Universidade de Trás-os-Montes e Alto Douro
Oxidative Status of Rabbits Fed With Olive Leaves Enriched
With Selenium
Dissertação de Mestrado em Engenharia Agronómica
Joana Margarida Machado Duarte Orientadores
Prof. Dr. Primo Proietti, Prof. Dr. Miguel Rodrigues
Oxidative Status of Rabbits Fed With Olive Leaves Enriched With Selenium
Dissertação Final de Mestrado
Mestrado em Engenharia Agronómica
Joana Duarte
Composição do Júri:
Presidente: Profº Doutor Virgílio Falco da Costa, Departamento de Agronomia Vogais: Profº Doutor Victor Manuel Carvalho Pinheiro, Departamento de Zootecnia
Profº Doutor Miguel António Machado Rodrigues, Departamento de Zootecnia
UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO VILA REAL, 2017
“As doutrinas expostas no presente trabalho são de exclusiva responsabilidade do autor.”
Esta tese foi elaborada com vista à obtenção do Grau de Mestre em Engenharia Agronómica apresentado à Universidade de Trás-os-Montes e Alto Douro.
vi
Acknowledgments
I would like to express my deep respect and appreciation to my supervisors Prof. Miguel Rodrigues from UTAD and Prof. Primo Proietti from UNIPG for all the help and guidance through this process.
I would like to offer my special thanks to Simona Mattioli, for all the guidance in this project and for her encouragement and availability at any time.
To Roberto D’Amato and Luca Regni with a special sense of gratitude for all the hospitality in the Department of Agricultural, Food and Environmental Sciences of the University of Perugia and for their patience and all the guidance through the experimental process. To Giovanni, for all the carpools to the rabbit farm and patience, in trying to understand and answer to all my questions, although my bad Italian.
I am also grateful to Prof. Victor Pinheiro for the valuable time spent in sharing ideas and advices for this thesis.
In a special way, I wish to express my gratitude to Filipe Pimenta, who with his support, patience and true friendship always brings the best in me.
I also wanted to acknowledge my family for being a model of courage and determination in life.
viii
Resumo
Estudos recentes têm comprovado o efeito positivo do selénio na nutrição animal e humana graças ao seu papel como suplemento de antioxidantes. Já foi comprovado o papel benéfico do selénio na protecção da oliveira do stress hídrico tendo ao mesmo tempo sido o seu azeite extra virgem nutraceuticamente fortificado. A incorporação das folhas destas oliveiras pré tratadas com selénio nas dietas animais é um método barato que aumenta a capacidade antioxidante da dieta e ao mesmo tempo providencia uma fonte de selénio. O objectivo deste estudo foi relacionar a influência da suplementação de 10% de folhas de oliveira não tratadas e folhas de oliveira tratadas com selénio na performance de coelhos e características físicas e químicas da sua carcaça. Foram escolhidos 30 coelhos de diferentes sexos, da raça branco neo-zelandês. Foram desmamados aos 30 dias de idade e alojados em gaiolas de rede bicelulares em 3 grupos homogéneos (10 animas por grupo) e submetidos a diferentes dietas: dieta controlo; dieta controlo enriquecida com 10% de folhas de oliveira (OL feed) e dieta controlo enriquecida com 10% de folha de oliveira tratada com selénio (Se feed). Aos 65 dias de idade os coelhos foram atordoados e sacrificados pelo corte da artéria carótida e veia jugular. A carcaça foi refrigerada (24h a 4ºC) e foram analisadas as características físicas e químicas. Foram realizadas determinações analíticas do alimento, das folhas de oliveira e da carne. Foi medido o pH e analisada a cor da carne, a sua capacidade de retenção de água e as perdas de água por cozedura. Foi medido o estado oxidativo do plasma e da carne e foi feita a quantificação do selénio total no músculo Longissimus dorsi. Os nossos resultados provaram que estas dietas não afectam a performance produtiva dos coelhos nem as características físicas da carne, no entanto verificou-se um aumento significativo de antioxidantes, nomeadamente α-tocopherol (control feed: 650.46nmol/mL; OL feed: 570.35 nmol/mL; Se feed: 892.34nmol/mL). Verificou-se um efeito de protecção da oxidação lipídica na carne (control feed: 0.11mg MDA/kg; OL feed: 0.09mg MDA/kg; Se feed: 0.06mg MDA/kg), no entanto houve um aumento na oxidação de proteínas para a dieta OL feed (control feed: 1.29nmol/mg proteína; OL feed: 2.98nmol/mg proteína; Se feed: 1.62nmol/mg proteína), o que não era esperado. A dieta Se feed promoveu um aumento significativo no conteúdo de selénio na carne (control feed: 143.31nmol/mL; OL feed: 306.07nmol/mL; Se feed: 1439.92nmol/mL). Estes resultados podem ser uma referência positiva, graças à sua concentração em antioxidantes e características nutraceuticas pode aumentar o consumo desta carne de animas com esta dieta, pois é um produto diferenciado. Ao mesmo tempo o conteúdo em antioxidantes permite prolongar o tempo de prateleira da carne no mercado.
x
Abstract
Recent studies have shown the positive role of selenium in animal and human nutrition for its role as an antioxidant supplement. Selenium has been proven to be beneficial in the protection of the olive tree from drought stress and at the same time as a way to nutraceuticaly fortify extra virgin olive oil. The incorporation of this olive leaves pretreated with selenium in animal diets is a cheap method that increases antioxidant capacity of the diet and at the same time provides as a source of selenium. The aim of this study was to relate the influence of dietary supplementation of 10 % olive leaves not treated or selenium treated on performance, physical and chemical characteristics of the rabbit carcass and the antioxidant content in serum plasma and meat. It was selected 30 new zealand white mixed sex rabbits that were weaned at 30 days of age and allocated into bicellular wire net cages in 3 homogeneous groups (10 animals per group) and subjected to different dietary treatments: control feed; control feed enriched with 10% olive leaves (OL feed) and control feed enriched with 10% olive leaves enriched with selenium. (SE feed). At 65 days of age the rabbits were stunned and sacrificed by cutting the carotid artery and jugular vein. The carcasses were refrigerated (24h at 4ºC) and its physical characteristics were analyzed. There was performed the analytical determinations of feed, olive leaves and meat. It was measured the color and pH of meat, its water holding capacity and its cooking loss. There was measured the oxidative state of meat and plasma and the quantification of total selenium content in Longissimus dorsi muscle. Our results proved that this diet does not affect the performance of rabbits neither physical characteristics of meat but there was a verified a increase of antioxidant compounds, namely α-Tocopherol in meat (control feed: 650.46nmol/mL; OL feed: 570.35 nmol/mL; Se feed: 892.34nmol/mL). It was verified a protective effect of lipid oxidation in meat (control feed: 0.11mg MDA/kg; OL feed: 0.09mg MDA/kg; Se feed: 0.06mg MDA/kg), nevertheless the results showed an increase in protein oxidation in OL feed (control feed: 1.29nmol/mg proteins; OL feed: 2.98nmol/mg proteins; Se feed: 1.62nmol/mg proteins) that was not expected. The Se feed proved a significant increase in the selenium content in meat (control feed: 143.31nmol/mL; OL feed: 306.07nmol/mL; Se feed: 1439.92nmol/mL). These results may be a positive reference thanks to its antioxidant and nutraceutical characteristics it may improve consumption of this rabbit meat because turns it into a differentiated product at the same time allows the improvement of its shelf-life duration.
xii
List of Contents
Acknowledgments ... vi Resumo ... viii Abstract ... x Chapter 1: Introduction ... 2Chapter 2: Literature Review ... 4
2.1 - Rabbit meat as a functional food ... 4
2.2 - Rabbit Digestive Tract ... 5
2.3 - Nutrient Requirements of the Rabbit ... 6
2.4 - Rabbits and selenium ... 9
2.5 - Free Radicals and Antioxidants ... 10
2.6 - Vitamin E ... 11
2.7 - Olive leaves in animal feeding ... 12
2.8 - The importance of selenium in nutrition ... 14
2.8.1 - Selenium characterization ... 14
2.8.2 - Selenoproteins ... 14
2.8.3 - Selenium deficiency and toxicity ... 17
2.8.4 - Human Selenium intake ... 18
2.8.5 - Selenium treatment in Olive europaea L. ... 19
Chapter 3: Experimental Work ... 22
3.1 – Objectives ... 22
3.2 – Materials and methods ... 22
3.2.1 - Animals and diets ... 22
3.2.2 - Productive performance ... 24
3.2.3 - Blood sampling and analysis ... 24
xiii
3.2.5 - Analytical determinations of feed, leaves and meat ... 26
3.2.6 - Oxidative status of the meat ... 27
3.2.7 - Analyses of selenium in LD muscle ... 28
3.2.8 - Statistical analyses ... 28
3.3 – Results and discussion ... 28
Chapter 4: Conclusions ... 36
xiv
List of Tables
Table 1 - Usual ingredient composition of feed for rabbits ... 7
Table 2 – Nutrient requirement of the fattening rabbits ... 7
Table 3 – Chemical composition and major phytochemicals in olive leaves ... 13
Table 4 – nown selenoproteins that carry out nutricional functions of selenium ... 16
Table 5 – Recommended selenium intae for selected populations sub-groups... 19
Table 6 – Formulation and chemical composition of feed and OL (% of wet weight) ... 23
Table 7 –Antioxidant compounds (mean ± sd) contents of OL ... 29
Table 8 – Antioxidant compounds (mean ±sd) contents of experimental diets ... 29
Table 9 – Productive performance of growing rabbits fed experimental diets ... 30
Table 10 –Chemical characteristics of the carcass of growing rabbits fed experimental diets 31 Table 11 – Chemical analysis of Longissimus dorsi meat of growing rabbits fed the experimental diets ... 32
Table 12 – Plasma antioxidant compounds and oxidative status of growing rabbits fed the experimental diets ... 33
Table 13 – Antioxidant compounds, oxidative status and selenium content of Longissimus dorsi meat of growing rabbits fed the experimental diets ... 34
xvi
List of Figures
Figure 1 - Schematic view of the digestive tract of the rabbit ... 5 Figure 2 - Various source of selenium and its content levels ... 18
xviii
Abbreviations
α-Tocopherol (αT)
2,4-dinitrophenylhydrazine (DNPH) Acid detergent fiber (ADF)
Acid detergent lignin (ADL) Digestible Energy (DE) Digestible protein (DP):
Gluthatione peroxidase (GSH-Px). Longissimus dorsi Muscles (LD) Neutral detergent fiber (NDF) Olive Leaves (OL)
Polyunsaturated fatty acids (PUFA)
Reactive oxygen/nitrogen species (ROS/RNS) Selenium (Se)
Selenocysteine (Sec) Selenomethonine (SeMet)
Thiobarbituric acid reactive substances (TBARS) Water Holding Capacity (WHC)
White muscle disease (WMD) Extra virgin olive oil (EVOO)
2
Chapter 1: Introduction
The importance of Selenium (Se) is mainly associated with its role as an essential part of important enzymes (for example glutathione peroxidase, with high antioxidant capacity) and its functions in reproduction, enhancement of the immune response, cancer, cardiovascular and type-2 diabetes prevention (Kumar and Priyadarsini, 2014).
Se enters the food chain through plants. Although it is harmful to plants in high concentrations, it exerts beneficial effects at low concentrations (Kuznetsov et al., 2003; Tadina et al., 2007; Proietti et al., 2013). Acording to D’Amato et al. (2014), the technique of fortifying olive tree canopies with sodium selenate showed an increased of the content of Se in the tree. This treatment has many benefits as a way of increasing the content of Se in extra virgin olive oil (EVOO) but also in order to increase the photosynthesis, fruit yield and maintaining the content of water in the leaves at a sufficiently high level (Proietti et al. 2013). In many regions of the world including Central Europe the recommended Se intake is not achieved. Consequently, increasing the Se content in animal products is a desirable feeding strategy. Se-enriched meat could be achieved through the fortification of diets with organic Se (selenomethionine) as it is incorporated into proteins in place of methionine (Rayman, 2004). It has been demonstrated that dietary Se supplementation increases Se concentration in the meat of rabbits (Dokoupilová et al., 2007; Marounek et al., 2009), pigs (Zhan et al., 2007) and lambs (Sushma et al., 2015). In this way rabbit meat could be considered a functional food since its nutritional properties such as the fatty acid profile, mineral and vitamin contents which could be further enriched via Se enrichment (Marounek et al., 2009; Dalle Zotte and Szendrő, 2011).
Rabbit feed frequently contains ingredients with high fiber content and low nutritive value, but with important physiological functions. The inclusion of olive leaves (OL) in growing rabbit’s diets affects apparent digestibility of crude protein and fat, although the effects on rabbit performance are not evident (Ribeiro et al., 2012).
According to these findings, the aim of this study was to evaluate the effect of the incorporation of olive leaves untreated or treated with sodium selenate on growth performance, carcass characteristics and meat quality. In order to determine this there was
3 performed the analytical determinations of feed, olive leaves and meat, as well as the productive performance of rabbits. It was measured the color and pH of meat, its water holding capacity and its cooking loss. There was measured the oxidative state of meat and plasma and the quantification of total selenium content in Longissimus dorsi muscle.
4
Chapter 2: Literature Review
2.1 - Rabbit meat as a functional food
Stress related disorders affect 450 million individuals worldwide and there is a strong potential for functional foods to propose stress management benefits (Hamer et al., 2005). A functional food is a food that aims to improve physiological functions or to reduce the risk of specific pathologies (Diplock et al., 1999). Meat can be considered a functional food by itself, it is a source of nutrients (Dalle Zotte and Szendrö, 2011), such as zinc and iron (mainly in red meats), Se (high in beef, chicken and rabbit meat) and B vitamins, phosphorus, magnesium and cobalt (abundant in all meats). Besides all this, it contributes to the intake of vitamin E, minerals and omega-3 fatty acids. Excessive meat intake is considered negative due to the high caloric content, high levels of saturated fatty acids, cholesterol and sodium (associated with cardiovascular diseases, hypertension, obesity and diabetes) (Department of Health and Human Services and Department of Agriculture, 2005).
The domestic rabbit has a white meat, known to be light, with high proportion of proteins and a desirable content of polyunsaturated fatty acids (PUFA), and a low content of cholesterol. It offers moderately high energy values, (603kJ/ 100g in the loin) depending on the protein content, 80% of energy value comes from it, these set of characteristics give this meat a positive dietary reference (Castelini and Dal Bosco, 1998; Hernàndez and Dalle Zotte, 2010). The consumption of 100g of rabbit meat contributes to 8% of daily B2 vitamin, 12% of B5 vitamin, 21% of B6 vitamin, 77% of B3 vitamin and fulfills the daily B12 requirements (Combes, 2004).
Hernández and Gondret (2006) considered that rabbit meat in human diet can improve human health doe to its richness in protein of high biological values, high content of unsaturated lipids, low cholesterol and sodium content and high amounts of phosphorus and a good source of complex B vitamins.
5
2.2 - Rabbit Digestive Tract
Rabbits are small nonruminant herbivores with a high metabolic rate and an enlarged hindgut, that is why they are selective eaters, selecting the nutritious plant parts allows them to satisfy their nutritional requirements (Redondo, 2006).
They own a large cecum that supports a population of microorganisms that uses nutrients not digested in the small intestine. Rabbits have a unique digestive strategy that allows them to utilize a forage-based diet, involving the selective separation of fiber (less dense particles, primarily lignocelluloses, that are moved rapidly by peristaltic movements of colon, forming the hard feces) from the non-fiber components that are used for fermentation in cecum. There are antiperistaltic movements that move the non-fiber components (starch granules, proteins and fluids) from colon to cecum (Pond et al., 1995). The cecum is the main fermentative area in rabbits digestive system, it accounts for about two-thirds of the fecal fiber digestion (Gidenne, 1992). At determined intervals, the cecal content is expelled as cecum contracts and mucus is secreted by the goblet cells in the lining of proximal colon forming mucus-coverd “soft feces” or cecotropes that are after consumed by the rabbit directly from the anus (MSD, 2010).
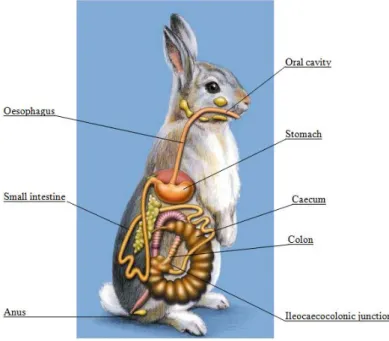
Figure 1 shows the schematic view of the digestive tract of the rabbit; it exemplifies the very large cecum, which is the site of the hind-gut fermentation
Figure 1 - Schematic view of the digestive tract of the rabbit
6 Cecotrophy (consumption of cecotropes) is an essential part of the digestive process of rabbits, as after ingestion cecotropes continue fermentation in the stomach, protected by the mucus from being broken by the acid and then continue digestion in the small intestine (Pond et al., 1995). This provides microbial protein, vitamins (all the B vitamins needed), and volatile fatty acids (that aid in the control of pathogenic organisms by maintaining pH in the cecum) (Carabaño et al., 2010; MSD, 2010).
Thanks to the selective separation and rapid excretion of large particles, fiber is digested poorly, and in order to minimize intestinal disease and promote intestinal motility a significant percentage of crude fiber in the diet is needed (~15% of crude fiber). Fiber is also known to absorb and eliminate through feces bacterial toxins, and low fiber diets have been lined to higher starch content. As starch is a substrate for the proliferation of pathogenic bacteria, it often results in a increased incidence of intestinal problems, such as, enterotoxemia. High-fiber diets (>20% of crude fiber) may result in cecal impaction and mucoid enteritis (MSD, 2010).
2.3 - Nutrient Requirements of the Rabbit
The rabbit feeding represents about 50-68 % of the expenses of rabbit production, being this significant, it is extremely important to monetize it the most (Redondo, 2006).
As mentioned before, rabbits are herbivorous animals and require high dietary fiber content. Rabbits are capable of achieving good growth performance on high fiber diets, the maximal growth rates have been associated with diets containing approximately 180-210g acid detergent fiber (ADF)/Kg which corresponds to 9.7–10.3 MJ digestible energy (DE)/Kg when no fat is added (Partridge et al.,1989; de Blas et al., 2010) An adequate content of dietary fiber is required to reduce total and caecal mean retention time and to maximize DE intake and weight gain, it is also important because it reduces total microbial growth (García et al., 2000), digestive disorders and fattening mortality. The recommended type of fiber includes a good level of lignin and minimum level for long fiber particles (de Blas and Mateos, 2010) The usual composition of feeds for rabbits can be observed in Table 1.
7
Table 1 - Usual ingredient composition of feed for rabbits
(Adapted from: de Blas and Mateos, 2010)
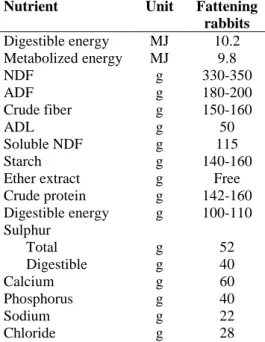
Table 2 – Nutrient requirement of the fattening rabbits
The nutrient requirements for fattening rabbits (based on the practical methods used in the industry) are present in Table 2 (Moughan et al., 1988; de Blas and Mateos, 2010).
Fat addition to feed has positive effects on energy digestibility and feed efficiency. The value of fat addition to a fattening feed should be established on an energy-cost basis, taking in
Ingredient Amount (g Kg-1)
Cereal grains (barley and wheat) 100-200
Animal and vegetable fats 5-30
Molasses 0-30
Beet, apple and citrus pulp, soy hulls 0-100
Cereal co-products (wheat bran, maize gluten feed and distillers co-products) 150-350
Lucerne hay 150-300
Lignified fibrous co-products (wheat straw, olive and grape co-products) 50-150
Protein concentrates (sunflower, soybean, rapeseeds and palm kernel meal) 120-220
Nutrient Unit Fattening rabbits Digestible energy MJ 10.2 Metabolized energy MJ 9.8 NDF g 330-350 ADF g 180-200 Crude fiber g 150-160 ADL g 50 Soluble NDF g 115 Starch g 140-160
Ether extract g Free
Crude protein g 142-160 Digestible energy g 100-110 Sulphur Total g 52 Digestible g 40 Calcium g 60 Phosphorus g 40 Sodium g 22 Chloride g 28
8 account the effects of fat quality to carcass quality and pellet stability (Fernández and Fraga, 1992). The fat addition should always be kept as constant as possible the digestible protein (DP):DE ratio (de Blas and Mateos, 2010).
Also, regarding the protein requirements of the rabbits, there should be a ratio between DP:DE, the optimal ratio is about 10g DP/MJ DE (de Blas and Mateos, 2010), and dietary ratios above or below this impair fattening performance and feed efficiency. Low DP:DE values (<9.5g/MJ) promote a decrease in water and protein and an increase in body fat, an excess of protein content related to energy increases environmental pollution (Xiccato, 2006). Soft feces represent only 0.14 of the total protein intake of the rabbit, so when feed formulation, special attention must be paid to this.
9
2.4 - Rabbits and selenium
The nutritional requirements of the micromineral Se regarding rabbits is about 0.15 mg kg-1 of the diet, the role of Se as detoxification of peroxides formed during metabolic processes, through gluthatione peroxidase (GSH-Px) activity is not very significant in rabbits as most of their GSH-Px does not have Se as a co-factor (Xiccato, 1996). According to Lee et al. (1979) rabbits do not develop symptoms of Se deficiency when fed diets deficient in Se, rabbit liver and kidneys contain a sufficient level of non Se dependent GSH-Px activity.
Se inclusion rate must be well defined as excessive Se feeding may led to the formation of selenite ion free radicals that have pro-oxidant characteristics combined with abundant GSH-Px can generate superperoxide anions and produce other types of reactive oxygen species (Erdélyi et al., 2000).
The supplementation of feed with Se has proved to increase the concentration in meat in various animals: broiler chickens (Ševčíková et al., 2006), pigs (Goehring et al., 1984) and calves (Pavlata et al., 2001). In reference to rabbits, Dokoupilová et al. (2007) conducted a study to investigate the deposition of Se in meat and the effect of supplemental Se on meat quality in rabbit fed with organic Se, and concluded that the supranutritional Se supply had no effect on GSH-Px activity and on oxidative stability of meat, but it had positive effect as a way to enrich that meat with Se. Also Marounek et al. (2009) studied the quality and selenium content in tissues of rabbits fed diets with supranutritional Se, and had similar results, although the oxidative stability of meat was not influenced, the Se supply allowed to obtain Se enriched meat.
In other hand, Ebeid et al. (2012) conducted a similar study and concluded that supplemented dietary Se had positive effects on growth performance, increased the final body weight and daily gain while the feed conversion ratio was reduced. As expected the deposition of Se in meat had increased and it appeared to improve the serum total antioxidant capacity and reduce the lipid peroxidation while it had no significant effect on meat chemical composition.
10
2.5 - Free Radicals and Antioxidants
During cellular metabolism there is the formation of free radicals that are high reactive molecules called reactive oxygen/nitrogen species (ROS/RNS), always trying to lose or gain an electron to achieve stability. They have an important role in cell signaling, apoptosis, gene expression and ion transportation. They must be converted into less reactive substances in order to avoid damaging to cell membranes, enzymes and cell nuclear material, that can led to an increased risk of cardiovascular disease, cancer autism and other diseases. ROS has such high significance that it has been considered to be a component of virtually every disease process (Dalle-Donne et al., 2003; Lü et al., 2010).
The generation of ROS may damage all types of biological molecules. Lipids for example are very susceptible because oxidation of polyunsaturated fatty acids proceeds as a self-perpetuating chain reaction – oxidation of polyunsaturated fatty acids produces hydroperoxides, which also damage cell tissues and more lipid free radicals (McDonald et al., 2010). In protein, the damage is greater as proteins are often catalysts rather than stoichiometric mediators (Dalle-Donne et al., 2003).
In order to maintain cell integrity, there are cells protection mechanisms that are provided by the antioxidant system – vitamins and enzymes containing trace elements. Superoxide dismutase eliminates superoxide radicals formed in the cell and prevents the reaction of the radical with biological membranes or their participation in the production of more radicals; GSH-Px and catalase detoxifies lipid hydroperoxides that are formed in the membrane during lipid peroxidation. Vitamin E is the main antioxidant, but works along with carotenoids and vitamin A and C (McDonald et al., 2010). Antioxidants can function directly be decreasing the oxidative damage by reacting with the free radicals or indirectly by inhibiting the activity or expression of free radicals generating enzymes or enhancing the activity or expression of intracellular antioxidant enzymes (Lü et al., 2010).
Protein carbonyl groups have been used as biomarkers of oxidative stress with positive results, because of its relative early formation and stability of carbonylated protein. The most common method for the measurement of protein oxidation is the measurement of protein carbonyl groups after its derivatisation with 2,4-dinitrophenylhydrazine (DNPH) using spectrophotometric assays (Dalle-Donne et al., 2003).
11
2.6 - Vitamin E
Vitamin E belongs to a family of naturally occurring, essential, fat-soluble vitamin compounds; it consists of four tocopherols and four tocotrienols (Jiang, 2014). All tocopherols and tocotrienols are potent antioxidants with lipoperoxyl radical-scavenging activities, (Brigelius-Flohé and Traber, 1999) but only tocopherols (with saturated lateral chain) have biological importance (McDonald et al.,2010).
Only until recently, most research on vitamin E has primarily focused on α-tocopherol (αT), because αT is the predominant form of vitamin E in tissues and low intake of this form results in vitamin E deficiency-associated ataxia (Brigelius-Flohé and Traber, 1999).
The four forms of tocopherols (α-; β-; γ- and δ-) are differentiated by their metil group and its position around the benzenic ring (Bondi, 1988).
A regular dietary source of vitamin E is necessary because it is not stored in the body in large amounts, but it is not a problem because this vitamin is widely distributed in foods, (McDonalds et al., 2010) nevertheless, vitamin E is dependent on adequate Se levels and since this nutrient level is normal there should be seldom deficiencies of vitamin E (Cullison and Lowrey, 1987).
Vitamin E and its metabolites have an impact on multiple regulatory pathways and may offer unique opportunities and advantage for prevention and treatment of diseases (Brigelius-Flohé and Traber, 1999). Vitamin E main functions are as an antioxidant – protects cells against oxidative damage, in association with GSH-Px and other vitamins and trace elements. It donates a hydrogen atom to the free radical in order to form a stable molecule, and its requirement increases with the increasing concentrations of polyunsaturated fatty acids in the diet. Vitamin E is also very important in the development and function of the immune system and in the regulation of cell signaling and gene expression (McDonald et al., 2010).
Previous studies with dietary vitamin E incorporation in livestock feed have shown positive results, vitamin E is known to accumulate within cell membranes, and preserving its integrity by preventing membrane phospholipids oxidation and thus improving meat oxidative stability (Dalle Zotte et al., 2000; Descalzo and Sancho, 2008; Ebeid et al., 2013).
With respect to the rabbits, Castellini et al. (1998) had conducted a study about the effect of vitamin E on the oxidative stability of the rabbit meat and concluded that despite dietary
12 vitamin E did not influenced weight gain, feed intake and dressing yield, it improved the physical traits of the meat by reducing shear value and increasing water-holding capacity. Dalle Zotte et al. (2000) also concluded that vitamin E has positive effects preventing oxidative damage and increasing shelf-life of stored rabbit meat.
2.7 - Olive leaves in animal feeding
Olive tree (Olea europaea) is one of the most economically valuable trees in the Mediterranean countries (Loreto et al., 2003). The utilization of olive by products (residues derived from olive tree cultivation and olive processing, mainly leaves and olive cakes) is a ecofriendly activity. As olive oil production grows (in 2010 the olive oil production in Europe was about 2,136,000 tons, and it has grown 78% between 1990 and 2010) also grows the olive mill wastes which imply severe environmental problems (Bhatnagar et al., 2014). These wastes are difficult to handle because of their high phenol, lipid and organic acid concentrations that turn them into phytotoxic materials that possess strong antimicrobial activity (Zervakis and Balis, 1996). On the other hand these wastes are valuable resources for their content in nutrients and organic matter that could be recycled (Roig et al., 2006).
Olive oil industry results in 35kg of solid waste and 100L of liquid wastewaters per 100kg of treated olives (Zervakis and Balis, 1996) and each year the olive pruning results in 25Kg of twigs and leaves per tree per year and in olive oil extraction, it represents 5% of the weight of olives. There are plenty alternative utilizations of these by-products, one of which is their utilization as animal feeding complement for its source of nutrients. OL are known to be a source of antioxidant compounds and are used in teas than to its diuretic, antihypertensive and antioxidant effects (Molina Alcaide and Nefzaoui, 1996; Roig et al., 2006).
Olive oil has always been recognized as important components to a healthy diet. The Mediterranean diet accents on incorporation of various fresh vegetables, beans, lean meat and the traditional use of olive oil. This diet has been recognized by promoting longevity and for its positive role in preventing obesity, cardiovascular disease, diabetes and cancer (Huang and Sumpio, 2008).
13 Numerous reports describe the beneficial properties of olive oil thanks to its 30 phenolic compounds that are strong antioxidants and radical scavengers (mainly oleuropein, hydroxytyrosol and tyrosol) (Tuck and Hayball, 2002).
Beyond the well-known benefits of olive oil, also leaves have proved its nutritional benefits, the phenol compounds existent in olive leaves are associated to antioxidant, antihypertensive, hypoglycemic, hypocholesteroemic and cardiopreventive activity (Özcan and Matthäus, 2016).
Ribeiro et al. (2012) reported the olive leaves composition from an oil-processing mill in the North of Portugal, shown in Table 3, but it is important to refer that its composition varies qualitatively and quantitatively depending on variety, water deficiency, salinity, fertilization geographical zone, period of the year, climatic conditions, pruning period, tree age, etc. (Molina Alcaide and Nefzaoui, 1996; Özcan and Matthäus, 2016). Olive leaves are considered fibrous with a low digestibility, especially of crude protein and must be adequately supplemented in animal feeding (Molina-Alcaide and Yáñez-Ruiz, 2008).
Table 3 – Chemical composition and major phytochemicals in olive leaves
Olive Leaves Chemical composition g/Kg dry matter
Dry matter (as feed basis) 916.0
Organic matter 909.0
Crude protein 94.0
Crude fat 40.0
Neutral detergent fiber 503.0
Starch 28.0
Phytochemical mg/g dry matter
Simple phenolics 0.537
Flavonoids 4.905
Iridoids 7.099
(Adapted from: Rbeiro et al., 2012)
Olive leaves have been studied for the incorporation in animal feeding for broiler chickens (Shafey et al., 2013), pigs (Paiva-Martins et al., 2009) and rabbits (Trebušak et al, 2014). García et al. (1996) referred that olive leaves inclusion in rabbit feeds up to 240g/Kg could decrease the digestibility values, due to its high fiber content (Molina-Alcaide and Yáñez-Ruiz, 2008), and increase voluntary intake. The amount of phytochemicals of olive leaves,
14 namely polyphenolic compounds, also contribute to the reduction in digestibility, but have not showed evident effects on rabbit performance (Ribeiro et al, 2012).
On the other hand it is important to know that olive leaves supplementation on rabbit meat have already showed small positive results in reducing the oxidative process in stored rabbit meat (Trebušak et al., 2014) and in pigs it resulted in increasing in back fat and intramuscular α-T content (Paiva-Martins et al., 2009).
2.8 - The importance of selenium in nutrition
2.8.1 - Selenium characterization
Se with atomic number 34, was discovered by the chemist Jons Jakob Berzelius in 1817, and since then it has been an controversial matter, firstly it was thought to be toxic and carcinogenic subsequently it was shown to be essential and anticarcinogenic (O’Dell and Sunde, 1997).
In 1935 selenium was identified as the toxic factor in forage causing a disease known as “Alkali disease”, but around 15 years later it was starting to be discovered that animals grazing in low selenium soils suffered from retard growth and reproductive problems that could be overcome by the feeding of the element. Later in the 70’s it was discovered that the element was a component of antioxidant enzymes like GSH-Px (McDonald et al., 2002) which catalyses the removal of toxic peroxides in the tissues (Cullinson et al., 1987), thereby protecting the cells from oxidative damage.
Today it’s known for being an essential mineral to human and animal health, most of all for its antioxidant activity, selenium works in concert with vitamin E in the maintenance of normal cell functions and membrane health (Cullinson et al., 1987), but it’s also important for its therapeutic aspects and anti-inflammatory and anti-viral properties (Rayman, 2000).
2.8.2 - Selenoproteins
Se occurs in two forms, the organic and the inorganic one. The entry point of Se in animals is via plants, which absorb the element from the soil. Se availability in the soils vary from
15 region to region, Byers et al. (1936) suggested that selenium in soils is derived manly from the volcanic gases or sublimates, and is carried into the soils by rain and there held in a highly insoluble form.
In plants, Se is converted in organic forms such as methylated low-molecular-weight selenium compounds and the aminoacids selenomethionine (SeMet) and selenocysteine (Sec). Se is mainly absorbed in the duodenum by enterocytes through aminoacid transport systems and catabolised into elemental selenium that gets incorporated into GSH-Px (Rayman, 2008; Fairweather-Tait, 2011). Then selenoproteins are transported into the liver, where they are converted into and distributed to various organs like brain, kidney, heart, spleen, muscles and gonads (Fairweather-Tait, 2011).
Se is the structural component of various selenoproteins, such as GSH-Px and thioredoxin redutases (TrxR) wich are part of the antioxidant defense of cells, iodothyronine deiodinases (DIO) an enzyme which converts the inactive precursor of thyroxine, tetraiodothyronine (T4) into the active form, tri-iodothyronine (T3), selenophosphate synthetases (SPS2) (Behne and Kyriakopoulos, 2001) and others like 15-kDa selenoprotein/Sep15, SelH, SelI, SelK, SelM, SelN, SelO, SelP/SepP, SelR, SelS, SelT, SelV and SelW (Papp et al.,2007). Table 4 details known selenoproteins that are responsible for nutritional functions of selenium.
16
Table 4 – nown selenoproteins that carry out nutricional functions of selenium
In humans, the biological roles of Se include the protection of tissues against oxidative stress, the maintenance of the body’s defense systems against infection, cancer prevention, and the modulation of growth and development, which are not exclusively linked to enzyme functions (FAO/WHO, 1998; Rayman, 2000).
Selenoprotein Function
Glutathione peroxidases
Antioxidant enzymes: remove hydrogen peroxide, and lipid and phospholic hydroperoxides (thereby maintaining membrane integrity, modulation eicosanoid synthesis, modifying inflammation and likelihood of propagation of further oxidative damage to biomolecules such as lipids,
lipoproteins and DNA) (Sperm) mitochondrial
capsule selenoprotein
Form of glutathione (GSH-Px4): Shields developing sperm cells from oxidative damage and later polymerises into strututral protein required for
stability/motility and mature sperm Iodothyronine
deiodinases
Prodution and regulation of level of active thyroid hormone T3, from Thyroxine T4
Thioredoxin redutases
Reduction of nucleotides in DNA synthesis; regeneration of antioxidant systems; maintenance of intracellular redox state, critical for cell viability
and proliferation; regulation of gene expression by redox control of binding of transcriptions factors to DNA
Selenophosphate synthetase, SPS2
Required for biocynthesis of selenophosphate, the precursor of selenocysteine, and therefore for selenoprotein synthesis Selenoprotein P Found n plasma associated with endothelial cells. Appears to protect
endothelial cells against damage from peroxynitrite Selenoprotein W Needed for muscle function
Prostate epithelial selenoprotein (15kDa)
Found in epithelial cells of ventral prostate. Seems to have redox function (resembles GSH-Px4), perhaps protecting secretory cells against
development of carcinoma DNA-bound spermatid
selenoprotein (34k)
Gluthatione peroxidase-like activity. Found in stomach and in nuclei of spermatozoa. May protect developing sperm
18Da Selenoprotein Important selenoprotein, found in kidney and large number of other tissues. Preserved in Se deficiency
17
2.8.3 - Selenium deficiency and toxicity
Usual diets in most countries satisfy selenium requirements, but Se deficiency is an important problem in some regions where the soils have a low content of this nutrient or where it is not bioavailable (between the inorganic forms, selenate is more mobile, soluble and less-well adsorbed than selenite) (Fordyce, 2013).
The concentration of the nutrient in the soil is mainly controlled by the underlying geology and its bioavailability is dependent on pH, redox conditions, amounts of organic matter in the soil, competing ionic species like sulfate, microbial activity, soil texture, compaction and mineralogy, soil temperature and level of rainfall during the growing season. The Se uptake by plants can be inhibited by high soil content of organic matter, iron hydroxides and clay minerals, that can adsorb or bind Se, so even soils containing adequate concentration of selenium can still produce selenium-deficient crops (Fordyce et al.,2000).
Se deficiencies in crops and livestock have been reported in all regions of the world like USA, UK, Finland, Denmark, Sri Lanka, New Zealand, Australia, India, Canada, Thailand, Africa and China, and it is associated to diseases like white muscle disease (WMD) that causes degeneration of the muscles, reduces appetite, affects growth and reproductive capacities and causes embryonic deformities; Keshan disease a fatal cardiomyopathy and Kanish-Beck disease a bone and joint condition that causes deformities. It also affects thyroid hormone metabolism (detrimental to growth and development), immune system function and reproduction and it is associated with cancer (Fordyce et al.,2000).
Seleniferous areas have been reported in Ireland, Israel, Australia, Russia, Venezuela, China, USA and South Africa (Fordyce, 2013) and are related with Se toxicity, some signs of it are hair and nail/hoof loss (Whanger et al, 1996) it’s known as Alkali disease. High doses of Se also have a negative effect on DNA integrity and repair and may have a carcinogenic effect (Papp et al., 2007).
Sensitive biochemical markers to prevent selenium intoxication have yet to be developed. In their absence, it is suggested that the upper tolerable nutrient intake level for selenium should be set at 400mg/day for adults. It is noteworthy that a maximum tolerable dietary concentration of 2mg/kg dry diet has been proposed for all classes of domesticated livestock and has proved satisfactory in use (Commission on Natural Resources, 1998). This suggests that the proposed upper tolerable level of 400mg/day for human subjects provides a fully
18 adequate margin of safety. The upper tolerable level for children and for pregnant or lactating women has yet to be determined (FAO/WHO, 2004).
2.8.4 - Human Selenium intake
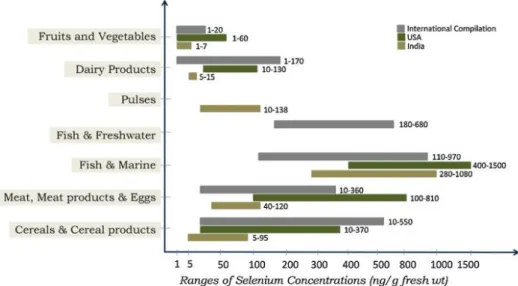
As previously mentioned, Se supply to humans comes from animal and vegetable sources and its availability is different depending if it is protein Se (80% absorption) or mineral Se (30% absorption). Good sources of Se are muscle meat, cereals, grains, eggs, vegetable and dairy products, sea food and edible organs are the richest source of Se (Rayman, 2008; Yamashita 2010) and even though brazil nuts are the richest source of Se, it is neither readily available nor commonly eaten (Rayman et al., 2008).
Various sources and levels of selenium are depicted in Figure 2, however the quantities may vary widely according to the soil content of selenium in different continents.
Figure 2 - Various source of selenium and its content levels
(Mahalingam et al., 1997)
Se needs can be affected by several factors like age, pregnancy, vitamin E supply, oxidative stress and growth (Dhur et al., 1990) and according to FAO/WHO (2006), usually diets in most countries satisfy Se requirements and in the case of industrialized countries, meat provides about half of the dietary Se.
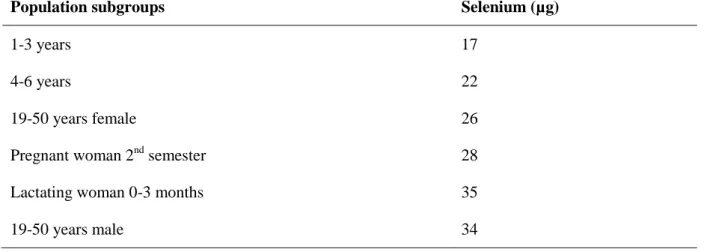
19 Table 5 shows the recommended nutrient intake for Se according to WHO (2004) , and it is important to keep in mind that WHO and FAO consider that it is neither essential nor desirable to maintain selenium status at a level which fully saturates blood GSH-Px activity.
Table 5 – Recommended selenium intae for selected populations sub-groups
(Adapted from, WHO, 2004)
In addition to dietary sources, Se is widely available as supplements and multivitamins, in inorganic and organic forms, in the form of SeMet or selenized yeast (Klein, 2009).
There are various possible forms of Se supplementation: sodium selenite, sodium selenate, selenomethionine, selenium-sulfide, etc. According to Dhur et al. (1990), SeMet seemed efficiently metabolized and incorporated into human tissues and quickly increases blood Se levels, and remain longer in the body and thus theoretically pose a higher risk of toxicity (FAO/WHO, 2006).
2.8.5 - Selenium treatment in Olive europaea L.
The content of Se in plants can be increased in different ways: by adding Se to the soil, soaking seeds in a Se solution before sowing, hydroponic and aeroponic cultivation in a nutrient solution containing Se, and the foliar application of Se solutions to plants (Zhao and Shewry, 2011).
Population subgroups Selenium (µg)
1-3 years 17
4-6 years 22
19-50 years female 26
Pregnant woman 2nd semester 28
Lactating woman 0-3 months 35
20 It has been proved that Se treatment (namely sodium selenate) by foliar spray, in olive trees subjected to water stress, results photosynthesis and fruit yield increase, and also maintain the level of leaf water content (Proietti et al., 2013).
According to D’Amato et al. (2014), the technique of fortifying olive tree canopies with sodium selenate showed an increased of the content of Se in the tree. This treatment has many benefits as a way of increasing the content of Se in extra virgin olive oil (EVOO).
22
Chapter 3: Experimental Work
3.1 – Objectives
The objectives of this experimental work were to determine the influence of OL untreated or treated with sodium selenate on growth, performance, carcass characteristics and meat quality (its antioxidant compounds and its Se content) in growing rabbits.
3.2 – Materials and methods
3.2.1 - Animals and diets
The experimental protocol was devised according to the Italian directives (Gazzetta Ufficiale, 1992) on animal welfare for experimental and other scientific purposes, and the research was carried out at the experimental farm of the Department of Agricultural, Food and Environmental Sciences of the University of Perugia (Italy).
Thirty New Zealand White mixed-sex rabbits were weaned at 30 days of age, allocated into three homogeneous groups (10animals/group) in bicellular wire net cages (60 x 25cm length x 35 cm height) and subjected to three different isoenergetic dietary treatments (Table 6) for 35 days until the slaughter:
Control feed;
Control feed enriched with 10% olive leaves (OLfeed);
Control feed enriched with 10% olive leaves enriched with 2.17 mg/kg selenium (Se feed).
The feeding program was adjusted according to previous studies (Martens and Villamide, 1998; de Blas and Mateos, 2010) and no drug substances were addicted.
23
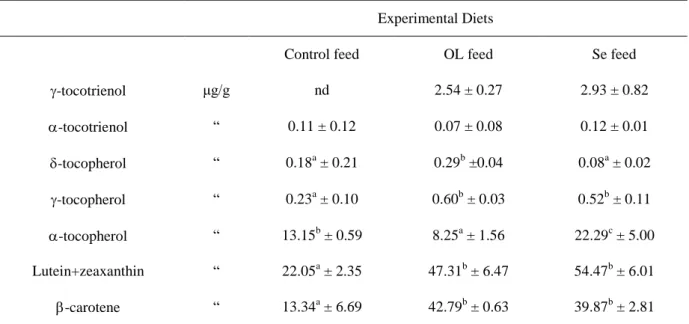
Table 6 – Formulation and chemical composition of feed and OL (% of wet weight)
Olive Leaves Experimental Diets Normal leaves Se leaves Control feed OL feed Se feed
Ingredients Alfa-alfa hay - - 45.00 38.00 38.00 Soybean meal 44% - - 14.50 14.50 14.50 Barley - - 31.00 31.00 31.00 Crusca - - 3.00 - -Soybean Oil - - 1.50 1.50 1.50 Olive Leaves - - - 10.00 10.00 Calcium-phosphate - - 1.40 1.40 1.40
Vitamin mineral premix* - - 1.20 1.20 1.20
Molasses - - 1.35 1.35 1.35 Salt - - 0.70 0.70 0.70 Calcium-Carbonate - - 1.00 1.00 1.00 DL-methionine - - 0.05 0.05 0.05 Chemical Composition Dry matter 92.29 92.98 89.26 88.69 88.62 Ether extract 2.24 2.54 2.58 2.86 2.92 Crude protein 10.74 10.28 15.14 15.20 15.29 Ash 5.29 5.16 8.38 8.03 8.22 Crude Fiber 14.78 13.90 14.47 12.78 12.78 NDF 34.98 30.88 30.59 26.95 26.91 ADF 27.60 22.66 19.77 17.59 17.41 ADL 15.35 12.50 3.92 3.64 3.72
* Added per kg: vit. A U.I. 11.000; vit. D3 U.I. 2.000; vit. B1 mg 2.5; vit. B2 mg 4; vit. B6 mg 1.25; vit. B12 mg 0.01; vit. E mg 25; biotine mg 0.06; vit. K mg 2.5; niacine mg 15; folic ac. mg 0.30; D-pantotenic ac. mg 10; coline mg 600; Mn mg 60; Cu mg 3; Fe mg 50; Zn mg 15; I mg 0.5; Co mg 0.5; lysine mg 50; methionine mg 40.
NDF : Neutral Detergent Fiber; ADF: Acid Detergent Fiber; ADL: Acid Digestible Lignin.
The OL used in the diets were descendant from Leccino varieties, with approximately 30 years old located in the Deruta municipal, Umbria, Italy. The soil was covered with permanent grass and the vase training system was used. Estimar o valor energetic da dieta
24 It was applied 3 treatments, mid July, mid August and mid September of sodium selenate solution (foliar spray), 100mg/L per plant.
Water was supplied ad libitum during the assay and the applied temperature and lighting schedules in the rabbit house were 15–18 °C and 16 L:8 D, respectively.
3.2.2 - Productive performance
The feed intake of rabbits was recorded daily and the live weight was recorded weekly to determine the growth, the feed intake and the feed efficiency (which was calculated as the ratio between the daily intake and the daily weight). The mortality was also controlled daily.
3.2.3 - Blood sampling and analysis
Blood samples (2.5mL) were collected from each animal immediately before slaughter from the marginal ear vein and were collected in vacuum containers and transported to the laboratory of the Department of Agricultural, Environmental and Food Science, at University of Perugia. Serum was obtained from blood samples coagulated at room temperature for 2 hours and then the collection tubes were rimmed and refrigerated at 4 °C for 24 hours before analysis. Plasma was obtained from blood samples collected into tubes containing Na2-EDTA
and immediately centrifuged at 2,500 G for 10 min at 4 °C to determine the haematic parameters.
TBARS of plasma: It was used 200 µL plasma with 2000 μL sol. A [0.2% TBA in acetate buffer, 2M, pH 4 (20:51 g Na-acetate dissolved in a few mL H2Od holder with acetic acid at a temperature of 50 °C under stirring, pH 4; add 49.16 mg of DPTA and brought volume to 125 mL with H2O. 100 mL were taken of this solution and dissolved 200 mg of TBA]. After vortex, the sample was put in incubate at 95 ° for 45 min.
After cooling and centrifuged at 10000g x 5’ it was withdrawal and transferred 1500μL in cuvettes and read at 532nm against the white (sol A). The values were expressed as nmol malondialdehyde (MDA)/mL of sample (Dal Bosco et al., 2009).
PGC of plasma: The detection of protein carbonyl groups followed the method of Dalle Donne et al. (2003). The plasma was diluted 1:40 with phosphate-buffered saline solution
25 (PBS) containing 10 mM sodium phosphate, pH 7.4, and 0.14 M NaCl, and this was centrifuged twice (5–10 min at 14,000 rpm in a tabletop microcentrifuge) to eliminate all particulate matter that might interfere with the reaction. The diluted proteins were precipitated with cold trichloroacetic acid (TCA, 20% final concentration) and then collected by centrifugation for 3–5 min. A solution of 10 mM DNPH in 2 N HCl was added to the protein pellet of each sample to give a final protein concentration of 1–2 mg/ml, with 2 N HCl only added to corresponding sample aliquot reagent blanks. Samples were allowed to stand in the dark at room temperature for 1 h with vortexing every 10 min; they were then precipitated with 10–20% TCA (final concentration) and centrifuged for 5 min. The supernatants were discarded, the protein pellets were washed once more with 10–20% TCA, and then washed three times with 1 ml portions of ethanol/ethyl acetate (1:1, v/v) to remove any free DNPH. Samples were then resuspended in 6 M guanidine hydrochloride (GdmCl, dissolved in 2 N HCl or in 20mM phosphate buffer, pH 2.3) at 37°C for 15 min with vortex mixing. Carbonyl contents were determined from the absorbance at 366 nm using a molar absorption coefficient of 22,000 M 1 cm. 1. The results were expressed as nmol/mg of proteins.
3.2.4 - Slaughtering, carcass dissection and meat sampling
At 65 days of age the rabbits were stunned and slaughtered by cutting the carotid artery and jugular veins, they didn’t underwent any kind of transportation
In animal slaughtered the caecal pH was measured in situ by direct insertion of a pH glass electrode with room temperature.
The slaughtering and carcass dissection procedures followed the World Rabbit Science Association (WRSA) recommendations described by Blasco and Ouhayoun (1996). The Longissimus dorsi muscles (LD) (between the 1st and 7th lumbar vertebrae) were excised from the two sides of refrigerated carcasses (24 h at 4°C), trimmed of all external fat and epimisyal connective tissue, and frozen at -80 °C for analyses of lipid profile, oxidative state and antioxidants content. There was also weighed the interscapular and perivisceral fat, liver and kidneys.
Physical characteristics of the LD: The LD were carefully removed, weighted and analyzed for color and pH.
26 The pH was measured 24 hours post mortem with a Phmeter Knick digital (Broadly Corp. Santa Ana CA, USA) by stabbing at the 7th-8th thoracic vertebra. The water holding capacity (WHC) was estimated (Nakamura and Katoh, 1985) after centrifugation of 1g of muscle to 4 minutes at 1,500 x g. The remaining water after centrifugation was quantified by drying the sample over night at 70 ° C. The WHC was calculated as follows: (sample weight after centrifugation – weight after drying) x 100 / initial weight.
The color parameters were determined on the muscle using an analyzer tristiomolo (Minolta Chroma Metre CR-200, Azuchi-Macgi Higashi-Ku, Osaka 541, Japan) with CIELAB color system (1976), the which gives the average of three subsequent measurements of brightness (L *), the parameter of the red (a *) and yellowness (b *).
About 10g of LD sample was cooked in the oven at 198ºC for 15 minutes, and after it was calculated the weight difference in order to determine the cooking loss (Mattioli et al., 2003)
3.2.5 - Analytical determinations of feed, leaves and meat
Chemical analysis: The chemical composition of the feed and leaves (moisture, protein, lipid, ash) was determined according to the Association of Analytical Chemists methods (AOAC, 1995). The crude fiber, neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) were analyzed according to Van Soest et al. (1991).
The meat proximal composition (moisture, protein, lipid and ash) was performance, according to AOAC (2000). Briefly, the moisture content was determined by oven drying meat samples (125ºC for 24h) (method 950.46). The fat content was determined gravimetrically using ether solvent extraction (method 960.30). The protein content was obtained multiplying the total Kjeldahl nitrogen (method 992.15) with a coefficient factor of 6.25. The ash content was determined using muffle furnace at 600ºC (method 923.03)
Tocopherols and Carotenes: The tocopherol (αT and its isomers β+γ and δ) and carotenoid contents of the feed and meat were quantified by HPLC according to the method described by Hewavitharana et al., (2004). 5 ml of distilled water and 4 ml of ethanol were added to 2 G of feed and leaves and 3g of meat samples and vortexed for 10 sec. After mixing, 4 ml of hexane containing BHT (200 mg/l) was added and the mixture was carefully shaken and centrifuged at 8,000 G for 10 min. An aliquot of supernatant (3 ml) was dried under a stream of nitrogen
27 and dissolved in 200 μl of acetonitrile; 50 μl were injected into the HPLC (pump model Perkin Elmer series 200, equipped with an autosampler system, model AS 950-10, Tokyo, Japan) on an Sinergy Hydro-RP column (4 µm, 4.6 x 100 mm; Phenomenex, Bologna, Italy). Tocopherols were identified using a FD detector (model Jasco, FP-1525 - excitation and emission wavelengths of 295 nm and 328 nm, respectively) and quantified using external calibration curves prepared with increasing amounts of pure tocopherols in ethanol. Retinol was identified using a UV-VIS spectrophotometer detector (Jasco UV2075 Plus) set at λ 325 nm and quantified by comparing the sample with a pure commercial standard in ethanol (Sigma-Aldrich, Steinheim, Germany; Extrasynthese, Genay, France).
3.2.6 - Oxidative status of the meat
Oxidative status of LD: Lipid extraction was performed according to the method described by Foch et al. (1957). 1mL of lipid extract was evaporated under a stream of nitrogen and the residue was derived by adding 3mL of sulfuric acid (3% in methanol). Following incubation at 80ºC for 1 hour. Lipid oxidation was evaluated with a spectrophotometer set at 532 nm (Shimadzu Corporation UV- 2550, Kyoto, Japan) that measured the absorbance of thiobarbituric acid-reactive substances (TBARs) and a 1,1,3,3-tetraethoxypropane calibration curve (Ke et al., 1977). Oxidation products were quantified as malondialdehyde equivalents (mg MDA/kg muscle).
PGC of LD: Carbonyl derivatives of proteins were detected by Lushchak et al. (2005) Methods. Briefly the pellets from TCA extracts were mixed with 1mL of 10mM 2,4-dinitrophenylhydrazine (DNPH) in 2M HCl. Control samples contained 1ml of 2M HCl instead the DNPH solution. Samples were incubated for 1 h at room temperature, then centrifuged as above. Supernatants were discarded and pellets were washed three times with 1ml of ethanol–butylacetate (1:1 v/v) in order to remove unreacted DNPH. Pellets were then dissolved in 1.5mL of 6M guanidine-HCl and centrifuged as above to pellet insoluble particles. The amount of PGC in the resulting supernatants was evaluated spectrophotometrically at 370nm using a molar extinction coefficient of 22 × 103M−1cm−1 (Lenz et al., 1989). The values were expressed as nanomoles of CP per protein milligram in the guanidine chloride solution. From the same TCA extract it was also evaluated the thiols
28 groups with the 5,5’dithio-bis (2-dinitrobenzoic acid) assay and it was used as extinction coefficient 13.6 * 103 M/cm and expressed as µmol SH – group/g.
3.2.7 - Analyses of selenium in LD muscle
Selenium concentration: For sample preparation, the previously lyophilized meat, was weighted and then suffered an acid digestion with a mixture of HNO3 and H2O2 (8:2, v/v)
(Method EPA3051). A volume of 8ml concentrated nitric acid and 2ml of hydrogen peroxide (30vol) were added to each vessel and the following heating program was applied: 1st step – 15min to 200°C, 2nd step – 15min to 200°. All steps were performed at 1000W of applied power. Maximum pressure was set at 120 bar.
The determination of Se in the digested materials was accomplished by using an atomic absorption spectrophotometer, equipped with graphite furnish and a deuterium lamp (Shimadzu AA-6800, GF-AAS, “Shimadzu Crop.”, Tokyo, Japan). The background correction was done using a matrix modifier (Pd (NO3)2, 0.5mol M in HNO3).
3.2.8 - Statistical analyses
For the statistical analysis it was used the ANOVA procedure with a linear model as diet with fixed effect using commercially available software program (SPSS 21.0 Chicago Illinois, USA, 2002). Multiple comparisons were performed using Bonferroni’s range test and significance was set at P<0.05.
3.3 – Results and discussion
Two rabbits died during the experiment, one from the standard diet group and other from the standard fed enriched with olive leaves with selenium.
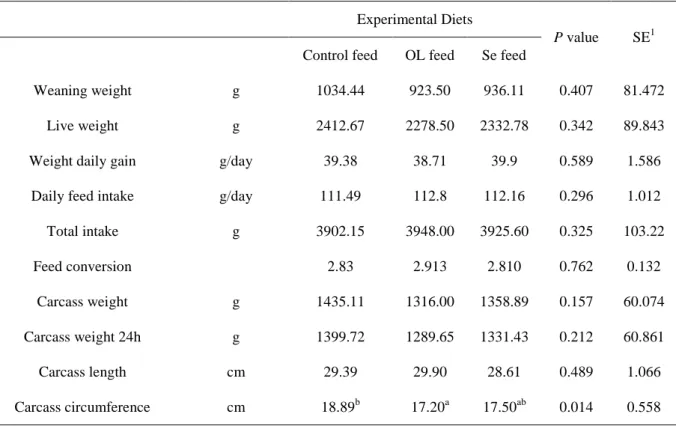
About the antioxidant compounds in OL (Table 7) it can be observed that in a general way the Se enriched OL have higher concentration (P<0.05) of tocopherol isofroms (δ-tocopherol, γ-tocopherol and αT), carotene and lutein + zeaxanthin. This result proves the positive influence of Se in the antioxidant content.
29
Table 7 –Antioxidant compounds (mean ± sd) contents of OL
Olive Leaves
Normal leaves Se leaves
-tocotrienol μg/g 5.88 ± 0.78 13.73 ± 6.94 -tocotrienol “ 0.14 ± 0.01 0.10 ± 0.02 -tocopherol “ 0.43a ± 0.55 1.90b ± 0.20 -tocopherol “ 2.04a ± 0.38 5.92b ± 1.06 -tocopherol “ 29.76a ±4.21 67.71b ± 3.45 Lutein+zeaxanthin “ 115.53a ± 8.08 229.78b ± 16.54 -carotene “ 79.95 ± 10.54 120.46 ± 56.18 a, b : P<0.05
Regarding the differences between OL and Se enriched OL, it would be expected that that the antioxidants in the Se feed were also higher than the antioxidants in OL. Nevertheless in the antioxidants of the diets (Table 8) Se feed only had the highest concentration for the α-tocopherol and lutein+zeaxanthin, while OL feed had the highest level of δ-α-tocopherol, γ-tocopherol and β-carotene.
Table 8 – Antioxidant compounds (mean ±sd) contents of experimental diets
Experimental Diets
Control feed OL feed Se feed
-tocotrienol μg/g nd 2.54 ± 0.27 2.93 ± 0.82 -tocotrienol “ 0.11 ± 0.12 0.07 ± 0.08 0.12 ± 0.01 -tocopherol “ 0.18a ± 0.21 0.29b ±0.04 0.08a ± 0.02 -tocopherol “ 0.23a ± 0.10 0.60b ± 0.03 0.52b ± 0.11 -tocopherol “ 13.15b ± 0.59 8.25a ± 1.56 22.29c ± 5.00 Lutein+zeaxanthin “ 22.05a ± 2.35 47.31b ± 6.47 54.47b ± 6.01 -carotene “ 13.34a ± 6.69 42.79b ± 0.63 39.87b ± 2.81 a, b, c : P<0.05
30 It is important to notice that in general, the concentration of antioxidant compounds was higher in the feed where it was added OL, this showed positive expectation about the antioxidant power of OL regardless if enriched with Se or not. It is known that carotenes in the diet may have a high scavenging power against free radicals and have a inhibitory power against lipid in vivo peroxidation (Bub et al., 2000; Osganian et al., 2003).
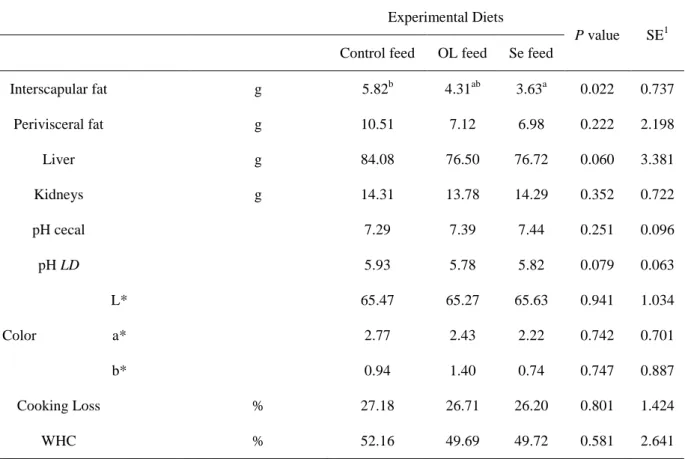
In general, the growing/productive performance of growing rabbits fed the experimental diets was not affected as observed in Table 9.
Table 9 – Productive performance of growing rabbits fed experimental diets
Experimental Diets
P value SE1
Control feed OL feed Se feed
Weaning weight g 1034.44 923.50 936.11 0.407 81.472
Live weight g 2412.67 2278.50 2332.78 0.342 89.843
Weight daily gain g/day 39.38 38.71 39.9 0.589 1.586
Daily feed intake g/day 111.49 112.8 112.16 0.296 1.012
Total intake g 3902.15 3948.00 3925.60 0.325 103.22 Feed conversion 2.83 2.913 2.810 0.762 0.132 Carcass weight g 1435.11 1316.00 1358.89 0.157 60.074 Carcass weight 24h g 1399.72 1289.65 1331.43 0.212 60.861 Carcass length cm 29.39 29.90 28.61 0.489 1.066 Carcass circumference cm 18.89b 17.20a 17.50ab 0.014 0.558 a, b : P<0.05
1SE: standard error
OL feed diet showed no significant (P<0.05) differences on feed intake of rabbits, suggesting that it didn’t have adversely affected the palatability of the diet and therefore its consumption, this was also shown in previous studies (Dal Bosco et al., 2004; Trebušak et al., 2014). This feed also resulted in a significantly lower carcass circumference regard control feed, this could be related with the carcass weight, and although there were no significant differences the mean values varied between groups
31 In previous studies in growing pigs the addiction of OL reduced feed intake and body weight (Paiva-Martins et al., 2009), in broiler chickens increasing the dietary level of OL reduced body weight gain (Shafey et al., 2013) and in previous studies with rabbits it has been reported that the OL diet has no effects on animal growth or feed intake and efficiency (Trebušak et al., 2014; Ribeiro et. al., 2012).
The nutritional recommendation of Se for rabbits is 0,05mg Se/kg diet (De Blas and Mateos, 2010). Due to this value it is not surprising that Se supplementation on Se feed did not significantly influence the rate of living weight or carcass yield This results are in agreement with the results of Dokoupilová et al., (2007) and Marounek et al., (2009), where the dietary Se supplementation on rabbits diets showed no effects on growing/productive performance. There are similar studies with other species like pigs (Kim and Mahan, 2001), lambs (Vignola et al., 2009; Sushma et al., 2015) and broiler chicken (Ryu et al., 2005) with data showing no influence regarding the growing/productive performance.
The chemical characteristics of the carcass of growing rabbits are shown in Table 10.
Table 10 –Chemical characteristics of the carcass of growing rabbits fed experimental diets
Experimental Diets
P value SE1 Control feed OL feed Se feed
Interscapular fat g 5.82b 4.31ab 3.63a 0.022 0.737 Perivisceral fat g 10.51 7.12 6.98 0.222 2.198 Liver g 84.08 76.50 76.72 0.060 3.381 Kidneys g 14.31 13.78 14.29 0.352 0.722 pH cecal 7.29 7.39 7.44 0.251 0.096 pH LD 5.93 5.78 5.82 0.079 0.063 Color L* 65.47 65.27 65.63 0.941 1.034 a* 2.77 2.43 2.22 0.742 0.701 b* 0.94 1.40 0.74 0.747 0.887 Cooking Loss % 27.18 26.71 26.20 0.801 1.424 WHC % 52.16 49.69 49.72 0.581 2.641 a, b : P<0.05
32 The carcass analysis showed significantly less (P<0.05) interscapular fat content in rabbits fed Se feed than in rabbits fed control feed, and it also resulted in less perivisceral fat content (although the differences were no significant). These results are in agreement with the results of Marounek et al., (2009). Shen et al. (2014) concluded that in mice fed OL extract diet significantly decreased visceral fat-pad weights and that this result appears to be mediated in part through down regulating the expression of molecules involved in thermo genesis in the visceral adipose tissue. However, this result may be directly correlated to the initial weight of rabbits, although the average weaning weight of groups were not statistical different, we can observe for example that the rabbits fed control fed were heavier at the weaning and showed a heavier carcass, which is related to the deposition of interscapular fat.
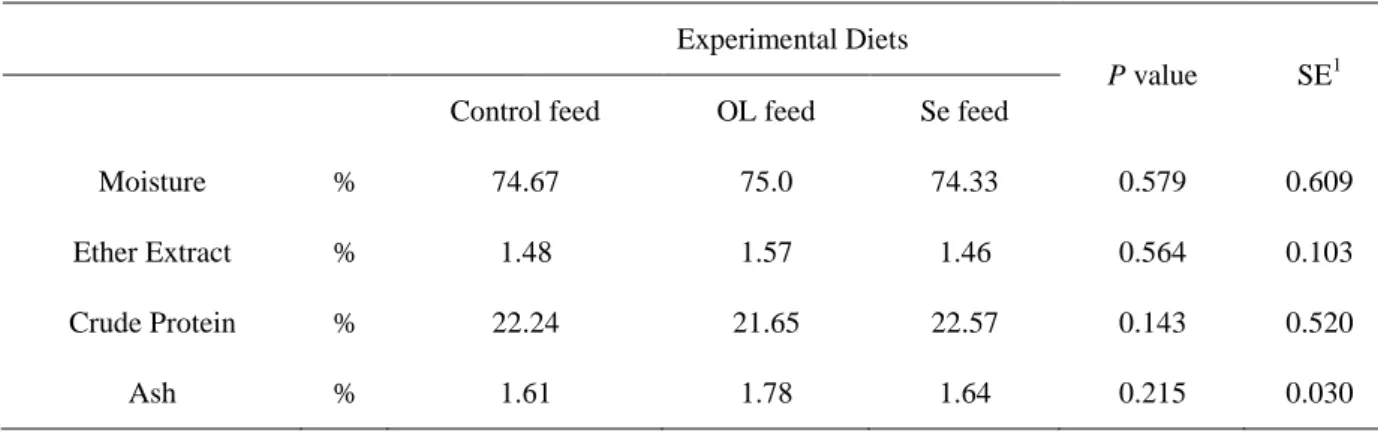
Regarding the chemical analysis of LD muscle, the data is shown in Table 11. As it can be observed, no differences were found for the moisture, ether extract, crude protein and ash parameters.
Table 11 – Chemical analysis of Longissimus dorsi meat of growing rabbits fed the experimental diets
Experimental Diets
P value SE1
Control feed OL feed Se feed
Moisture % 74.67 75.0 74.33 0.579 0.609
Ether Extract % 1.48 1.57 1.46 0.564 0.103
Crude Protein % 22.24 21.65 22.57 0.143 0.520
Ash % 1.61 1.78 1.64 0.215 0.030
1
SE: standard error
Table 12 and 13 shows the results relatively to the plasma and meat antioxidant compounds and status, analyzed using thiobarbituric acid reactive substances (TBARS) assay.
In the plasma lipid oxidative status (TBARS) there were no significant differences between groups. It is not surprising that there is not a direct relationship between the feed intake and the plasma results because many other factors could be interfered (for example the stress of the blooding sampling or the housing condition) and also because the plasma is a metabolic product Ebeid et al., (2013) obtained a significant increase of blood serum antioxidative properties when vitamin E or Se were supplemented in the diet.