswithpotent bacterio-staticorbactericidalactivity,whichreducetheminimumtimerequiredtocuretuberculosis infection.
©2013ElsevierEditoraLtda.Allrightsreserved.
Introduction
The pathogen responsible for tuberculosis (TB) infection,
Mycobacteriumtuberculosis,wasfirstidentifiedbyRobertKoch
in1882.However,theglobalTBepidemicremains undimin-ishedandisexpectedtoreach9,8millionnewcasesin2010, morecasesthaninanypreviousyearinhistory.About80%of thesenewTBcaseswillbefoundinthe20–25highest-burden countries, withmorethan one-third inIndiaand China. A reviewoftheTBcasesreportedby134countriesbetween1998 and2007foundthatonly35hadpercapitaratesofdecline
∗ Correspondingauthor.
E-mailaddress:yselee@knu.ac.kr(Y.S.Lee).
exceeding5%peryear.1However,surveillanceand
mathemat-icalmodelingsuggeststhatthetotalTBincidencepercapita isfallingatanestimated1%peryear,afindingthatindicates thattheglobalincidenceratewilldecreaseby2015.However, theworld’spopulationisgrowingatabout2%peryear,and thusthetotalnumberofnewTBcasesremainsontherise.2
Thisfindingrevealstherelativefailureoftheexisting man-agementstrategiesforTBandtheinadequateeffectivenessof publichealthsystems,mainlyinunderdeveloped countries. Inspiteoftheavailabilityofanti-TBdrugsdevelopedoverthe lastfivedecades,one-thirdoftheworld’spopulationretainsa
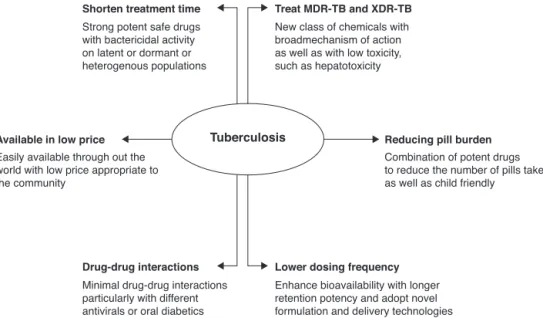
Shorten treatment time Treat MDR-TB and XDR-TB
Reducing pill burden
Lower dosing frequency Drug-drug interactions
Available in low price
Strong potent safe drugs with bactericidal activity on latent or dormant or heterogenous populations
New class of chemicals with broadmechanism of action as well as with low toxicity, such as hepatotoxicity
Combination of potent drugs to reduce the number of pills taken as well as child friendly
Enhance bioavailability with longer retention potency and adopt novel formulation and delivery technologies Minimal drug-drug interactions
particularly with different antivirals or oral diabetics
Tuberculosis Easily available through out the
world with low price appropriate to the community
Fig.1–Aschematicrepresentationfornewtuberculosis(TB)drugs.Thedesiredprofileofeachproductisenlightenedby thebiologicalcharacteristicspreferredtoachievetherespectivefeatures.
dormantorlatentformofM.tuberculosis.Thesepopulations are generally asymptomatic but remain at lifelong risk of diseasereactivationandrepresentahighriskfactorforthe spreadofthedisease(Fig.1).
Currently,the TBepidemicisfurtherexacerbatedbythe existenceofmultidrugresistant-TB(MDR-TB)andextremely drugresistant-TB(XDR-TB)strainsand decliningtreatment optionsasleastthreeeffectivedrugsarerequiredtobeused incombinationinordertosuccessfullytreatthediseasewhile preventingthedevelopmentoffurtherdrugresistance.3The
lastdrugtherapy discoveredandapproved, whichemploys anewmechanism ofactionfortreatmentofTB,was com-bination dosing using isoniazid (INH) and rifampicin (RIF). However,MDR-TBisresistanttoINHandRIF.Casesof MDR-TB and XDR-TB result from either primary infection with a drug-resistant bacteria strain or non-optimal treatment durationsorregimens.Inspiteoftheintroduction through-out the world many years ago of concomitant chemicals foruseinTBtreatment,lower cureratesforMDR-TB have been observed, ranging from 50 to 70%.4 XDR-TB is
resis-tanttoINHandRIF,allfluoroquinolones,andatleastoneof thesecond-lineanti-TBinjectabledrugsincludingamikacin, kanamycin and/or capreomycin. These types of resistance TB infections produce very high mortality rates.3
Further-more,theoccurrenceofdrug–druginteractionsexcludesthe co-administrationofsomeexistingTBdrugswithother medi-cationsusedinchronicdiseases.5Multipleeffectivetherapies
needtobedevelopedthatproducemultiplefunctionssuch asshorteningoftreatmenttime,efficacyagainstMDR-TBand XDR-TBstrains,simplertreatmentregimensincludinglower dosingfrequencies,andtherapiesthatcanbeco-administered withmedications usedinother chronicdiseases.The chal-lengeofmeetingthespectrumofexpectationsforthisdesired targetproductprofilecomplicatesdrugdiscovery efforts as manyoftheforthcomingcompoundsare eitherderivatives ofpresentlyusedcompounds ormodulate the same cellu-larprocesses as drugs currently in use.5,6 In addition, the
analoguesandderivativesindevelopmentarefarfrom trans-lationintotheclinic,maybesubjecttocross-resistance,and maybehamperedbytheunfavorableeconomicssurrounding TBdrugdevelopmentandlackofproperpolicyincentives.
Theaimofthisstudy wastodeterminewhether ornot thereareasufficientnumberofpromisingcompoundsinthe TBpipelinenecessaryinordertoachieveglobalcontrolofthis epidemic. Wepresenteda mechanismofactionagainstTB infection,providedaperspectivewiththegoalofimproving thefocusofdiscoveryanddevelopmentefforts,andidentified theunderlyingknowledgegapsandscientificobstaclesinTB drugdevelopment.
Drug
resistance
mechanisms
in
Mycobacterium
tuberculosis
Theexistingsituationsurroundingthegrowingdrug-resistant TBpatientpopulationissignificantlydisquietingand repre-sentsasignificantweaknessintheabilitytocontroldiseaseon aglobalscale.5,6Anyviralinfectioncausinghumanimmune
systemdeficiencycanreactivatetheTBinfection5 and
gen-erate the potential of producing new TB cases. In recent reports, the percentageof MDRcasesdemonstrating resis-tancetovariousclassesofantibioticsrangedfrom0to22.3%.6
Around40.000casesofXDR-TBareprojectedtoemerge world-wide each year.5,7 It isofutmost importance tofigureout
themechanismsthroughwhichthemycobacterialcellsresist anti-tuberculosisdrugs.Understandingthemechanismwill enablethedesignofnewanti-tuberculosisdrugsaswellas methods for interfering with the expression of such drug resistance.7
regimenliesinthefactthatthechromosomallociresponsible forresistancetovariousdrugsarenotlinked.Thus,the pos-sibilityofdevelopingresistancetothreedrugsadministered simultaneouslybecomes10−18 to10−20,anoutcomewhose probabilityisextremelyloworalmostnegligible.8Enormous
effortshavebeenmadetoidentifythemolecularbasisofdrug actionandresistanceintheTBpathogen.Thedrugactionand thebacterialresistancemechanismofafewcommonlyused drugshave been briefly summarizedto highlight the need fornoveldrugdesignthatcouldreducetreatmenttimeand avoidtheoccurrenceofdrugresistanceinthetreatmentofTB infections.
Resistance
to
anti-TB
drugs
Isoniazid(INH)
Sinceits introduction asan anti-TBdrug in1951,INH has beenthe mostwidelyusedtreatmentforTBandits latent infections.9,10INHpenetratesintothecellasapro-drug
acti-vated bykatG, the gene encodingcatalase-peroxidase. The peroxidaseactivityofthis enzymeisessentialinactivating INHandenablingitsinteractionswithvarioustoxicreactive speciesinthebacterialcell.11Thereactivespeciesusually
con-sist ofoxides, hydroxylradicals,and organic moieties that deterioratecomponentsofthecellwallwhichresultsinthe lossof cellularintegrity, and finally, bacterialdeath.12 The
resistancefrequency of 105,6 for INH ismuch higher than
thatformostanti-tuberculosisdrugs.13InhAenzyme
(enoyl-acylcarrierproteinreductase),involvedintheelongationof fattyacids inmycolicacid synthesis,isconsideredthe pri-marytargetofINHinhibition.14Thereplacementofanamino
acid in the NADH bindingsite of Inh A apparently results inINHresistance,preventingtheinhibitionofmycolicacid biosynthesis.Variousgeneticmutationshavebeenobserved tooccurusuallybetweencodons138and328;theSer315Thr mutationismostfrequentandfoundinabout40%ofall INH-resistant strains.15–17 ThemutationatSer315Thrresults in
anenzymelackingtheabilitytoactivateINH,butpreserves approximately50%ofitscatalase-peroxidaseactivity.18Thus,
themodifiedcatalase-peroxidaseoffershigh-levelresistance toINHwhilemaintainingahighlevelofoxidativeprotection sufficienttofacilitatetheorganisminsustainingits detoxify-ingactivityagainsthostantibacterialfreeradicals.
Rifampicin(RIF)
Rifampin, rifapentine, and rifabutin have been commonly usedasfirst-linetherapiesincombinationwithotherdrugs forthetreatmentofTBinfections.TheuseofRIFin combina-tionwithPZA/INHledtotheestablishmentofshorttherapy courses,whichreducedroutineTBtreatmentfrom1yearto 6months.RIFisbelievedtoinhibitbacterialDNA-dependent RNApolymerase.RNApolymeraseiscomposedoffour differ-entsubunits(␣,,′,and),andthegeneticlocicodingfor thesesubunitsincludetherpoA,rpoB,rpoC,andrpoDgenes, respectively.RMPinterfereswith RNAsynthesisbybinding tothesubunitoftheRNApolymerase,hindering transcrip-tionandtherebykillingtheorganism.ResistancetoRIFarises
due to missense mutationsin the gene. In M. tuberculosis, resistancetoRIFoccursatafrequencyof10−7to10−8,5The resistance in RIF develops due to mutations in a distinct, 81-base-pair(bp) (27-codon)centralregion ofthe genethat encodes the -subunit ofRNApolymerase.19 About 96%of
allmutationsarefoundinthe81-bpcoreregionofthegene betweencodons507and533,withthemostcommonchanges occurringincodonsSer531Leu,His526Tyr,andAsp516Val.20
Pyrazinamide(PZA)
PZA isavitalfirst-linedrugforthetreatmentof tuberculo-sis. PZAhasanexcellent sterilizingeffecton semidormant tubercle bacilli. By killing semidormantbacilli inan acidic environment, PZA,along with INHand RIF, formsthe cor-nerstone ofmodern TBtreatmenttherapy. PZA hasplayed an important role in reducing the duration of TB treat-ment from the previous 9–12 months to the current 6 months.21 PZA is a prodrugthat is converted to its active
form pyrazinoic acid (POA) by the mycobacterial enzyme pyrazinamidase/nicotinamidase. PZA, produced intracellu-larly, diffuses into the tuberculosis pathogen in a passive manner,andisconvertedintoPOAbypyrazinamidasewithin thecell.Theinefficienteffluxsystemofthemycobacterialcell enablesmassiveaccumulationofPOAinthebacterial cyto-plasm,leadingtodisruptionofthemembranepotential.22,23
TheexactmechanismofPZAresistanceremainsunknown. However, it is known that PZA-resistant bacterial strains usually lose their pyrazinamidase activity.24 Cloning and
sequencingstudiesofthegenethatencodespyrazinamidase revealed that 72–97%ofallPZA-resistant clinicallyisolated specimenscarryamutationeitherinthestructuralgeneor intheputativepromoterregionofthegene.25
Ethambutol(EMB)
EMB is a first-line drug that is used in combination with INH, RIF, and PZA in order to prevent the emergence of drugresistancespecifictomycobacterium.EMBisanactive bacteriostatic agent for bacilli that are growing but has no effect on non-replicating bacilli. EMB interferes with themycobacterialcellwallthroughasyntheticmechanism andinhibitsarabinosyl-transferase,whichisinvolvedincell wall biosynthesis.26 Encoded byarabinosyl-transferase and
involved in the synthesis of arabinogalactan, arabinosyl-transferase hasbeen proposed asthe target ofEMBaction withinthetuberculosisorganism.27Studieshaveshownthat
resistance to EMB is due to random spontaneous genetic mutations occurring at a rate of approximately 1 in 107
organisms; mutations most commonly result in increased productionoftheenzymearabinosyl-transferase,which over-whelmstheinhibitoryeffectsofEMB.Studieshaveidentified five mutationsin codon306 including ATG-GTG,ATG-CTG, ATG-ATA,ATG-ATC,andATG-ATTthatresultinthreedifferent aminoacidsubstitutions(Val,Leu,andIle)inEMB-resistant strains.28,29Thesefivemutationsareassociatedwith70–90%
Current
challenges
in
the
TB
treatment
Thecurrentanti-TBdrugs,discoveredprimarilyinthe1950s to1970s, were developedthrough aseries ofclinical trials that extended into the 1980s.30 The subsequent 30 years,
untilapproximately2000,representedafallowperiodinTB drugresearchanddevelopment,avoidcontributinggreatlyto thesignificantchallengescurrentlyfacedbythecommunity of drug developers focused on markedly improving treat-mentsforactiveMDR-TBand XDR-TB.Atpresent,MDR-TB istreatedusingacombination ofdifferentdrugswith ther-apieslasting 18–24 months,with only four of thesedrugs developedforthepurposeoftreatingTB.31Suboptimal
ther-apy leadsto almost 30% ofMDR-TB patients experiencing treatment failure.32 The treatment options forXDR-TB are
verylimitedbecauseXDR-TBbacilliareresistant,notonlyto INHandRIF,butalsotofluoroquinolonesincludinginjectable drugssuchas aminoglycosides.In addition, thereare seri-ous side effects observed with most MDR-TB and XDR-TB drugs,includingnephrotoxicityandototoxicitywiththeuseof aminoglycosides,hepatotoxicitywiththeuseofethionamide, anddysglycemiawiththeuseofgatifloxacin.33
Thevast majorityofTBcasesanddeathsoccur inpoor countriesandaboutoneinfourofthedeathsoccuramong HIV-positive people. It has been reported that 9,4 million patientsdiagnosedwithTBin2009(11–13%)were alsoHIV positivewith approximately 80% ofthese morbidities con-finedtothe Africanregion.34 Thisisdue tolimited health
careaccessaswellasdiminishedcompliancewith therapeu-ticregimensassociatedwithincreasedpillburden,drug–drug interactions,andoverlappingtoxicsideeffects.Theprimary interactionbetweenHIVandanti-TBtreatmentagentsis RIF-inducedoverexpressionofthehepaticcytochrome(CYPP450) oxidasesystem.35StudieshaveshownthatCYPincreasedthe
pharmacokineticrateand decreased theefficacy ofseveral concomitantmedicationsincludingHIVproteaseinhibitors.36
Itisnotedthat withthe use ofCYP450 inhibitors,such as ritonavir,normaltrenchlevelsofvariousclassesofprotease inhibitorscannot beregained. Accordingly,they havebeen showntocompetewithrifampicininintracellular phosphor-ylation. Thus, these drugs should not be co-administered. However, the presence of ritonavir in a protease cock-tailincreasestheserum concentrationofrifabutin,thereby increasingitsaccompanyingtoxicity.37Multipledrug
interac-tionstudiesare fewformostsecond-lineTBdrugssuchas ethionamide,cycloserine,kanamycin,amikacin,capreomycin andpara-aminosalicylate,whichhavebeeninusesincethe lastdecade fortreating HIVpatientswithMDR-or XDR-TB strainco-infections.38 Therefore,specificactivecase-finding
strategiesareneededtotargetHIV-infectedindividualsaswell astheinteractionofanti-retroviralswithcurrentlyavailable second-line TBdrugs, aswell asthose currentlyinclinical development.
Like HIV, diabetes is also associated with the suppres-sionofcell-mediatedimmunityandisknowntoincreasethe riskofdevelopingactiveTBbythree-fold.39Studiesin2000
revealedthatapproximately20%ofsmear-positiveTBcases wereattributedtoconcurrentdiabetesinIndia.40Inthe
mean-time,iftheprojectedrisefrom25milliondiabetescasesin
2000to80millionin2030comestofruition,andtheriskratio remains the same,then42% ofsmear-positiveTBcasesin India willbeattributabletodiabetesby2030.EachTBcase causedbydiabeteswouldalsopotentiallyleadtothe infec-tionofotherpeople,addingtotheoverallTBburdeninthe community.41 Thebiological basisforthe limitedresponse
ofdiabeticstoanti-TBagentsandfortheirincreasedriskof developingMDR-TBispoorlyunderstood.But,itisbelieved that cell-mediatedimmunityissuppressedindiabetes and fostershigherTBinfectionrates.
Future
clinical
TB
drugs
to
overcome
drug
resistance
Development of drugs for the treatment of TB and other epidemicdiseaseshasbeenlackingfordecades.The conven-tionalapproachtoTBdrugdevelopmentrequiressubstituting each approveddrug inthe currentmulti-drugregimen,but onlyafterthenewdrughasbeenapprovedfortreatmentas asingleagent.TBcontrolinitiativeprogramforthetestingof newcompoundssimultaneouslycoulddrasticallyshortenthe developmenttimeline,butethicalimplicationsshouldbe con-sideredintheidentificationofapracticalwaytoimplement suchclinicaltrialdesigns.5,6
Adenosinetriphosphate(ATP)synthaseinhibitors
TheselectionofnewTBdrugtargets hasbeendetermined mainly by accessibility to the genome sequence ofthe TB pathogen. But studies dealing with the invention of new drugs have shown that genome-derived and target-based approachesgenerallyhavemodesttherapeuticeffectsinthe areaofantibacterials.42Studieshavealsoshownthat
chang-ingtheselectionapproachfromsingle-enzymetargetstothe wholebacterialcelllevelgreatly improvestheeffectsofTB drugs. However,the whole-cell screening approach is hin-deredbyalackofunderstandingofthemechanismofaction aswell asidentificationoftherelevantligandsforinternal infections.43 Current achievements utilizing the whole-cell
approacharehighlightedbytheidentificationofnewTBdrug agentssuchasTMC207,adiarylquinolinethatinhibitsATP synthesis, and BTZ043, a benzothiazine that blocks arabi-nansynthesis.44,45 TMC207(R207910orthe‘J’compound)is
activeagainstdrug-sensitiveanddrug-resistantM. tuberculo-sisisolates.46,47Alongwithbactericidalcharacteristicagainst
dormant (non-replicating) tubercle bacilli, it also has the potentialtominimizethetreatmentduration.48TMC207has
shownsameinhibitoryeffectsinmurinemodelof tubercu-losislikelyshownbyisoniazid, rifampin,andpyrazinamide inacombination.However,asignificantincreaseintherate of bacilli clearance was reported by using TMC207 along withtriple-drugregimenandthesynergisticinteractionwith pyrazinamide.49Moreover,ithasbeenreportedthatTMC207
incaseofmurinemodelofdrug-sensitiveenhancesthe effec-tivenessofsecond-linedrugcombination.50TMC207hasalso
onsetofresponse.Thistreatmentwasassociatedwith accept-able adverse-events.51 Additionally, controlled trial having
consistingofan(8 weeks) followed byaseparate proof-of-efficacystage(24weeks),wasconductedtoevaluateTMC207 efficacy in patients with newly diagnosed, smear-positive pulmonaryinfectioncausedbymultidrug-resistantM.
tuber-culosis.TMC207reducedthetimeintervaltoconversiontoa
negativesputumculture.51Theseexamplesdemonstratethat
multi-dimensionalsignalingpathwayinhibitioncanbeused asthebasisfortheidentificationofnewTBtreatment scaf-folds.
Proteinsynthesisinhibitors
Remodelingofpresentregimensisalsoaviablestrategyfor fuelingmomentumintheantibioticdevelopmentpipelineand discoveringnewtreatmentscaffoldsforuseagainstresistant bacterialstrains. Differentmembersofeachantibioticclass shareacommoncorestructure,anduponsynthetic modifica-tion,thecoreoftheantibioticisleftintactbutitsfunctional groupsarerearrangedtopotentiatethedrug’sactivity.
The tailored forms of oxazolidinones (linezolid against Gram-positiveinfections)haveprovidedaplatformfornew structuressuch as PNU-100480 and AZD-5847, which have been found to enhance antibiotic activity.44 For the very
firsttime,4-amino-1,2-oxazolidin-3-one(cycloserine),anew classofantibacterialagent,wasused.Cycloserineisa broad-spectrum antibiotic that has been used as a second-line antituberculosisdrugsince1955.52,53Inthemodernerawhen
severalbacterialstrainsarebecomingresistantagainst antibi-otics,linezolid (Zyvox)is theonly agentapprovedbyFood andDrugAdministration(FDA)intheclassandreleasedin 2000byUpjohnforthetreatmentofnosocomialpneumonia andskinandsofttissueinfectionscausedbyGram-positive bacteria.52AgainstMDR-TB,linezolidwasthefirst
oxazolidi-nonethathasbeenusedoff-label,However,itslong-termuse hasnotbeenprescribedbecauseofcertainsideeffects asso-ciatedwith its continuous use, suchas thrombocytopenia, anemia,andperipheralandopticneuropathy.54ForM.
tubercu-losis,minimuminhibitoryconcentration(MIC)isintherange
of0,125–1,0g/mL;because ofits severeside effectsitwas replacedbyPNU-100480(Sutezolid),ananalogueoflinezolid developedbyPizerwithsimilarMICsforM. tuberculosis,for betterinvivo activityand lesstoxicity.52,55,56 Ina
compari-sonstudyofthemurinemodelofTB,PNU-100480hasshown more potent bactericidal activity compared with linezolid, evenatlowerdrugexposures.44Additionally,theintroduction
ofPNU-100480 enhanced the bactericidal activitiesof regi-menscontainingsomefirst-linedrugstocertainfolds,giving aclue thatitmaybeeffectiveatminimizingthetreatment durationfordrug-susceptibleTBby1–2months.57Anearlier
study investigatedits safety,tolerability,pharmacokinetics, andpharmacodynamicsinhumansbygivingdifferentdoses, i.e.,100,300,or600mgtwicedailyor1200mgoncedailyfor 14days,or600mgtwicedailyfor28days,towhichPZAwas addedondays27and28.Asixthcohortwasgivenlinezolid at300mgdailyfor4days.Noneofthedosesgivenshowed anylethaleffectandwerewelltolerated.58C
maxlevelwas0,94
or2,01g/mL,andhalf-lifewas2,92or3,38h,respectively,in healthyvolunteersreceivingtwice-daily600mgoronce-daily
1200mgofPNU.Troughconcentrationswere maintainedat orabovetheMIC.AnEBAanalysissuggestsdailytestingof 600mgand1200mgfor14days.AZD-5847(posizolid)isa lead-containingcompoundthatisusedtocureTBbyAstraZeneca. ItpossessesanMICanalogoustothatoflinezolidand PNU-100480 for curing M. tuberculosis, and has shown profound effectinmousemodelexperimentofTB.53At600mgoral
dos-ing,Cmax is2,60g/mLinfastingsubjectsand5,66g/mLin fedsubjects, with ahalf-life closeto 8h but it hascaused nauseainthetestorganism.53Adailyoraldoseof800,1600
and2400mgfor14daysinhealthyvolunteerswaswell tol-erated, and hascaused anincreased Cmax up to 10g/mL; however, the increasewas notinproportiontothe dose.53
Similarly,cephalosporinssuchascefaclorandceftazidimeare moreimpervioustodestructionbytheresistanceenzyme -lactamase,andenterthebacterialmembranemoreefficiently. Furthermore,new-lactamasescancleavethird-generation cephalosporins. Thus,fourth-generation moleculessuchas cefepimeweredevelopedthatarelesssusceptibletocleavage by-lactamase.45 Cephalosporins andother semi-synthetic
antibioticsharbor64%ofthenewchemicalscaffoldsthatwere filedbetween1981and2005.59
Nitromidazole(PA-824),Delaminid(OPC67683)
PA-824 is a prodrug derived from metronidazole that has shownstrongactivityagainstanaerobicbacteriaand proto-zoa (trichomoniasis, amoebiasis).PA-824has demonstrated significant sterilizingandanti-tuberculosisactivity,withan MIC90of0,125g/mLagainstboth drug-resistantand non-drug-resistant strains ofM. tuberculosis.60 It is bactericidal
against actively replicating and latent (non-replicating or slowly replicating) TB bacteria. Itsbactericidal mechanism againstreplicatingaerobicspeciesinvolvestheinhibitionof synthesisofcertainproteinsandcellwalllipidcomponents requiredforthesurvivalandreplicationofbacterialspecies. Thelatentbacteriaarekilledbyreleasingnitricoxidegas dur-ing enzymatic nitro-reduction that poisons the respiratory machinery.61InmurinemodelsofTB,PA-824demonstrated
bactericidalactivitynotonlyduringinitialphasebutalsoin thecontinuationphasewhereitkilledthebacteriathathad survivedforinitialtwomonths.62Theindividual
administra-tion ofPA-824hasshownbactericidal activitiescomparable tothatofmoxifloxacinandisoniazid.63Theevaluationof
PA-824incombinedtherapieswithfirst-linedrugstoshortenthe treatmenttimeproducedencouragingresults.Itsdailyintake incombinationwithmoxifloxacinand PZAhascontributed toanimpressivesterilizingregimen,signifyingthepotential toshorten the treatmenttime.64 Astudy compiledby
sub-stitutingisoniazidwithPA-824remarkablylowerlungsCFU after2monthstreatmentaswellasledtoarapid culture-negativeconversionwhencomparedfirst-linecombinationof rifampin, INHand PZA.65 Itssubstitution withrifampin in
first-line drug during intensivephase therapy issuggested to drastically reduce the treatment of multidrug-resistant tuberculosis.66DifferentamountsoforaldosesofPA-824were
beincludedinregimenforquickandeffectivetreatmentof drug-susceptibleanddrug-resistantTB.67
DNAgyraseinhibitors
Fluoroquinolonesare agroup ofantimicrobialsthatkill M.
tuberculosisviadouble-strandedbreaksinDNA,bybindingto
DNAgyrase.DNAgyraseconsistsoftwoAandtwoBsubunits encodedbythe gyraseA (gyrA)and gyrase B(gyrB)genes, respectively.68 Previouslydrugs suchasofloxacinand
levo-floxacinwereinuseratherthanthenalidixicacidderivatives moxifloxacinandgatifloxacin,whicharebeingdeveloped cur-rentlyforDS-TB.ThegatifloxacinbytheOflotubConsortium (NCT00216385)andmoxifloxacinbyBayerandtheTBAlliance (NCT00864383)havebeenevaluatedinPhaseIIItrial, substi-tutedwiththefirst-lineregimenforethambutol(gatifloxacin, moxifloxacin)andisoniazid(moxifloxacin)toshorten treat-mentofDS-TB.69Severalstudieshaveshownthepotentialof
moxifloxacinandgatifloxacininvitroandinmousemodels
ofTB70,71 aswellasinhumans.72,69Additionally,the
poten-tial toxicitiesof thesedrugs are mostly group related, but amongthem,gatifloxacinhasbeenreportedtodisturbglucose metabolicstateindiabeticandagedindividuals.73Incontrast,
thistypeofdrawbackwasnotassociatedwithmoxifloxacin treatment.74Studieshavealsoshownthereduceddrug–drug
interaction of gatifloxacinand moxifloxacin because these drugsarenotstronginhibitorsorinducersoftheCYPenzyme systemandalsonotmetabolizedtoagreatextent.75 RIF is
firstlineTBdrug,whichreducedapproximately30%plasma concentrations of moxifloxacin but according to the phar-macokinetic and dynamic condition of moxifloxacin, RIF induceglucuronidationorsulphation whichreduced moxi-floxacinplasmalevel in18 outof19patients.76,77 Wehope
that the Phase III trials will elaborate the safety and effi-cacyofa4-month,fluoroquinolone-basedtreatmentfordrug susceptible-TB,whichcouldbetranslatedtotheclinicforthe treatmentofTBby2015.
Conclusion
InthisreportweprovidedexamplesofpersistentTB trans-missionandofenhancedsusceptibilityoflargepopulations toinfectionanddisease.Itisclearthatallcurrentlyapproved TBdrugsarefacingacertainlevelofresistancegovernedby specific geneticmutations in the disease-causing
Mycobac-terium. Long-termuse ofaspecific drugresults inthe loss
ofitsefficiencywithincreasinglevelsofbacterialresistance. Therefore,the rightdrugshouldbetakenattherighttime inordertoavoiddevelopmentofseverebacterialresistance problems.Thisstudyalsohighlightedsomepromising bacte-ricidalagentsforquickpreventionandeffectivetreatmentof drug-susceptibleanddrug-resistantTB.
Anothersuggestion byWHO istochange thedrug dos-ingatregularintervalswithinaprescribedformat.78Besides
theseapproaches,itisimportanttodevelopnoveldrugsthat avoidthemanifestationofdrugresistanceinbacterialcells. Weconcludethatcontrolprogramshavebeenlesseffective thanexpectedinreducingtheoccurrenceofTBtransmission, mainlybecausepatientsarenotdiagnosedandcuredquickly
enough.Wedohopethatenhancingfeaturesofantibioticswill helpindevelopingimprovedtoolsforTBcontrol.TheseTB controleffortswillneedtobereinforcedandredoubledifTB istobeeliminatedasaglobalpublichealthcrisis.Thepriority nowshouldbetomaintainthebasicprinciplesoftreatment usingchemotherapysuchasgatifloxacinandmoxifloxacinbut implementtheseeffortswithgreatervigor.
Conflict
of
interest
Allauthorsdeclaretohavenoconflictofinterest.
r
e
f
e
r
e
n
c
e
s
1. LönnrothK,JaramilloE,WilliamsBG,etal.Driversof tuberculosisepidemics:theroleofriskfactorsandsocial determinants.SocSciMed.2009;68:2240–6.
2.Globaltuberculosiscontrol:ashortupdatetothe2009report. Geneva,WorldHealthOrganization2009,
WHO/HTM/TB/2009.426.
3.ZhangY,YewWW.Mechanismsofdrugresistancein
Mycobacteriumtuberculosis.IntJTubercLungDis.
2009;13:1320–30.
4.WrightA,ZignolM,VanDeunA,etal.Epidemiologyof antituberculosisdrugresistance2002–07:anupdatedanalysis oftheGlobalProjectonAnti-TuberculosisDrugResistance Surveillance.Lancet.2009;373:1861–73.
5.DyeC,WilliamsBG.Thepopulationdynamicsandcontrolof tuberculosis.Science.2010;328:856–61.
6.SharmaSK,MohanA.Multidrug-resistanttuberculosis. IndianJMedRes.2004;120:354–76.
7.JohnsonR,StreicherEM,LouwGE,etal.Drugresistancein
Mycobacteriumtuberculosis.CurrIssuesMolBiol.2006;8:97–111.
8.SlaydenRA,BarryCE.Thegeneticsandbiochemistryof isoniazidresistanceinMycobacteriumtuberculosis.Microbes Infect.2000;2:659–69.
9.HeymB,Saint-JoanisB,ColeST.Themolecularbasisof isoniazidresistanceinMycobacteriumtuberculosis.TuberLung Dis.1999;79:267–71.
10.ZhangY,HeymB,AllenB,etal.Thecatalase-peroxidasegene andisoniazidresistanceofMycobacteriumtuberculosis.Nature. 1992;358:591–3.
11.BarryCE,LeeRE,MdluliK,etal.Mycolicacids:structure, biosynthesisandphysiologicalfunctions.ProgLipidRes. 1998;37:143–79.
12.WinderF.Modeofactionoftheantimycobacterialagentsand associatedaspectsofthemolecularbiologyofmycobacteria. In:RatledgeC,StanfordJ,editors.Thebiologyof
mycobacteria,vol.1.NewYork:AcademicPress;1982.p. 354–438.
13.BanerjeeA,DubnauE,QuemardA,etal.inhA,agene encodingatargetforisoniazidandethionamidein
Mycobacteriumtuberculosis.Science.1994;263:227–30.
14.ZhangY,TelentiA.Geneticsofdrugresistancein
Mycobacteriumtuberculosis.In:HatfulGF,JacobsJrWR,editors.
Moleculargeneticsofmycobacteria.WashingtonDC:ASM Press;2000.p.235–54.
15.RamaswamyS,MusserJM.Moleculargeneticbasisof antimicrobialagentresistanceinMycobacteriumtuberculosis: 1998update.TuberLungDis.1998;79:3–29.
originatingfromtheSt.PetersburgareainRussia.Antimicrob AgentsChemother.1998;42:2443–5.
17.RouseDA,DeVitoJA,LiZ,etal.Site-directedmutagenesisof
thekatGgeneofMycobacteriumtuberculosis:effectson
catalase-peroxidaseactivitiesandisoniazidresistance.Mol Microbiol.1996;22:583–92.
18.TelentiA,ImbodenP,MarchesiF,etal.Detectionof
rifampicin-resistancemutationsinMycobacteriumtuberculosis. Lancet.1993;341:647–50.
19. HerreraL,JiménezS,ValverdeA,etal.Molecularanalysisof rifampicin-resistantMycobacteriumtuberculosisisolatedin Spain(1996–2001).DescriptionofnewmutationsintherpoB geneandreviewoftheliterature.IntJAntimicrobAgents. 2003;21:403–8.
20.ZhangY,WadeMM,ScorpioA,etal.Modeofactionof pyrazinamide:disruptionofMycobacteriumtuberculosis
membranetransportandenergeticsbypyrazinoicacid.J AntimicrobChemother.2003;52:790–5.
21.SalfingerM,CrowleAJ,RellerLB.Pyrazinamideand pyrazinoicacidactivityagainsttuberclebacilliincultured humanmacrophagesandintheBACTECsystem.JInfectDis. 1990;162:201–7.
22.ZhangY,ScorpioA,NikaidoH,etal.RoleofacidpHand deficienteffluxofpyrazinoicacidinuniquesusceptibilityof
Mycobacteriumtuberculosistopyrazinamide.JBacteriol.
1999;181:2044–9.
23. SheenP,FerrerP,GilmanRH,etal.Effectofpyrazinamidase activityonpyrazinamideresistanceinMycobacterium
tuberculosis.Tuberculosis(Edinb).2009;89:109–13.
24.ScorpioA,ZhangY.MutationsinpncA,ageneencoding pyrazinamidase/nicotinamidase,causeresistancetothe antituberculousdrugpyrazinamideintuberclebacillus.Nat Med.1996;2:662–7.
25.TakayamaK,KilburnJO.Inhibitionofsynthesisof
arabinogalactanbyethambutolinMycobacteriumsmegmatis. AntimicrobAgentsChemother.1989;33:1493–9.
26.TelentiA,PhilippWJ,SreevatsanS,etal.Theemboperon,a geneclusterofMycobacteriumtuberculosisinvolvedin resistancetoethambutol.NatMed.1997;3:567–70. 27.LeeHY,MyoungHJ,BangHE,etal.MutationsintheembB
locusamongKoreanclinicalisolatesofMycobacterium
tuberculosisresistanttoethambutol.YonseiMedJ.
2002;43:59–64.
28.RamaswamySV,AminAG,GökselS,etal.Moleculargenetic analysisofnucleotidepolymorphismsassociatedwith ethambutolresistanceinhumanisolatesofMycobacterium
tuberculosis.AntimicrobAgentsChemother.2000;44:326–36.
29.VanNiekerkC,GinsbergA.Assessmentofglobalcapacityto conducttuberculosisdrugdevelopmenttrials:dowehave whatittakes?IntJTubercLungDis.2009;13:1367–72. 30.MitnickC,BayonaJ,PalaciosE,etal.Community-based
therapyformultidrug-resistanttuberculosisinLima,Peru.N EnglJMed.2003;348:119–28.
31.MaZ,LienhardtC,McIlleronH,etal.Globaltuberculosisdrug developmentpipeline:theneedandthereality.Lancet. 2010;375:2100–9.
32.WorldHealthOrganization.TheglobalplantostopTB 2011–2015:transformingthefighttowardseliminationof tuberculosis.WorldHealthOrganization;2010.
33.NiemiM,BackmanJT,FrommMF,etal.Pharmacokinetic interactionswithrifampicin:clinicalrelevance.Clin Pharmacokinet.2003;42:819–50.
34.L’hommeRF,NijlandHM,GrasL,etal.Clinicalexperience withthecombineduseoflopinavir/ritonavirandrifampicin. AIDS.2009;23:863–5.
35. KhachiH,O’ConnellR,LadenheimD,etal.Pharmacokinetic interactionsbetweenrifabutinandlopinavir/ritonavirin
HIV-infectedpatientswithmycobacterialco-infection.J AntimicrobChemother.2009;64:871–3.
36.BurmanWJ,GallicanoK,PeloquinC.Therapeutic
implicationsofdruginteractionsinthetreatmentofhuman immunodeficiencyvirus-relatedtuberculosis.ClinInfectDis. 1999;28:419–29.
37.DooleyKE,ChaissonRE.Tuberculosisanddiabetesmellitus: convergenceoftwoepidemics.LancetInfectDis.
2009;9:737–46.
38. StevensonCR,CritchleyJA,ForouhiNG,etal.Diabetesand theriskoftuberculosis:aneglectedthreattopublichealth? ChronicIlln.2007;3:228–45.
39.LönnrothK,WilliamsBG,CegielskiP,etal.Aconsistent log-linearrelationshipbetweentuberculosisincidenceand bodymassindex.IntJEpidemiol.2010;39:149–55.
40.PayneDJ,GwynnMN,HolmesDJ,etal.Drugsforbadbugs: confrontingthechallengesofantibacterialdiscovery.NatRev DrugDiscov.2007;6:29–40.
41.PetheK,SequeiraPC,AgarwallaS,etal.Achemicalgenetic screeninMycobacteriumtuberculosisidentifies
carbon-source-dependentgrowthinhibitorsdevoidofinvivo efficacy.NatCommun.2010;1:57.
42.KoulA,DendougaN,VergauwenK,etal.Diarylquinolines targetsubunitcofmycobacterialATPsynthase.NatChem Biol.2007;3:323–4.
43. MakarovV,ManinaG,MikusovaK,etal.Benzothiazinoneskill
Mycobacteriumtuberculosisbyblockingarabinansynthesis.
Science.2009;324:801–4.
44.WilliamsKN,StoverCK,ZhuT,etal.Promising
antituberculosisactivityoftheoxazolidinonePNU-100480 relativetothatoflinezolidinamurinemodel.Antimicrob AgentsChemother.2009;53:1314–9.
45.CharestMG,LernerCD,BrubakerJD,etal.Aconvergent enantioselectiveroutetostructurallydiverse
6-deoxytetracyclineantibiotics.Science.2005;308:395–8. 46.AndriesK,VerhasseltP,GuillemontJ,etal.Adiarylquinoline
drugactiveontheATPsynthaseofMycobacteriumtuberculosis. Science.2005;307:223–7.
47.HuitricE,VerhasseltP,AndriesK,HoffnerSE.Invitro antimycobacterialspectrumofadiarylquinolineATP synthaseinhibitor.AntimicrobAgentsChemother. 2007;51:4202–4.
48.HaagsmaAC,Abdillahi-IbrahimR,WagnerMJ,etal. SelectivityofTMC207towardsmycobacterialATPsynthase comparedwiththattowardstheeukaryotichomologue. AntimicrobAgentsChemother.2009;53:1290–2.
49.KoulA,VranckxL,DendougaN,etal.Diarylquinolinesare bactericidalfordormantmycobacteriaasaresultofdisturbed ATPhomeostasis.JBiolChem.2008;283:25273–80.
50.IbrahimM,AndriesK,LounisN,etal.Synergisticactivityof R207910combinedwithpyrazinamideagainstmurine tuberculosis.AntimicrobAgentsChemother.2007;51:1011–5. 51.LounisN,VezirisN,ChauffourA,Truffot-PernotC,AndriesK,
JarlierV.CombinationsofR207910withdrugsusedtotreat multidrug-resistanttuberculosishavethepotentialto shortentreatmentduration.AntimicrobAgentsChemother. 2006;50:3543–7.
52.KeshavjeeS,FarmerPE.Tuberculosis,drugresistance,andthe historyofmodernmedicine.NEnglJMed.2012;367:931–6. 53.GlobalAllianceforTBDrugDevelopment.Evaluationofearly
bactericidalactivityinpulmonarytuberculosis.USNational InstitutesofHealth,ClinicalTrials.gov.
http://www.clinicaltrials.gov[ClinicalTrials.govidentifier NCT00944021].
55.MiglioriGB,EkerB,RichardsonMD,etal.Aretrospective TBNETassessmentoflinezolidsafety,tolerabilityandefficacy inmultidrug-resistanttuberculosis.EurRespirJ.
2009;34:387–93.
56.DiaconAH,DawsonR,HanekomM,etal.Thesafety, tolerabilityandearlybactericidalactivityand pharmacokineticsofPA824inpreviouslyuntreated, uncomplicated,sputumsmear-positivepulmonary tuberculosispatients.AntimicrobAgentsChemother. 2010;54:3402–7.
57.WilliamsKN,BricknerSJ,StoverCK,etal.Additionof PNU-100480tofirst-linedrugsshortensthetimeneededto curemurinetuberculosis.AmJRespirCritCareMed. 2009;180:371–6.
58.WallisRS,JakubiecWM,KumarV,etal.Biomarker-assisted doseselectionforsafetyandefficacyinearlydevelopmentof PNU-100480fortuberculosis.AntimicrobAgentsChemother. 2011;55:567–74.
59.FischbachMA,WalshCT.Antibioticsforemergingpathogens. Science.2009;325:1089–93.
60.VandenBoogaardJ,KibikiGS,KisangaER,BoereeMJ, AarnoutseRE.Newdrugsagainsttuberculosis:problems, progress,andevaluationofagentsinclinicaldevelopment. AntimicrobAgentsChemother.2009;53:849–62.
61. LienhardtC,RaviglioneM,SpigelmanM,etal.Newdrugsfor thetreatmentoftuberculosis:needs,challenges,promise, andprospectsforthefuture.JInfectDis.2012;205: S241–9.
62.TyagiS,NuermbergerE,YoshimatsuT,etal.Bactericidal activityofthenitroimidazopyranPA-824inamurinemodel oftuberculosis.AntimicrobAgentsChemother.2005;49: 2289–93.
63.LenaertsAJ,GruppoV,MariettaKS,etal.Preclinicaltestingof thenitroimidazopyranPA-824foractivityagainst
Mycobacteriumtuberculosisinaseriesofinvitroandinvivo
models.AntimicrobAgentsChemother.2005;49:2294–301. 64.GinsbergAM.Drugsindevelopmentfortuberculosis.Drugs.
2010;70:2201–14.
65.NuermbergerE,RosenthalI,TyagiS,etal.Combination chemotherapywiththenitroimidazopyranPA-824and first-linedrugsinamurinemodeloftuberculosis.Antimicrob AgentsChemother.2006;50:2621–5.
66.NuermbergerE,TyagiS,TasneenR,etal.Powerfulbactericidal andsterilizingactivityofaregimencontainingPA-824, moxifloxacin,andpyrazinamideinamurinemodelof tuberculosis.AntimicrobAgentsChemother.2008;52:1522–4. 67.DiaconAH,DawsonR,HanekomM,etal.Earlybactericidal
activityandpharmacokineticsofPA-824insmear-positive tuberculosispatients.AntimicrobAgentsChemother. 2010;54:3402–7.
68.CollinF,KarkareS,MaxwellA.ExploitingbacterialDNA gyraseasadrugtarget:currentstateandperspectives.Appl MicrobiolBiotechnol.2011;92:479–97.
69. RustomjeeR,LienhardtC,KanyokT,etal.AphaseIIstudyof thesterilisingactivitiesofofloxacin,gatifloxacinand moxifloxacininpulmonarytuberculosis.IntJTubercLungDis. 2008;12:128–38.
70.RodríguezJC,RuizM,LópezM,etal.Invitroactivityof moxifloxacin,levofloxacin,gatifloxacinandlinezolidagainst
Mycobacteriumtuberculosis.IntJAntimicrobAgents.
2002;20:464–7.
71.CynamonM,SklaneyMR.Gatifloxacinandethionamideas thefoundationfortherapyoftuberculosis.AntimicrobAgents Chemother.2003;47:2442–4.
72.JohnsonJL,HadadDJ,BoomWH,etal.Earlyandextended earlybactericidalactivityoflevofloxacin,gatifloxacinand moxifloxacininpulmonarytuberculosis.IntJTubercLung Dis.2006;10:605–12.
73. Park-WyllieLY,JuurlinkDN,KoppA,etal.Outpatient gatifloxacintherapyanddysglycemiainolderadults.NEnglJ Med.2006;354:1352–61.
74.GavinIIIJR,KubinR,ChoudhriS,etal.Moxifloxacinand glucosehomeostasis:apooled-analysisoftheevidencefrom clinicalandpostmarketingstudies.DrugSaf.2004;27:671–86. 75.HooperDC,Rubinstein3rdE,editors.Quinoloneantimicrobial
agents.3rded.Washington,DC:ASMPress;2003.
76.WeinerM,BurmanW,LuoCC,etal.Effectsofrifampinand multidrugresistancegenepolymorphismonconcentrations ofmoxifloxacin.AntimicrobAgentsChemother.
2007;51:2861–71.
77.NijlandHM,RuslamiR,SurotoAJ,etal.Rifampicinreduces plasmaconcentrationsofmoxifloxacininpatientswith tuberculosis.ClinInfectDis.2007;45:1001–7.