Influence of kaolin particle film in antioxidant activities of leaf
and berry extracts from Vitis vinifera L. (cv. Touriga Nacional)
– Versão Definitiva –
Dissertação de Mestrado em Engenharia Agronómica
Sara Silva Bernardo
Orientadores: Doutora Lia-Tânia Rosa Dinis
Professor Doutor José Manuel Moutinho Pereira
“Most people say that is the intellect which makes a great scientist. They are wrong: it is character.”
v First of all, I would like to thank my parents, Celeste and Pedro Bernardo, and my grandmother Elísia for being always there for me, for supporting my decisions and to make this step of my life possible with all that I needed. I’m proud to say that I am the reflection of the values, love and guidance that you generously gave me.
I would like to express my deepest gratitude to my coordinators, Doctor Lia-Tânia Dinis and Professor José Manuel Moutinho Pereira, for their excellent guidance, caring, patience, friendship and for providing me with an excellent atmosphere for doing research. Thanks so much for the knowledge transmitted along this journey, it wouldn’t be possible without the trust that you have placed in me.
I would like to thank all the Professors and technicians who patiently accompanied all the work done throughout the year, especially to Doctor Helena Ferreira who provided me self-confidence with lab work and for all the helpful advices and precious words.
I thank my fellow labmates in for the stimulating discussions and the good moments and laughs, always willing to help and give their best suggestions.
My sincere thanks to my friends and family, particularly to my cousin Camila and to Daniel for the patience, affection and friendship, and for never stop trying to cheer me up with a good advice and for believing in me, most of the times more than myself.
Last but not the least I would like to thanks my lovely Rita for being the sister, friend and person that anyone would like to have constantly in their lives. Lucky me, you are just mine.
vii O aumento do stresse estival associado às alterações climáticas tem vindo a ter consequências negativas para o património vitícola duriense, sendo necessário adotar medidas que preservem a produtividade e a qualidade das uvas. A aplicação de caulino constitui uma medida de mitigação a curto prazo, de baixo custo, apresentando um forte poder refletor da radiação incidente e impactos na maturação do fruto. Este trabalho teve como principal objetivo estudar o efeito deste mineral no sistema antioxidante de extratos de folhas e bagos de videiras (cv. Touriga Nacional) submetidas ao stresse estival através de métodos químicos (ABTS+,
FRAP e DPPH) e bioquímicos. Os resultados obtidos revelam que a aplicação exógena de caulino apresenta um efeito estimulante da atividade dos sistemas antioxidantes, enzimáticos e não enzimáticos, diminuindo os níveis de espécies reativas de oxigénio (ROS) e de peroxidação lipídica (TBARS) e potenciando a produção de compostos antioxidantes, tais como fenóis, flavonoides, antocianinas e vitamina C, que influenciam a qualidade das uvas. As atividades das enzimas SOD, CAT e APx indicam uma maior eficácia de resposta a condições de stresse nas plantas tratadas com caulino. Uma vez que as plantas tratadas com caulino estão sob menores condições de stresse sugere-se que existam mecanismos regulatórios complexos ao nível da síntese de fenóis, flavonoides, carotenoides, vitamina C e antocianinas que possam ser desencadeados ao longo do processo de aclimatação. No fruto, a aplicação foliar de caulino promoveu a homeostase redox dos sistemas antioxidantes através da atividade de enzimas que participam na regulação da produção e eliminação de ROS, diminuindo a produção de peróxido de hidrogénio (H2O2) e consequentemente, o dano oxidativo em vinhas sujeitas ao stresse
estival. Apesar dos dados obtidos, este estudo poderá ainda representar um ponto de partida para outros trabalhos na área da enologia de forma a compreender se o tratamento de videiras com caulino poderá ter impactos benéficos na qualidade do vinho. O efeito do caulino como medida de mitigação poderá ainda ser explorado em regiões vitícolas com condições climáticas mais extremas, como o Douro Superior, de forma a evidenciar as propriedades refletoras deste mineral na proteção foliar, designadamente na eficiência fotossintética e na capacidade antioxidante da planta, assim como na consequente qualidade dos frutos.
ix
A
BSTRACTIncreased summer stress related to climate change has negative consequences for the Douro wine-growing heritage, being necessary to adopt measurements that preserve grapes productivity and quality. The application of kaolin is a short-term mitigation measure, inexpensive, with a strong incident radiation reflective effectiveness and impacts on fruit ripening. This work aimed to study the effect of this mineral on the antioxidant system of grapevine (cv. Touriga Nacional) leaves and fruits extracts to summer stress, using chemical (ABTS+, FRAPe DPPH) and biochemical methods. The results show that kaolin exogenous
application enhanced the activity of enzymatic and non enzymatic antioxidants systems, reducing reactive oxygen species (ROS) levels and lipid peroxidation levels (TBARS) and promoting the production of antioxidants such as phenols, flavonoids, anthocyanins and vitamin C, which influence grapes quality. The enzymatic activity of SOD, CAT and APx indicates a better response efficiency to stress conditions of kaolin treated plants than control ones. Since kaolin treated plants are under lower stress conditions, there might be complex regulatory mechanisms beneath the synthesis of phenols, flavonoids, carotenoids, vitamin C and anthocyanins which can be triggered during the acclimation process. In fruit, kaolin exogenous foliar application improved redox homeostasis of the antioxidant systems through the activity of enzymes that participate in the regulation of production and elimination of ROS, decreasing hydrogen peroxide (H2O2) levels and consequently, oxidative damage in vineyards subjected to
summer stress. Besides all the collected data, this study may still represent a starting point for other studies in oenology area in order to understand if kaolin treated grapevines may have benefits in wine quality. The effect of kaolin as a mitigation measure can still be explored in wine-growing regions with more extreme environmental conditions such as “Douro Superior” sub-region, in order to highlight the properties of this mineral in foliar protection, namely in plant photosynthetic efficiency and antioxidant capacity, as well as in the consequent fruit quality.
xi
T
ABLE OF CONTENTS ACKNOWLEDGMENTS ... V RESUMO ... VII ABSTRACT ... IX TABLE OF CONTENTS ... XILIST OF FIGURES ... XIII
LIST OF TABLES ... XV
ABBREVIATIONS ... XVII
CHAPTER 1–INTRODUCTION ... 1
1.1 Douro wine region and an impending challenge: climate change ...2
1.2. Impacts of summer stress for grapevine phenology, growth and yield ...3
1.3. Reactive oxygen species (ROS) and oxidative damage ...5
1.4. Antioxidative defense: Protection by scavenging systems ...7
1.5. Adaptation measures for viticulture under climate change ...10
1.6. Objectives ...12
2. References ...12
CHAPTER 2–INFLUENCE OF KAOLIN PARTICLE FILM ON NON-ENZYMATIC ANTIOXIDANT DEFENSES ... 17
KAOLIN EXOGENOUS APPLICATION BOOSTS ANTIOXIDANT CAPACITY AND PHENOLIC CONTENT IN BERRIES AND LEAVES OF GRAPEVINE UNDER SUMMER STRESS ... 18
ABSTRACT ... 18
1.INTRODUCTION ... 19
2.MATERIAL AND METHODS ... 21
2.1.Chemicals ...21
2.2. Plant material and samples preparation ...21
2.3. ROS quantification ...22
2.4. Determination of total antioxidant components ...23
2.5. Antioxidant activity by chemical and biochemical assays...24
2.5.1. ABTS+ radical-scavenging activity ... 24
xii
2.5.3. Ferric reducing activity (FRAP assay) ... 25
2.5.4. Inhibition of lipid peroxidation using the β-carotene linoleate mode system ... 25
2.5.5. Hydroxyl radical-scavenging activity ... 26
2.6. RNA extraction and gene expression analysis by Real Time qPCR ...26
2.7. Statistical analysis ...27
3.RESULTS AND DISCUSSION ... 27
3.1.Kaolin decreases ROS levels and stimulates changes in antioxidants components in berries and leaves ...27
3.2. Kaolin application boosts the antioxidant activity of berry tissues ...31
3.3. Kaolin application regulates the biosynthesis of phenolics compounds at the transcriptional level ...35
4.CONCLUSIONS ... 35
5.ACKNOWLEDGMENTS ... 37
6.REFERENCES ... 37
CHAPTER 3–INFLUENCE OF KAOLIN PARTICLE FILM ON ENZYMATIC ANTIOXIDANT DEFENSES ... 41
GRAPEVINE ANTIOXIDATIVE SYSTEMS: MODULATION OF SUMMER STRESS BY KAOLIN EXOGENOUS APPLICATION ... 42
Abstract ...42
1.INTRODUCTION ... 42
2.MATERIAL AND METHODS ... 44
2.1. Plant material and sampling ...44
2.2. Quantification of TBARS and H2O2 concentrations ...45
2.3. Proline concentration ...45
2.4. Antioxidant enzyme extraction and assays ...46
3.RESULTS AND DISCUSSION ... 47
3.1. Kaolin protects leaves and fruits against oxidative damage ...47
3.2. Kaolin protects by boosting antioxidant enzymatic defences ...49
4.ACKNOWLEDGEMENTS ... 54
5.REFERENCES ... 55
xiii
L
IST OF FIGURESCHAPTER 1
Figure 1 - Differences in the number of days with the maximum temperatures
equal or above 40 °C (2041-2070 less 1961-2000) 3
Figure 2 – Sources of production of reactive oxygen species in plants 6
Figure 3 – ROS and antioxidants defense systems 10
Figure 4 – Kaolin 5 % preparation for exogenous application 12
CHAPTER 2 Figure 1 – Monthly values of maximum temperature and precipitation at the experimental site during 2014 21
Figure 2 – ROS concentration of leaf and fruit extracts in control and Kaolin treated grapevine plants in different development stages 28
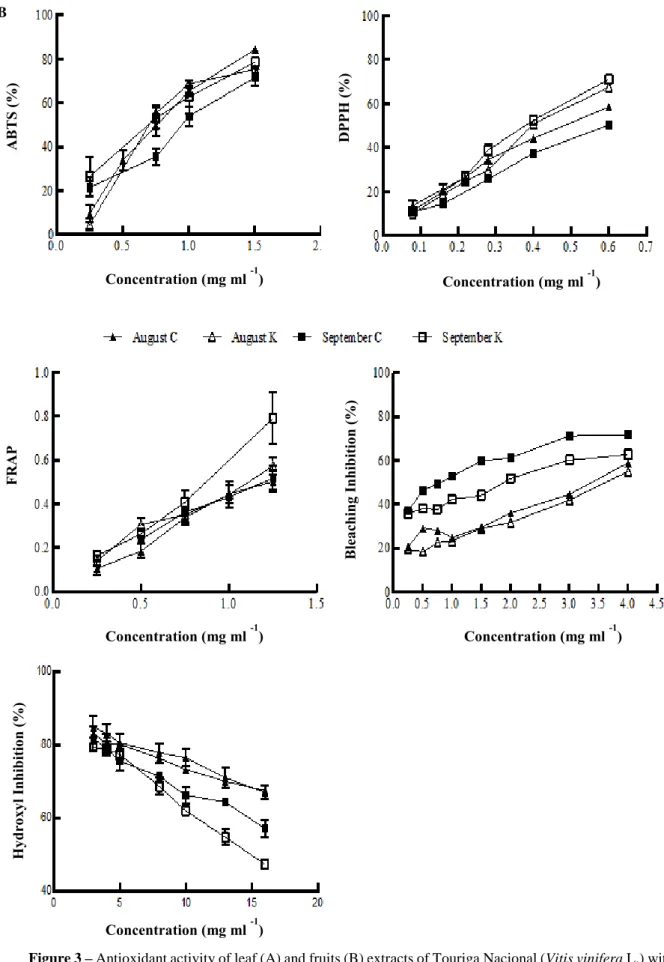
Figure 3 – Antioxidant activity of leaf and fruits extracts of Touriga Nacional (Vitis vinifera L.) with and without Kaolin in different growth stages. Scavenging activity on ABTS, DPPH and hydroxyl radicals, FRAP and bleaching inhibition 32-33 Figure 4 – The effect of Kaolin application in the transcript levels of the grapevine VvPAL1 and VvCHS1 in grape berries 35
CHAPTER 3 Figure 1 – Proline concentrations of grapevine leaves and fruits in response to kaolin exogenous application 49
Figure 2 – Superoxide dismutase activities of grapevine leaves and fruits in response to kaolin exogenous application 50
Figure 3 – Catalase activities of grapevine leaves and fruits in response to kaolin exogenous application 51
Figure 4 – Ascorbate peroxidase activities of grapevine leaves and fruits in response to kaolin exogenous application 52
Figure 5 – Glutathione reductase activities of grapevine leaves and fruits in response to kaolin exogenous application 53
Figure 6 – Glutathione peroxidase (GPx) activities of grapevine leaves and fruits in response to kaolin exogenous application 53
xv
L
IST OF TABLESCHAPTER 2
Table 1 – Seasonal variation of total phenols, flavonoids, vitamin C and anthocyanin
concentrations on leaves and fruits of grapevine with and without Kaolin 29 Table 2 - Seasonal variation of β-carotene and lycopene concentrations and total carotenoids
concentration on grapevine leaf and fruit extracts with and without Kaolin 30 Table 3 - EC50 values and p-values obtained in the antioxidant activity assays of grapevine
leaves and fruits with and without Kaolin 34
CHAPTER 3
Table 1 – Hydrogen peroxide and thiobarbituric acid reaction substances concentrations
xvii
A
BBREVIATIONS·O2, hydroxyl radical
1Chl*, chlorophyll excited state 1O2, singlet oxygen
3Chl*, chlorophyll triplet state ABA, abscisic acid
ABTS·+, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) APx, ascorbate peroxidase
Asc, ascorbate
BHT, butylated hydroxytoluene Car, carotenoids
CAT, catalase
cDNA, complementary DNA Chl, chlorophyll
CO2, carbon dioxide DCF, dichlorofluorescein
DCFH-DA, 2’,7’-dichlorofluorescein diacetate DHA, dehydroascorbate
DHAR, dehydroascorbate reductase DNA, deoxyribonucleic acid DTT, dithiothreitol
DW, dry weight
EDTA, ethylenediamine tetraacetic acid ETC, electron transport chain
ɛ, molar absorption coefficient
FRAP, ferric reducing antioxidant power GAE, gallic acid equivalents
GPx, glutathione peroxidase GR, glutathione reductase GSH, reduced glutathione GSSG, oxidized glutathione H2O2, hydrogen peroxide HCl, hydrochloric acid LPO, lipid peroxidation
xviii M, molar
MDHA, monodehydroascorbate
MDHAR, monodehydroascorbate reductase mg, microgram
MW, molecular weight
NADPH, nicotinamide adenine dinucleotide phosphate NBT, nitro blue tetrazolium chloride
nm, nanometer O2ˉ, superoxide anion O3, ozone
PAR, photosynthetically active radiation PCR, polymerase chain reaction
PMSF, phenylmethanesulfonyl fluoride PVPP, polyvinyl polypyrrolidone qPCR, quantitative real-time PCR RCOO·, peroxyl radical
RNA, ribonucleic acid RO·, alkoxyl radical
ROS, reactive oxygen species RSA, radical scavenging activity SD, standard deviation
SOD, superoxide dismutase SSA, sulfosalicylic acid
TBARS, thiobarbituric acid reactive substances TBHQ, tert-butylhydroquinone
TCA, trichloroacetic acid Tmax, maximum temperature TPTZ, 2,4,6-tripyridyl-s-triazine UV, ultra violet radiation
VvACT1, actin gene
VvCHS1, chalcone synthase gene
VvGAPDH, glyceraldehyde-3-phosphate dehydrogenase gene VvPAL1, phenylalanine ammonia lyase gene
WST, water-soluble tetrazolium rpm, rotation per minute
1
2
1.1 Douro wine region and an impending challenge: climate change
The Douro valley is outstanding for its viticultural potential which has strong socioeconomic and cultural impact on the region, considering the importance of the wine industry in the national development and economic stability (Fraga et al. 2015). Located in northeast Portugal, the Douro region comprises steep slopes areas with specific soil characteristics and climacteric gradients, along with the terroir, composed not only by the climate, soil and vineyard management practices but also by the grapevines varieties grown, making this region suitable for unique wine production (Reis et al. 2007, Magalhães 2008). The Mediterranean-like areas, such as Douro region, are characterized by hot and dry summers and long growing seasons, which exerts a deep influence on the grapevine physiology and affecting consequently vegetative growth and yield (Ferreira et al. 2012, Dinis et al. 2014). Microclimate (climate within a surrounding plant canopy) plays an essential role in the characterization of varieties suitability for high quality wines production (Fraga et al. 2012). Moreover, appropriate climate patterns are essential for a well-balanced ripening (Hannah et al. 2013). In fact, along with other elements, climate conditions variations are a key factor to understand the dynamic behind vines development. So knowing the mechanisms by which plants respond to such alterations has become imperative, since environmental changes can affect grapevines quality and consequently their economic value (Pedreira dos Santos et al. 2007, Fraga et al. 2015).
It is known that a controlled exposure of grapes to sunlight is valuable for wine quality, particularly for red wine, however it is uncertain if the advantageous effects of sun exposure emerge from higher light or from higher temperature, which are both consequences of solar radiation (Jackson and Lombard 1993, Keller 2010). The bioclimatic rates projections made for South Europe concerning climate change point to an increase in the number of days with temperatures above 40 ºC, especially in the North of Portugal (Figure 1), related with severe drought during the growing season, and thus affecting negatively the vine development and the quality of wine (Fraga et al. 2013). Temperature gradient variations are also followed by a decrease in precipitation compared to evapotranspiration rates, so that droughts will increase considerably in regions and seasons that are already relatively dry (Manabe et al. 2004, Keller 2010). Additionally, the predicted increase of temperature in spring and autumn temperatures will also attend to longer growing seasons and will increase the probability of summer heat waves, either in frequency and severity (IPCC 2007, Keller 2010). Furthermore, the combination of all this factors may arise new potential viticultural areas in North Europe and
3 in higher altitudes, since it is expected a decay in 80% of the Mediterranean area for grapevine production (Hannah et al. 2013).
1.2. Impacts of summer stress for grapevine phenology, growth and yield
Vineyards are often located in the mediterranean-like climatic regions, being regularly exposed to high UV-B radiation and temperature levels, along with strong atmospheric and soil water deficits, thus affecting grapevine productivity (Moutinho-Pereira et al. 2004, Martinez-Luscher et al. 2013). Since crop development rate is strongly temperature dependent, an increasing in this abiotic factor may earlier the occurrence of key phenological stages and influence grape physiology and fruit metabolism (Coombe 1987).
Despite of some particular cases, for instance a part of the native varieties of the Douro region which optimal photosynthetic temperature is about 30 – 35 ºC, the ideal temperature for grapevine leaf net photosynthesis and stomatal conductance is thought to be around 25 - 28 ºC
Figure 1 – Differences in the number of days with the maximum temperatures equal or above 40 °C
4 (Moutinho-Pereira et al. 2001, Keller 2010). In grapevines, heat stress during berry ripening is linked with a decline in yield due to stomatal and mesophyll limitations to photosynthesis, as well as injures in other physiological processes (Moutinho-Pereira et al. 2004). Moreover, high temperatures during grape ripening can cause poor fruit set and small berries, inhibition of color development since daytime temperature appears to be the main driver for anthocyanin accumulation, and increase volatilization of aroma compounds (Moutinho-Pereira et al. 2004, Moriondo and Bindi 2007). Although high temperatures tend to accelerate grape maturation, too much heat can inhibit or even denature berry proteins, leading to symptoms of sunburn (Keller 2010). Besides that, along with the fact that water supply is becoming shorter in many regions, it is perceptive that climate change will cause some modifications in vineyard management practices as well as in the winemaking processes and quality (Delrot et al. 2010, Teixeira et al. 2013). Ripening stage will tend to be longer although phenolic and aromatic ripeness are not always achieved as well as harvest, which becomes sooner and sooner. The predicted decrease in wine acidity will have potential effects on wine maturation stage (Teixeira et al. 2013).
Abiotic stresses induces changes in the plant body depending on the strength of the stresses effects, which can be positive, like the acclimation mechanism and negative, such as growth inhibition, plant senescence and damage to plant organs (Mittler 2006, Maksymiec 2007). One of the main consequences of abiotic stress regarding physiological injures and redox imbalances remains in the inevitable leakage of electrons to oxygen (O2 ) from the electron
transport chain activities of mitochondria, chloroplasts and plasma membranes and in the formation of byproducts of several metabolic pathways in different cellular compartments (Sharma et al. 2012).
Chloroplasts contains a well-organized thylakoid membrane system that provides all structural properties for optimal light harvesting in order to harbor photosynthesis, which embrace a series of redox reactions in which light produces NADPH, acting as the reducing molecule for carbon dioxide (CO2) fixation through Calvin cycle (Gill and Tuteja 2010,
Hajiboland 2014). However, environmental stresses, such as heat, high light, drought and salinity can directly or indirectly disrupt the activities of Calvin cycle, resulting in a decline of NADP+ regeneration and therefore in an overreduction of the electron transport chain (Hajiboland 2014). Furthermore, high temperature stress shifts membrane fluidity and permeability, culminating in impairment of membrane linked processes and can lead to full denaturation and inactivation of enzymes, resulting in an imbalance in metabolic pathways
5 (Howarth 2005, Wahid et al. 2007). The consequences of these adverse effects on membranes and proteins include reduce photosynthesis, defective translocation of assimilates and decrease carbon achievement, causing modified growth and reproduction (Rizhsky et al. 2004).
1.3. Reactive oxygen species (ROS) and oxidative damage
Water and atmospheric oxygen are requirements for sustainable aerobic life, therefore, plant metabolism must be deeply regulated in order to grant an effective integration of a distinct range of biosynthetic pathways that are naturally reductive (Apel and Hirt 2004, Foyer and Noctor 2005). Oxygen itself is a totally harmless molecule as in its ground state it has two unpaired electrons with parallel spin, making this molecule unreactive (Apel and Hirt 2004). The adverse effects of oxygen depends on its activation, which is an inevitable result of the photosynthetic electron transfer under aerobic conditions, being taken by physical or chemical mechanisms (Hajiboland 2014). Physical activation of oxygen happens by direct transfer of excitation energy from a photo-activated pigment, for instance, an excited chlorophyll (Chl*) molecule to O2, absorbing enough energy to switch the spin on one of the unpaired electrons,
thus forming singlet oxygen (1O2) (Sharma et al. 2012). The 1O2 state is a prevalent reactive
species characterized by being highly diffusible through the membranes and able to react with many organic molecules, hence damaging the photosynthetic apparatus (Arora et al. 2002). Chemical activation occurs by stepwise univalent reduction of O2, meaning the addition of
electrons one by one (Arora et al. 2002). The three intermediates of monovalent reduction between the full reductions of O2 to water comprises superoxide anion (O2̇·ˉ) hydrogen peroxide
(H2O2) and the hydroxyl radical (·OH), considered to be the most common reactive oxygen
species that are chemically reactive and biologically toxic (Moller et al. 2007). The toxicity is reflected by their short half-lives before reacting with cellular compounds comparatively to that of O2 (Sharma et al. 2012). Furthermore, reactive oxygen species may also pull an electron
from an organic molecule and turning it a radical, such as the peroxyl (RCOO·) and alkoxyl (RO·) radicals which are capable of propagating a chain reaction (Moller et al. 2007).
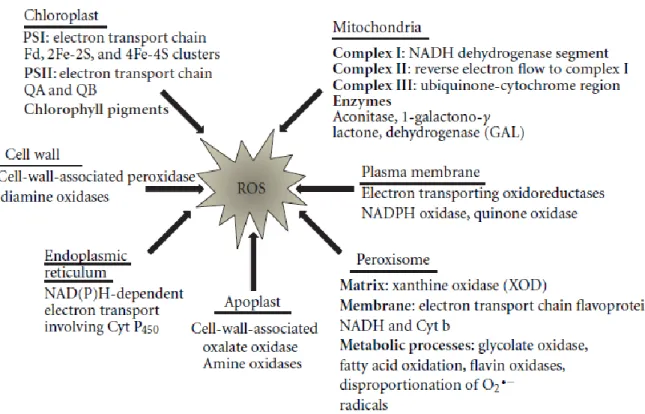
ROS are produced under steady state and stress conditions at several locations in chloroplasts, mitochondria, plasma membranes, peroxisomes, apoplast, endoplasmic reticulum and cell wall (Gill and Tuteja 2010, Sharma et al. 2012). ROS can also be generated by enzymes, such as NADPH-oxidase, xanthine oxidase, peroxidases and amine oxidase, however, in green
6 plant organs in the light, photochemical events as well as photorespiration are the main ROS sources (Figure 2) (Schmidt and Schippers 2014).
Figure 2 – Sources of production of reactive oxygen species in plants. Adapted from (Sharma et al. 2012).
Superoxide is the first reduction product of the ground state oxygen and could be subjected to both oxidation and reduction reactions, arising a cascade of reactions in the cell to produce other ROS, either directly or through enzyme or metal catalyzed processes depending on the cellular compartment (Sharma et al. 2012, Hajiboland 2014). O2̇·ˉ is moderately reactive
and is able to accept one electron and two protons to form H2O2 spontaneously or enzymatically
(Arora et al. 2002).
Hydrogen peroxide function as oxidant or reductant in many cellular reactions and is produced under normal and stressful conditions, such as drought, chilling, UV irradiation, wounding and invasion by pathogens (Moller et al. 2007). Electron transport chain (ETC) of chloroplast and mitochondria, endoplasmic reticulum and plasma membrane, β-oxidation of fatty acids and photorespiration are the major sources of this molecule that, unlike O2̇·ˉ, is very
diffusible trough membranes causing consequently oxidative damage away from the site of its synthesis (Moller et al. 2007, Gill and Tuteja 2010). Due to it relatively stability and its unique capacity to diffuse through aquaporins among the others ROS, H2O2 has received particular
7 consideration as a signal molecule involved in the regulation of specific biological processes, like stomatal closure and root growth, and triggering tolerance against various environmental stresses (Bienert et al. 2007, Hajiboland 2014). However, the main concern regarding by both O2̇·ˉ and H2O2 lies in their potential to generate highly reactive ·OH (Moller et al. 2007).
The hydroxyl radical is the most reactive among all ROS and it’s generated by the reaction of H2O2 withO2- (Haber-Weiss reaction), reactions of H2O2 with Fe2+ (Fenton reaction)
and decomposition of ozone (O3) in apoplastic space (Gill and Tuteja 2010, Sharma et al. 2012,
Hasanuzzaman et al. 2013). The electronic configuration, composed by a single unpaired electron, gives this ROS the ability to react nonspecifically to all biological molecules, causing subsequent cellular damages. Due to its short lifetime and strongly positive redox potential its site of reaction is close to its point of formation (Hajiboland 2014).
The state of oxidative stress is characterized by an overproduction of ROS that exceeds the defense mechanisms and can be reinforced by environmental stresses (Foyer and Noctor 2005, Mittler 2006). Enhanced levels of ROS can cause damage to biomolecules, such as proteins, lipids, DNA, pigments, and other fundamental cellular molecules. Furthermore, it have been reported that ROS participate in the control and regulation of cell growth, cell cycle, programmed cell death, hormone signaling and plant responses to biotic and abiotic stresses (Saed-Moucheshi et al. 2014). Due to the multifunctional roles of ROS it is crucial for plants to produce scavengers expressed by enzymatic and nonenzymatic antioxidants (Gill and Tuteja 2010).
1.4. Antioxidative defense: Protection by scavenging systems
Abiotic stresses, like heat stress and/or high irradiance, boosts oxidative damage so that plants have evolved a number of mechanisms to scavenge the oxygen intermediates formed in the cells (Gill and Tuteja 2010). Antioxidants can act in two different ways in plants: either they can prevent ROS formation, or scavenge the unavoidably formed ROS (Pinto-Marijuan and Munne-Bosch 2014). In order to protect themselves against photo-oxidative stress, plants acquire complex antioxidative defense system which are found in several organelles, such as chloroplasts, mitochondria and peroxisomes (Pang and Wang 2008). One of the first photoinhibition avoidance mechanisms comprises leaves and chloroplasts movement or solar radiation decay by phenolic compounds (Takahashi and Badger 2011). Chlorophyll is very efficient in absorbing light and its excited state (1Chl*) allows the excitation energy to be
8 converted into an electrochemical potential (Hajiboland 2014). However, as reported in the presence of excessive light or in a limiting availability of CO2, disturbances in the balance
between light harvesting and utilization of energy induces the production of the triplet state Chl (3Chl*), which can react with ground state oxygen forming superoxide anion. The triplet state of Chl can be quenched directly by carotenoids that are not far from the site of the reaction or by α-tocopherol, located in the thylakoid membrane (Moller et al. 2007).
Among the non enzymatic components, ascorbate (Asc) and glutathione, as well as tocopherol, carotenoids, phenolic compounds and proline, are the major cellular redox buffers in the antioxidative system (Sharma et al. 2012, Hasanuzzaman et al. 2013).
Ascorbate is a powerful and hydrophilic antioxidant that occurs in all plant compartments, usually being concentrated in photosynthetic cells and meristems (Gill and Tuteja 2010). Due to its electron providing capacity in a number of enzymatic and non enzymatic reactions, ascorbate performs an important role in several physiological processes in plants, including growth, differentiation and metabolism (Sharma et al. 2012). Asc particularly scavenges H2O2 and is also a substrate for ascorbate peroxidase (APx), which leads to the
production of dehydroascorbate, the oxidized form of Asc. Furthermore, ascorbate reestablish α-tocopherol from α-tocopheroxyl radicals preventing lipid peroxidation and also act as a cofactor for violanxanthin de-epoxidase, sustaining this way the dissipation of excess excitation energy (Asada 2006, Demmig-Adams et al. 2013).
Under physiological circumstances glutathione occurs mostly in the reduced form (GSH), while a small amount is oxidized (GSSG), playing a central role in various processes, including signal transduction, pathogen resistance, regulation of sulfate transport, detoxification of xenobiotics and participates in the regulation of the cell cycle, cell division, differentiation and senescence (Foyer et al. 1997, Noctor et al. 1998). This antioxidant scavenges 1O2, O2̇·ˉ, ·OH and H2O2, and it also determines GSH/GSSG ratio, which represents
a good redox balance indicator. Moreover, GSH participates in the regeneration of Asc as the cofactor of dehydroascorbate reductase (DHAR), being reduced by glutathione reductase (GR) (Foyer and Halliwell 1976, Noctor et al. 1998).
Regarding grapevines, phenolic compounds are divided into non-flavonoid (hydroxybenzoic acids, hydroxycinnamic acids, volatile phenols and stilbenes) and flavonoid compounds, such as flavones, flavanols, flavanones, flavan-3-ols and anthocyanins (Teixeira et al. 2013). Flavonoids feature the most bioactive plant secondary metabolites, performing the ability to either being proton or electron donor, thus they not only can participate in the
9 quenching of H2O2 but also act as transition metal chelators, ceasing the Fenton reaction
(Blokhina et al. 2003, Gill and Tuteja 2010). Another mechanism that justify their antioxidant potential is the ability of flavonoids to change peroxidation kinetics through decreasing the fluidity of the membranes, which can obstruct the diffusion of ROS and regulate peroxidative reactions (Karuppanapandian et al. 2011).
Enzymatic ROS scavenging mechanisms in plants include a group of 4 enzymes: superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APx) and glutathione peroxidase (GPx), which play an important role in ROS detoxification during abiotic stresses (Apel and Hirt 2004). As referred above, ascorbate and glutathione perform a crucial and interrelated role in antioxidative defenses since they act both chemically and as substrates in enzyme detoxification reactions (Alscher et al. 1997). These enzymes arise in various subcellular areas and operate all together when cells are exposed to oxidative stress (Sharma et al. 2012). SOD function as the first defense line against ROS, dismutating superoxide anion into H2O2 and O2 in a reaction that is spontaneous and extremely fast, thus preserving cells from
damage by superoxide radical reaction products (Kochhar et al. 2003, Apel and Hirt 2004). H2O2 is then reduced to water by CAT and APx. CAT has remarkably high catalytic rates but
low substrate affinity, since that one of the requirements of the reaction is the simultaneous access of two H2O2 molecules to the active site. H2O2 detoxification by APx result from the
oxidation of ascorbate to monodehydroacorbate (MDHA), constituting an integrative step of the Halliwell-Asada cycle or ascorbate-glutathione cycle (Noctor et al. 1998, Kochhar et al. 2003). Both ascorbate and glutathione can scavenge ROS through this cycle, considered to be an effective way for plant cells to compartmentalize H2O2 in the places where this component
is produced, using a series of reactions catalyzed by APx, MDHAR, DHAR and GR (Figure 3) (Apel and Hirt 2004). APx needs two molecules of ascorbate to reduce H2O2 into water, with
the adjuvant production of two molecules of MDHA, which represents a radical with a short lifetime that, if not rapidly reduced, disproportionates to ascorbate and dehydroascorbate (DHA). MDHA can be reduced directly to ascorbate, using the reduction of ferredoxin or NADPH to donate electrons.
10 The latter reaction is catalyzed by MDHAR. Even though the enzymatic or non enzymatic regeneration of ascorbate being directly from MDHA, an accelerated disproportionation of the MDHA means that some dehydroascorbate is produced when ascorbate is oxidized in plant tissues. Dehydroascorbate is reduced to ascorbate by the action of DHAR, using reduced glutathione. This reaction generates oxidized glutathione (GSSG) which is in turn reduced again to reduce glutathione by NADPH, a reaction catalyzed by GR (Noctor et al. 1998). .
In the course of the scenarios predicted by climate change, particularly for vines, it becomes essential to develop heat stress mitigation techniques, both in terms of cultural management practices and scientific research in order to preserve and perhaps improve grapevine quality production through enhancing antioxidant defense system and ROS detoxification pathways, as well as in alleviating their formation.
1.5. Adaptation measures for viticulture under climate change
It has long been known the benefits of a suitable exposure of grapes to sunlight for wine quality, however, the projected increase in the frequency of hot summer days will certainly intensify sunburn in grape berries, especially on the afternoon side of canopies (Keller 2010). This perspective may require short and long term adaptations in vineyards. Because grape varieties differ in their suitability and adaptability to different climates, long-term adaptations may include shifts in the variety profile of different regions, though shifts entail that some
11 vineyards located in the warmest or driest regions may be discarded, which has implications for the quality of life in rural areas. In addition, rootstocks selection and genetic improvement may also constitute long-term measures for future vineyards (Keller 2010, Jelly et al. 2014). Short-term adaptation measures are considered the first protection strategy and should be center at specific threats in order to improve yield (Fraga et al. 2012). Recently, exogenous applications of protectants, for example osmoprotectants like proline, glycine betaine and trehalose, phytohormones (abscisic acid, gibberellic acids, jasmonic acids, salicylic acid, etc.), signaling molecules such as nitric oxide, trace elements (selenium, silicon, etc.) and nutrients (nitrogen, phosphorus, potassium, calcium, etc.) have been found useful in alleviating the damage of heat stress in plants (Waraich et al. 2012, Hasanuzzaman et al. 2013). Proline accumulation is one of the most prevailing described modifications induced by water deficit and salt stress in plants and it is usually considered to be involved in stress tolerance mechanisms (Kahlaoui et al. 2015).
Kaolin, a white chemically inert clay mineral, is considered to be a valuable component regarding short-term measures against heat stress in grapevines, mainly in the warmest and driest regions (Figure 4) (Glenn 2012, Boari et al. 2015). Some kaolin characteristics are comparable to natural plant defenses consisting of increasing cuticle thickness and pubescence to reduce water and heat stress (Glenn 2012). Due to its excellent reflective properties found to reduce leaf and fruit surface temperatures, along with an influence on fruit maturation and internal quality, kaolin exogenous application improves photosynthetic efficiency in plants subjected to high photosynthetic ative radiation (Cantore et al. 2009, Chamchaiyaporn et al. 2013). Moreover, kaolin use is authorized in organic farming, promoting alterations in insect/pathogen behaviour on the plant, being considered a biopesticide (Glenn 2012). Previous studies showed that kaolin particle film revealed to increase fruit average weight (Chamchaiyaporn et al. 2013), constituting an opportunity when concerning grapevine’s yield in the Douro valley, hence decreasing by consequence the adverse impacts of abiotic stresses on crop plants.
Besides all the techniques, measurements and treatments that can be taken regarding heat stress mitigation, it is necessary to take into account that interdisciplinary and applied reasearch is fundamental for viticulturists and eonologists and can be used as a basis to evolve practical outcomes.
12
1.6. Objectives
The present work aims to evaluate the potential of kaolin exogenous application as a foliar protector in a vineyard located in the Douro region, studying the effects of its application on the antioxidant defense systems of Vitis vinifera L. leaves and fruits extracts. For that purpose, it was made a targeted approach in the fields of biochemistry and plant physiology in order to better understand some of the defense mechanisms and pathways used by grapevines. In addition, this study intends to verify if kaolin constitutes a short-term mitigation measure against summer stress that does not compromise grapes’ quality, as well as the physiology of the same.
2.
R
EFERENCESAlscher, R. G., J. L. Donahue and C. L. Cramer (1997).Reactive oxygen species and antioxidants: relationships in green cells. Physiologia Plantarum 100: 221-233.
Apel, K. and H. Hirt (2004).Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373-399.
Arora, A., R. K. Sairam and G. C. Srivastava (2002).Oxidative Stress and antioxidative system in plants. Current Science 82: 1227-1238.
Asada, K. (2006).Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 141(2): 391-396.
Bienert, G. P., A. L. Moller, K. A. Kristiansen, A. Schulz, I. M. Moller, J. K. Schjoerring and T. P. Jahn (2007).Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Journal of Biological Chemistry 282(2): 1183-1192.
13 Blokhina, O., E. Virolainen and K. V. Fagerstedt (2003).Antioxidants, oxidative damage and
oxygen deprivation stress: a review. Annals of Botany 91 Spec No: 179-194.
Boari, F., A. Donadio, M. I. Schiattone and V. Cantore (2015).Particle film technology: A supplemental tool to save water. Agricultural Water Management 147: 154-162. Cantore, V., B. Pace and R. Albrizio (2009).Kaolin-based particle film technology affects
tomato physiology, yield and quality. Environmental and Experimental Botany 66: 279-288.
Chamchaiyaporn, T., K. Jutamanee, P. Kasemsap, P. Vaithanomsat and C. Henpitak (2013).Effects of kaolin clay coating on mango leaf gas exchange, fruit yield and quality. Kasetsart Journal : Natural Science 47: 479-491.
Coombe, B. G. (1987).Influence of temperature on composition and quality of grapes. Acta Horticulturae 206: 23-36.
Delrot, S., H. Medrano, E. Or, L. Bavaresco and S. Grando (2010).Methodologies and results in Grapevine Research. Springer: New York: 5-6.
Demmig-Adams, B., C. M. Cohu, V. Amiard, G. Zadelhoff, G. A. Veldink, O. Muller and W. W. Adams (2013).Emerging trade-offs - impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins as regulators of development and defense. New Phytologist 197: 720-729.
Dinis, L.-T., C. M. Correia, H. F. Ferreira, B. Gonçalves, I. Gonçalves, J. F. Coutinho, M. I. Ferreira, A. C. Malheiro and J. Moutinho-Pereira (2014).Physiological and biochemical responses of Semillon and Muscat Blanc à Petits Grains winegrapes grown under Mediterranean climate. Scientia Horticulturae 175: 128-138.
Ferreira, M. I., J. Silvestre, N. Conceição and A. C. Malheiro (2012).Crop and stress coefficients in rainfed and deficit irrigation vineyards using sap flow techniques. Irrigation Science 30: 433-447.
Foyer, C. and G. Noctor (2005).Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell and Environment 28: 1056-1071.
Foyer, C. H. and B. Halliwell (1976).The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 133(1): 21-25. Foyer, C. H., H. Lopez-Delgadp, J. F. Dat and I. M. Scott (1997).Hydrogen peroxide and
glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum 100: 241-254.
Fraga, H., A. C. Malheiro, J. Moutinho-Pereira and J. A. Santos (2013).Future scenarios for viticultural zoning in Europe: ensemble projections and uncertainties. International Journal of Biometeorology 57(6): 909-925.
Fraga, H., A. C. Malheiro, J. M. Moutinho-Pereira and A. J. Santos (2012).An overview of climate change impacts on European viticulture. Food and Energy Security 1: 94-110.
14 Fraga, H., A. J. Santos, A. C. Malheiro, A. A. Oliveira, J. Moutinho-Pereira and G. V. Jones (2015).Climatic suitability of Portuguese grapevine varieties and climate change adaptation. International Journal of Climatology.
Fraga, H., Santos J.A., Malheiro A.C. and J. Moutinho-Pereira (2012).Climate change projections for the portuguese viticulture using a multi-model ensemble. Ciência e Técnica Vitivinícola 27: 39-48.
Gill, S. S. and N. Tuteja (2010).Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48(12): 909-930. Glenn, D. M. (2012).The mechanisms of plant stress mitigation by kaolin-based par-ticle films
and applications in horticultural and agricultural crops. HortScience 47: 710-711. Hajiboland, R. (2014). Reactive oxygen species and photosynthesis. Oxidative damage to
plants. P. Ahmad. United States of America, Elsevier Inc.: 1-63.
Hannah, L., P. R. Roehrdanz, M. Ikegami, A. V. Shepard, M. R. Shaw, G. Tabor, L. Zhi, P. A. Marquet and R. J. Hijmans (2013).Climate change, wine, and conservation. Proceedings of the National Academy of Sciences of the United States of America 110(17): 6907-6912.
Hasanuzzaman, M., K. Nahar, M. M. Alam, R. Roychowdhury and M. Fujita (2013).Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International Journal of Molecular Sciences 14(5): 9643-9684.
Howarth, C. J. (2005). Genetic improvements of tolerance to high temperature. Abiotic stresses: Plant resistance through Breeding and Molecular approaches. M. Ashraf and M. P. J. C. Harris. New York, Howarth Press Inc.
IPCC. (2007). Climate Change 2007: The Physical Science Basis. Summary for Policymakers. from http://www.ipcc.ch.
Jackson, D. I. and P. B. Lombard (1993).Environmental and management practices affecting grape composition and wine quality - A review. American Journal of Enology and Viticulture 44: 409-430.
Jelly, N. S., L. Valat, B. Walter and P. Maillot (2014).Transient expression assays in grapevine: a step towards genetic improvement. Plant Biotechnology Journal 12(9): 1231-1245. Kahlaoui, B., Hachicha, M., Misle, E., Fidalgo, F., Teixeira, J (2015). Physiological and
biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. Journal of Saudi Society of Agricultural Sciences. http://dx.doi.org/10.1016/j.jssas.2015.12.002
Karuppanapandian, T., J.-C. Moon, C. Kim, K. Manoharan and W. Kim (2011).Reactive oxygen species in plants: their generation, signal transduction and scavenging mechanisms. Australian Journal of Crop Science 5: 709-725.
15 Keller, M. (2010).Managing grapevines to optimise fruit development in a challenging environment: a climate change primer for viticulturists. Australian Journal of Grape and Wine Research 16: 56-69.
Kochhar, S., C. B. Watkin, P. L. Conklin and S. K. Brown (2003).A quantitative and qualitative analysis of antioxidant enzymes in relation to susceptibility of apples to superficial scald. Journal of the American Society for Horticultural Science 128: 910-916.
Magalhães, N. (2008). Tratado de viticultura: a videira, a vinha e o terroir. Lisboa.
Maksymiec, W. (2007).Signaling responses in plants to heavy metal stress. Acta Physiologiae Plantarum 29: 177-187.
Manabe, S., R. T. Wetherald, P. C. D. Milly, T. L. Delworth and R. J. Stouffer (2004).Century-scale change in water availability: CO2-quadrupling experiment. Climate Change 64:
59-76.
Martinez-Luscher, J., F. Morales, S. Delrot, M. Sanchez-Diaz, E. Gomes, J. Aguirreolea and I. Pascual (2013).Short- and long-term physiological responses of grapevine leaves to UV-B radiation. Plant Science 213: 114-122.
Mittler, R. (2006).Abiotic stress, the field environment and stress combination. Trends in Plant Science 11(1): 15-19.
Moller, I. M., P. E. Jensen and A. Hansson (2007).Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 58: 459-481.
Moriondo, M. and M. Bindi (2007).Impact of climate change on the phenology of typical mediterranean crops. Italian Journal of Agrometeorology 3(5-12).
Moutinho-Pereira, J. M., C. M. Correia, B. M. Gonçalves, E. A. Bacelar and J. M. Torres-Pereira (2004).Leaf gas exchange and water relations of grapevines grown in three different conditions. Photosynthetica 42: 81-86.
Moutinho-Pereira, J. M., N. Magalhães, L. F. Torres de Castro, M. Manuela Chaves and J. M. Torres-Pereira (2001).Physiological responses of grapevine leaves to Bordeaux mixture under light stress conditions. Vitis 40: 117-121.
Noctor, G., A. C. M. Arisi, L. Jouanin, K. J. Kunert, H. Runnenberg and C. H. Foyer (1998).Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. Journal of Experimental Botany 49: 623-647.
Pang, C. H. and B. S. Wang (2008).Oxidative stress and salt tolerance in plants. Progress in Botany: 231-245.
Pedreira dos Santos, T., C. M. Lopes, M. L. Rodrigues, C. R. de Souza, J. M. Ricardo-da-Silva, J. P. Maroco, J. S. Pereira and M. M. Chaves (2007).Effects of deficit irrigation strategies on cluster microclimate for improving fruit composition of Moscatel field-grown grapevines. Scientia Horticulturae 112: 321-330.
16 Pinto-Marijuan, M. and S. Munne-Bosch (2014).Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. Journal of Experimental Botany 65(14): 3845-3857.
Reis, A. P., L. Menezes de Almeida, E. Ferreira da Silva, A. J. Sousa, C. Patinha and E. C. Fonseca (2007).Assessing the geochemical inherent quality of natural soils in the Douro river basin for grapevine cultivation using data analysis and geostatistics. Geoderma 141: 370-383.
Rizhsky, L., H. Liang, J. Shuman, V. Shulaev, S. Davletova and R. Mittler (2004).When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology 134(4): 1683-1696.
Saed-Moucheshi, A., H. Pakniyat and H. Pirasteh-Anosheh (2014). Role of ROS as signaling molecules in plants. Oxidative damage to plants. P. Ahmad. USA, Elsevier Inc. 20. Schmidt, R. and J. H. M. Schippers (2014).ROS-mediated redox signaling during cell
differentiation in plants. Biochimica et Biophysica Acta.
Sharma, P., A. B. Jha, R. S. Dubey and M. Pessarakli (2012).Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012: 26.
Takahashi, S. and M. R. Badger (2011).Photoprotection in plants: a new light on photosystem II damage. Trends in Plant Science 16(1): 53-60.
Teixeira, A., J. Eiras-Dias, S. D. Castellarin and H. Geros (2013).Berry phenolics of grapevine under challenging environments. International Journal of Molecular Sciences 14(9): 18711-18739.
Wahid, A., S. Gelani, M. Ashraf and M. R. Foolad (2007).Heat tolerance in plants: an overview. Environmental and Experimental Botany 61: 199-223.
Waraich, E. A., R. Ahmad, A. Halim and T. Aziz (2012).Alleviation of temperature stress by nutrient management in crop plants: A review. Journal of Soil Science and Plant Nutrition 12: 221–244.
17
C
HAPTER2
–
I
NFLUENCE OFK
AOLIN PARTICLE FILM ON NON-ENZYMATIC ANTIOXIDANT DEFENSES
18
K
AOLIN EXOGENOUS APPLICATION BOOSTS ANTIOXIDANT CAPACITY AND PHENOLIC CONTENT IN BERRIES AND LEAVES OF GRAPEVINE UNDER SUMMER STRESSDinis, L.-T., Bernardo, S., Conde, A.,Pimentel, D., Ferreira, H., Félix, L.,Gerós, H., Correia, C.M., and Moutinho-Pereira, J.
Accepted in: Journal of Plant Physiology
Abstract
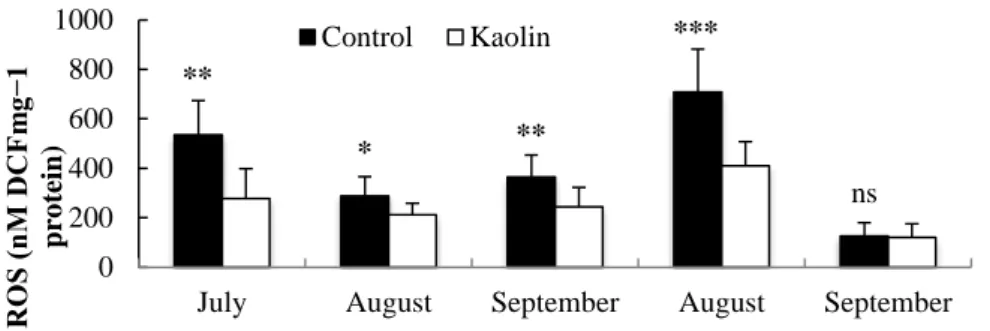
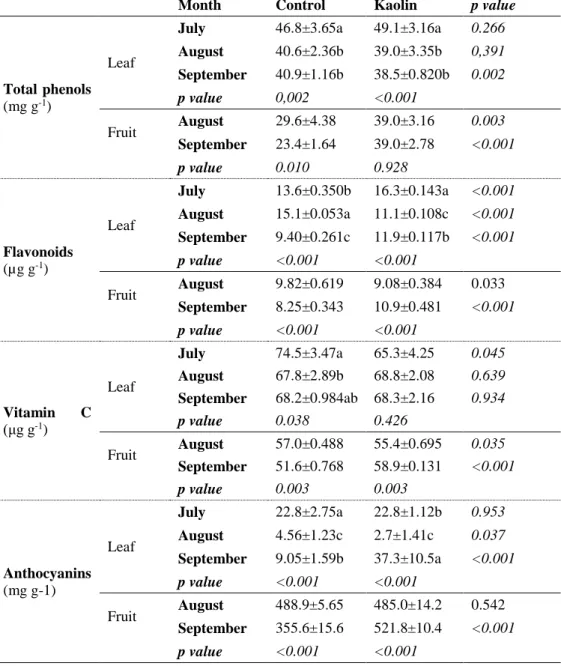
Heat waves, high light intensities and water deficit are becoming important threats in many important viticultural areas worldwide, so the implementation of efficient and cost-effective mitigation strategies is crucial for the production of premium wines while maintaining productivity. In this context, the foliar application of kaolin, a chemically inert mineral with excellent reflective properties, is being developed and experimented as a strategy to reduce the impact of heat and drought in Douro vineyards (Northern Portugal), already revealing promising results. In the present study we investigated if an improved antioxidant capacity is part of the beneficial effects of kaolin, by studying changes in the enzymatic and nonenzymatic antioxidant system in leaves and berries (cv Touriga Nacional). Results showed that mature grape berries contained higher amounts of total phenols (40%), flavonoids (24%), anthocyanins (32%) and vitamin C (12%) than fruits from control vines, and important changes were also measured in leaves. In parallel, kaolin application improved the antioxidant capacity in berries, which was correlated with the observed increased content in secondary metabolites. Kaolin application also regulated secondary metabolism at the transcriptional level through the increase in the transcript abundance of genes encoding phenylalanine ammonia lyase and chalcone synthase.
19
1. Introduction
Douro Demarcated Region (DDR), recognized in 2001 by UNESCO as World Heritage, is
one of the iconic wine regions in the world. This widespread area of vineyards, with a crucial
socioeconomic and cultural relevance, has peculiar soil and climatic characteristics providing a suitable “terroir” for premium wine production (Likar et al., 2015). Excess of temperature, high irradiance and water scarcity are well-known environmental stress factors that severely limit grapevine productivity in Douro, which are becoming particularly frequent in the context of ongoing climate change (Fraga et al., 2014) .
High irradiance, including UVB radiation, is absorbed by cellular components such as proteins and nucleic acids, resulting in biomass reduction, impaired photosynthesis, reduced protein synthesis, damage to DNA and to other chloroplast functions (Schultz, 2000). Also, leads to oxidative stress (Molassiotis et al., 2006) when excess of reactive oxygen species (ROS) are produced from the disruption of metabolic activities and through the activation of membrane localized NADPH oxidase (Majer and Hideg, 2012). It is well known that oxygen radicals are remarkably reactive and cytotoxic in all organisms, since they can react with unsaturated fatty acids and thus induce the peroxidation of essential membrane lipids or intracellular organelles (Zimmermann and Zentgraf, 2005). Peroxidation leads to cell leakage, fast dehydration and finally cell death. Damage to intracellular membranes may influence mitochondrial respiration and induce the degradation of pigments and a loss of the CO2 fixation
ability as well as photoinhibition (Zimmermann and Zentgraf, 2005).
The negative impacts of extreme heat, water scarcity and high irradiance in vineyards prompted the search for short mitigation strategies through the application of exogenous compounds that could maintain or even improve plant productivity under such environmental stresses. Although the literature reports encouraging results in other crops, these strategies are so far less explored in grapevine. Indeed, exogenous mannitol application to salt-stressed wheat, a plant unable to synthesize this polyol, significantly increased its salt tolerance, mainly by stimulating the activity of antioxidant enzymes (Seckin et al., 2009). Also, in maize, an exogenous application of glycinebetaine limited the adverse effects of water stress by modulating water relations (Nawaz and Ashraf, 2007), and its application in tomato (Park et al., 2006), a fleshy fruit, enhanced tolerance to chilling by protecting membranes and macromolecules directly and by inducing antioxidant enzymatic mechanisms. Abscisic acid (ABA) is an important phytohormone responsible for activating drought resistance, and has
20 been successfully experimented as an exogenous protective compound against abiotic stress in maize (Hose et al., 2000), tomato (Aroca et al., 2008), spring wheat and poplar (Du et al., 2013).
From a set of promising compounds, we experimented the exogenous application of kaolin, which is a chemically inert mineral with excellent reflective properties that has yielded promising results. Kaolin reduced leaf surface temperatures, and improved fruit maturation and quality in apple (Wand et al., 2006), and reduced leaf surface temperature and increased CO2
assimilation rates in olive (Nikoleta-Kleio et al., 2012). In tomato, kaolin application reduced the number of sunburned fruits, exhibited protective properties against insect attack (Cantore et al., 2009) and influenced the physiological response to salinity (Boari et al., 2014). Also, in Merlot grape (Vitis vinifera L.), kaolin application enhanced the total amount of berry anthocyanins (Song et al., 2012) and we recently showed that in Touriga Nacional it induced a protective effect on photosystem II structure and function (Dinis et al., 2015). The protective properties of kaolin make imperative to investigate in more detail the protective properties of kaolin in grapevine against heat waves, high irradiance and water scarcity, and if an improved antioxidant capacity is part of its beneficial effects also due to its potential useful role in water management.
In the present study the effect of kaolin exogenous application was investigated on the enzymatic and nonenzymatic antioxidant capacity of leaves and berries from grapevine (cv. Touriga Nacional) through approaches that included ROS-scavenging assessment by non-enzymatic methods, such as ABTS+ and β-carotene and hydroxyl radical-scavenging activity assays, the quantification of key secondary compounds and antioxidants, and molecular biology approaches to study the effect of kaolin on the expression of two important genes of the secondary metabolism, VvPAL1 and VvCHS1, which encode phenylalanine ammonia lyase (PAL) and chalcone synthase (CHS), respectively. The observed lower ROS levels, increased hydroxyl radical scavenging and enhanced production of antioxidants compounds, including phenolics, flavonoids and anthocyanins, all key metabolites in berry, are likely important mechanisms underlying the mitigation effects of kaolin on adverse abiotic climatic stresses in grapevines.
21
2. Material and methods
2.1.Chemicals
All chemicals and reagents were of analytical grade and were obtained from commercial sources (Sigma/Aldrich, Merck, and Pronalab). Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA).
2.2. Plant material and samples preparation
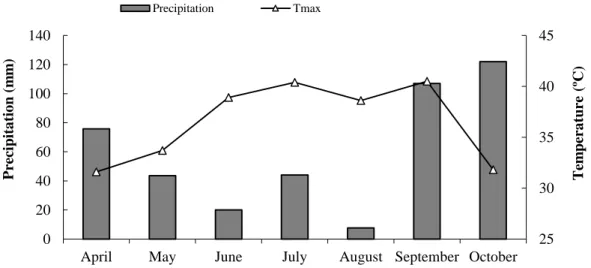
Samples were obtained from Touriga Nacional (Vitis vinifera L.) cultivar grafted on 110 R located in a commercial vineyard “Quinta do Vallado” in the Douro Demarcated Region (Denomination of Origin Douro/Porto) located at Peso da Régua (41º09'44.5''N 07º45'58.2''W), in northern Portugal. Touriga Nacional is considered the finest Portuguese red grape variety, and in Douro wine region is particularly important in the production of Port. The climate is typically Mediterranean-like, with a warm-temperate climate and dry and hot summers (Kottek et al. 2006), with higher precipitation during the winter months and very low during the summer. The soil, essentially of schist origin with a loam-dominated texture, is classified as dystric-surribi aric anthrosols (Agroconsultores and Coba 1991). Vines were managed without irrigation and grown using standard cultural practices as applied by commercial farmers. Monthly maximum temperature (Tmax) and precipitation values (April to October) are shown in
Figure 1. Temperature values were higher in July and September, while precipitation values were lower in June and August.
25 30 35 40 45 0 20 40 60 80 100 120 140
April May June July August September October
T em pera ture (º C) P re cipita tio n ( m m ) Precipitation Tmax
Figure 1 – Monthly values of maximum temperature (Tmax) and precipitation at the experimental site
22 Three vineyard rows, with twenty plants each, were sprayed soon after veraison (17th July 2014) with 5 % (w/v) Kaolin (Surround WP; Engelhard Corp., Iselin, NJ). A second application in the same day was done to ensure Kaolin adhesion uniformity. Other three vineyard lines, with twenty plants each, were maintained as control, i.e. without Kaolin application. All rows are located side-by-side (ensuring the same edaphoclimatic conditions) on a steep hill with an N-S orientation. The vines, with 7 years, were trained to unilateral cordon and the spurs were pruned to two nodes each with 10-12 nodes per vine.
Leaves (six fully expanded leaves of the first third of the shoot per treatment) were sampled in three different dates: 23th July (one week after pulverization), 21st August (one month after pulverization) and 3rd September (one month and half after pulverization). The berry samples were randomly collected (n = 200 per row, from different positions in the clusters and in the vine) in two different dates: 28th August and 12th September (close to harvest). Samples (leaves and berries) were kept at −80 °C and protected from light prior to further use. For extraction of antioxidant compounds, samples were lyophilized for 48 h and converted to a fine dried powder before analysis (ground with liquid nitrogen). Sample extraction was carried out combining 0.5 g (triplicated per sample – one for each treatment row) of each sample with 5 ml of 50 % (V/V) methanol under shaken for 1h. This step was repeated three times to increase the yield of extraction. All samples were dissolved in the same solvent at a concentration of 20 mg/ml.
2.3. ROS quantification
Reactive oxygen species (ROS) were determined with 2’,7’-dichlorofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, Germany) (Kong et al. 2014). A 25 mM solution was prepared in dimethyl sulphoxide for pending use. Twenty μl of each sample were loaded into a small well ELISA plate containing 0.2 ml of PBS buffer (pH 7.4) and 12 μM of DCFH-DA and incubated for 20 min at 25 ºC.
Fluorescence was measured at 485 nm and 530 nm (excitation and emission wavelength, respectively), in a CARY 50 Bio (Eclipse, Australia) every 15 min until 60 min after the incubation. A calibration curve was prepared with 2’,7’-Dichlorofluorescein and the results were expressed as nM DCF mg-1 protein.
23 2.4. Determination of total antioxidant components
The concentration of total phenolics was quantified by the Folin–Ciocalteu colorimetric method (Singleton and Rossi 1965) using a gallic acid (GAE) calibration curve, and the results were expressed as mg of gallic acid equivalents/g of dry weight (GAEs).
Flavonoids concentrations in the extracts were determined by a colorimetric method at 510 nm (Jia et al. 1999). A calibration curve was prepared with (+)-catechin and the results were expressed as μg of (+)-catechin equivalents per g of dry weight (DW).
The concentrations of β-carotene and lycopene were determined according to previously published method (Nagata and Yamashita 1992), and the following equations were used:
β-carotene (mg/100 ml) = 0.216 A663 – 1.220 A645 − 0.304 A505 + 0.452 A453 Lycopene (mg/100 ml)= − 0.0458 A663 + 0.204 A645 − 0.304 A505 + 0.452 A453
The results were expressed as μg /g (DW) of extract.
For the determination of the total concentration of carotenoids (Car) (Lichtenthaler 1987), leaves were ground with liquid nitrogen and crushed in 80% acetone. The absorbance was measured at 663, 645 and 470 nm. The results were expressed as μg per g DW of extract.
Ascorbic acid concentration was measured at 515 nm (Klein and Perry 1982). A calibration curve was prepared with L-ascorbic acid and the results were expressed as μg per g DW.
Total anthocyanin concentration was estimated using the pH differential method (Meng et al. 2012). Extracts were diluted with buffers at pH 1.0 and 4.5 to attain the same dilution. Absorbance was measured at 520 and 700 nm in both pH 1.0 and 4.5 buffers. Total anthocyanin (expressed in terms of cyanidin-3-glucoside per DW) were calculated using the following formula:
Anthocyanin concentration = A × DF × MW/ (ε × C)
A = (A520 – A700)pH1.0 − (A520 – A700)pH4.5
where MW is the molecular weight of cyanidin-3-glucoside (449 g/ mol); DF is the dilution factor; ε is the molar extinction coefficient of cyanidin-3-glucoside (29,600); C is the concentration of extracted volume.
24 2.5. Antioxidant activity by chemical and biochemical assays
2.5.1. ABTS+ radical-scavenging activity
The radical-scavenging activity of leaf and fruit extracts were determined by ABTS+ (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical cation decoloration assay (Re et al. 1999). The ABTS+ radical was generated through the reaction of 2,20-azinobis-(3-ethylbenzo- thiazoline-6-sulphonic acid) diammonium salt (ABTS, 7 mM), with potassium persulphate (2.45 mM), in water. The mixture was left to stand for 12–16 h in the dark at room temperature. Absorbance of the reactant was later adjusted to 0.70 ± 0.02, at room temperature, at a wavelength of 734 nm in a UV/Vis VARIAN spectrophotometer (CARY 100 Bio, Australia). The radical-scavenging activity (RSAABTS) was calculated as a percentage of ABTS+ decoloration, using the equation: % RSAABTS = [(AABTS − AS)/AABTS] × 100, where
AABTS is the absorbance of the ABTS+ solution and AS the absorbance of the solution with the
sample extract (Dinis L-T. et al. 2012). The extract concentration providing 50 % of radical-scavenging activity relative to the tested concentration (EC50) was calculated from the graph of RSAABTS percentage against extract concentration in the range between 0.25 and 1.5 mg ml-1 of Trolox (hydrophilic homologous of α-tocopherol) used as standard (y = −0.0287x + 0.5535; R2 = 0.9976).
2.5.2. DPPH radical-scavenging activity
Various concentrations of leaf and fruit extracts (0.5 ml) were mixed with methanolic solution containing DPPH radicals (60 μl mol/l) (Dinis L-T. et al. 2012). The mixture was vigorously shaken and left to stand for 5 min in the dark. The reduction of the DPPH radical was measured by monitoring continuously the absorbance at 517 nm. The radical-scavenging activity (RSADPPH) was calculated as a percentage of DPPH reduction, using the same equation as for ABTS. The extract concentration providing 50 % of radical-scavenging activity relative to the tested concentration range (relative EC50) was calculated from the graph of RSADPPH percentage against extract concentration in the range between 0.1 to 0.6 mg ml-1 of extract. Trolox was used as standard (y = −0.021x + 0.3825; R2 = 0.9976).
25 2.5.3. Ferric reducing activity (FRAP assay)
The FRAP assay was carried out according to a modification of the original medicinal biochemical assay (Benzie and Strain 1996). The antioxidants present in the samples reduce the Fe(III)/tripyridyltriazine (TPTZ) complex to the blue ferrous form, with an increase in absorbance at 593 nm. The working FRAP reagent was freshly prepared by mixing 300 mM acetate buffer, pH 3.6, with 10 mM tripyridyltriazine (TPTZ) in 40mM hydrochloric acid and 20 mM ferric chloride (FeCl3) in water solution. A reagent blank reading was taken at 593 nm
(Areagentblank; t = 0 min). Different concentrations of extracts (0.2ml) were added to freshly FRAP reagent, and were shaken for 30 min (Dinis L-T. et al. 2012). Afterwards, the absorbance was read and selected as final reading (Asample). Sample blank reading, using extract and adequate volume of acetate buffer, was taken also (Asampleblank). The difference between Asample and Asample blank was calculated as A1sample. The FRAP value was calculated according the change in
absorbance of A1sample against Areagent blank. The extract concentration providing 0.5 of absorbance relative to the tested concentration range, (relative EC50) between 0.25 and 1.5 mg
ml-1 of extract, was calculated from the graph. Aqueous solutions of known FeII concentrations, in range of 10–100 mM (FeSO47H2O) and trolox were used as standard (y = −0.0019x + 0.157; R2 = 0.9934).
2.5.4. Inhibition of lipid peroxidation using the β-carotene linoleate mode system
The antioxidant activity of leaf and fruit grapevine extracts was evaluated by the β-carotene linoleate model system (Mi-Yae et al. 2003). A solution of β-β-carotene was prepared dissolving 2 mg of β-carotene in 10 ml of chloroform. Two ml of this solution were transferred into a 100 ml round-bottom flask. After removing the chloroform at 40 °C under vacuum, 40 mg of linoleic acid, 400 mg of Tween 80 emulsifier, and 100 ml of distilled water were added to the flask with vigorous shaking. This emulsion (4.8 ml) was transferred to different test tubes containing 0.2 ml of different concentrations of the extracts. The tubes were shaken and incubated at 50 °C in a water bath. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm. Absorbance readings were then recorded at 20 min intervals until the control sample had changed colour. A blank, devoid of β-carotene, was prepared for background subtraction. Lipid peroxidation (LPO) inhibition was calculated using the following equation: LPO inhibition = (carotene concentration after 2 h of assay/initial β-carotene concentration) × 100. The extract concentration providing 50 % antioxidant activity