J. Braz. Chem. Soc. vol.22 número9
Texto
Imagem
![Figure 1. Chemical structure of efavirenz, [(4S)-6-chloro-4-(2- [(4S)-6-chloro-4-(2- cyclopropylethynyl)-4-(triluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one].](https://thumb-eu.123doks.com/thumbv2/123dok_br/18995030.461927/1.892.563.688.927.1055/figure-chemical-structure-efavirenz-cyclopropylethynyl-triluoromethyl-dihydro-benzoxazin.webp)


Documentos relacionados
A simple conirmatory method for HPLC-UV determination of ketoconazole and clotrimazole residues in cow’s milk after solid phase extraction (SPE) is reported in this article..
Using the same electrochemical low-through cell under galvanostatic conditions, the response surface methodology (RSM) was employed to ind the optimum conditions for Pb 2+
The results showed that after 7 h of solar irradiation, dissolved organic matter (DOM) was degraded promoting the increase of labile, free Cu 2+ ions and dissolved inorganic
The QuEChERS method of sample preparation was used for the determination of six pesticides (buprofezin, carbofuran, endosulfan-α, endosulfan-β, endosulfan sulfate and
Although Ti/Pt electrodes exhibit higher peak current density related to ethanol electrooxidation compared to bimetallic electrodes, in Figure 3b, it is possible to observe
The predictive power of the best HQSAR model derived using the training set molecules (model 8; fragment distinction A/B/H/Ch, and fragment size 4-7) was assessed by predicting
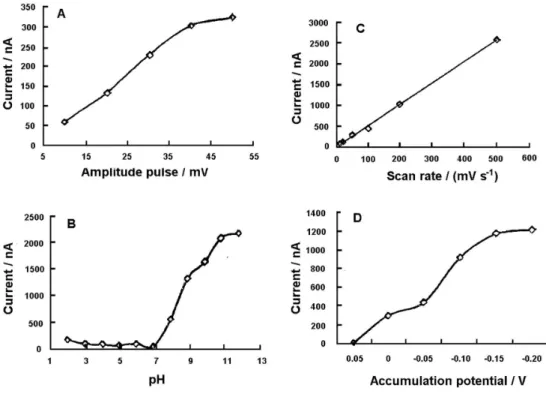
The use of an accumulation potential directly in the analyzed sample enables using very short preconcentration time and allowing the development of a very fast and
A signiicant improvement of the chemical yields (up to 88%), stereoselectivity (> 99:1) and enantiomeric excesses (up to 98%) of ( L )-proline catalyzed direct asymmetric
