Towards an understanding of ketamine teratogenicity
in zebrafish early development
A Thesis Submitted for the Degree of
Doctor of Philosophy in Chemical and Biological Sciences
LUÍS MANUEL LOURENÇO FÉLIX
Advisor: Doctor Ana M. Coimbra
Co-advisor: Prof. Dr. Luís Antunes
Towards an understanding of ketamine teratogenicity
in zebrafish early development
A Thesis Submitted for the Degree of
Doctor of Philosophy in Chemical and Biological Sciences
Candidate: Luís Manuel Lourenço Félix
Advisor: Doctor Ana Maria Monteiro Paiva Coimbra Postdoctoral researcher at the Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB) from the University of Trás-os-Montes and Alto Douro (UTAD)
Co-advisor: Prof. Dr. Luís Miguel Joaquim Marques Antunes Associate professor with aggregation at the School of Agricultural and Veterinary Sciences (ECAV) from the University of Trás-os-Montes and Alto Douro (UTAD)
Jury Composition:
President: Prof. Dr. Luís Herculano Melo de Carvalho
Professor at the Life Sciences and Environment School (ECVA) from the University of Trás-os-Montes and Alto Douro (UTAD)
Vowels: Prof. Dr. Francisco Manuel Pereira Peixoto
Associate professor with aggregation at the Life Sciences and Environment School (ECVA) from the University of Trás-os-Montes and Alto Douro (UTAD)
Prof. Dr. Miguel Alberto Fernandes Machado e Santos
Assistant professor at the Biology Department from the University of Porto (UP)
Doctor Paula Inês Borralho Domingues
Postdoctoral researcher at the Biology Department and at the Centre for Environmental and Marine Studies (CESAM) from the University of Aveiro (UA)
Doctor Ana Maria Monteiro Paiva Coimbra
Postdoctoral researcher at the Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB) from the University of Trás-os-Montes and Alto Douro (UTAD)
I, Luís Félix, declare that this thesis entitled, “Towards an understanding of ketamine teratogenicity in zebrafish early development” and the work presented in it are my own.
I confirm that:
• This thesis has not been and will not be, submitted in whole or in part to another University for the award of any other degree.
• The work presented in this thesis was done wholly while in candidature for a research degree at this University.
• Where published work of others was consulted, this was always clearly attributed.
• Where the work of others was quoted, the source was always given. With the exception of such quotations, this thesis is entirely my own work.
• All main sources of help were acknowledged.
• This thesis conforms to an ‘article format’ in which the middle chapters consist of discrete articles written in a style that is appropriate for publication in peer-reviewed journals in the field. The first and final chapters present synthetic overviews and conclusions of the field and of the research undertaken.
Signed:
Date: 04 / July / 2016
Any quotation from the thesis or use of any of the information contained in it must acknowledge this thesis or its publications as the source of the quotation or information.
The work presented in this thesis was conducted in partnership between the Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB) from the University of Trás-os-Montes and Alto Douro (UTAD), the Institute for Research Innovation in Health (i3s) and the Institute for Molecular and Cell Biology (IBMC) from the University of Porto (UP). This work was supported by European Investment Funds by FEDER/COMPETE/POCI – Operational Competitiveness and Internationalization Programme, under the projects POCI-01-0145-FEDER-006958 and FCOMP-01-0124-FEDER-028683 and National Funds by FCT - Portuguese Foundation for Science and Technology, under the projects PTDC/CVT-WEL/4672/2012 and UID/AGR/04033/2013.
To my Family, Lover(s) and Friends
“Nothing exists except atoms and empty space; everything else is opinion” Democritus Greek philosopher (460 BC - 370 BC)
Acknowledgements
A PhD experience is more than just performing experiments, analysing data and
getting published. In many ways, getting a PhD is like running a marathon.There must
be a balance between lifestyle, achievements and the desire to achieve the finish line. However, reaching the goal comes with a price: courage, determination, persistence,
dedication, flexibility, time and effort. Despite the overwhelming challenge that the
PhD/marathon presents, it can be overcome by the support from friends and family. There are many people that in some way have contributed to the achievement of this PhD. However, I hope people understand the brevity of the part in which the acknowledgments will not be made at the extent they deserve.
I would like to express my gratitude to my advisor, Doctor Ana M. Coimbra. She gave me enough freedom to pursue the research that led to this thesis. Because of that, I
have become more confident and have energy to face future life and work challenges.I
also acknowledge her feedback on this thesis.
I would like to express my deep appreciation to my co-supervisor, Prof. Dr. Luís Antunes, for giving me the opportunity to work with the Laboratory Animal Science group at the Institute for Molecular and Cell Biology. I also thank the practical and financial opportunities as well as his criticism and comments which have been arranged during this work.
My gratitude goes also to Ana Maria Valentim for her insightful comments and constructive criticisms at different stages of this research. Besides her friendship, I also thank her for the financial opportunities which have been arranged in the course of this thesis.
I am also appreciative of all the help from the undergraduates and master's, Cindy Serafim, Maria João Martins and Ana Vidal, who have helped in carrying out the research present in this thesis. To all of you I am thankful for significantly reducing the workload throughout this thesis.
A special thanks goes to Ana Luzio. For her friendship, for always being there, for her enthusiasm and optimism, for her support, for her energy, for all the good times we had, for the vivid experiences, for the conversations and confidences, and for many
other different reasons. It would have been a more challenging journey without her.I hope
this will be just the beginning of a long friendship.
I also want to acknowledge Dércia Santos and Sandra Pereira. Thanks for your friendship and help at the beginning of my PhD.
Dr. Manuela Matos, Doctor Ana Rita Álvaro and Prof. Dr. Fernando Nunes for their experimental assistance and constructive contributions.
I am also grateful to Sónia Campos and Stefanie Santos for the vivid experiences outside of the laboratory.
A special thanks goes also to Lia-Tânia Dinis, for her friendship, words, humour and “whatever”.
To my friends, Céu Sousa and André Polónia, a word of great honour for their friendly and fellowship words when needed. Also, a special thanks to Sérgio Vilela, my
academic “partner”, and Vânia Graça.To all of them I recognize the unforgettable human
qualities.
I gratefully acknowledge the lived moments on Fridays in “sala de bolseiros”. Joana Faria, Beatriz Ferreira, Vera Cardoso, Kelly Machado and Eugénia Teixeira thank you for these moments that helped to relieve the PhD tension. I am also grateful to Mariana Fernandes, for her strength, support and appreciation of my potential. I greatly value your friendship and I deeply appreciate your belief in me.
To the teachers at the University of Trás-os-Montes and Alto Douro who accompanied me and have always dedicated a little of their time to say a few friendly words, I highlight Prof. Dr. Francisco Peixoto, Prof. Dr. Maria Manuel Oliveira, Dr. Romeu Videira, Prof. Dr. João Carrola and Prof. Dr. Carlos Venâncio. To all of you, my most heartfelt thanks.
A word of appreciation also goes to the members of the Laboratory Animal Science group at the Institute for Molecular and Cell Biology.
I am also grateful to Cesaltina Carvalho, Helena Ferreira, Ana Fraga, Luís Ferreira, Donzília Costa, Carlos Matos at the University of Trás-os-Montes and Alto Douro for their various forms of support in the course of this thesis.
I would also like to thank other friends that somehow passed my way and helped in any way to this work. Ana Pinto, Marita Cardoso, Fátima Martins, Sofia Santos, Maria Themudo, Ana Santos, Enoque Dinis, Margarida Monteiro, Insaf Ayadi, Iva Prgomet, Elisabete Santos, Francisco Morinha, Márcia Carvalho, Miguel Ribeiro, Cátia Brito, Sara Bernardo, Ermelinda Silva, Luís Rocha, Ana Abraão, André Lemos and Sandrine Ferreira. Thank you for something. I also would like to thank all the people that I might have forgotten to mention for sharing time and that somehow have contributed to this work.
Words cannot express how grateful I am to my mother-in law, Júlia Leal, for her constant unconditional support.
I owe a special thanks to my family, my parents and my brother Carlos who supported me and helped me throughout my life and during this study. I dedicate this work to you all. A special thanks to my parents. I could not thank you enough for the opportunity to be where I am today and be as I am. You always stimulated me to keep on wondering and to believe in my potential.
I also dedicate this Ph.D. thesis to my grandparent, Manuel Lourenço, for the value he placed on Family. He has played an important role in the development of my personal identity and helped shape who I am today.
Finally, I would like to acknowledge the most important person in my life, Ana Leal. She has been a constant source of strength and inspiration that kept me going even in the worst moments of this thesis. Your determination and constant encouragement made it possible for me to see the light at the end of the tunnel. I can't thank you enough for your support, friendship, understanding, help, guidance, advice, care, concern and love. I love you for everything.
Luís Félix Vila Real, Portugal July 04, 2016
Resumo
A cetamina é um antagonista não-competitivo do recetor N-metil D-aspartato (NMDA). Este composto é um anestésico dissociativo e analgésico, utilizado clinicamente em seres humanos e em animais há mais de 40 anos. Devido a várias das suas propriedades clínicas, a cetamina é ainda amplamente utilizada, quando comparada com outros agentes anestésicos, com especial ênfase em situações de emergência em países em desenvolvimento. No entanto, e apesar de potenciais novos usos clínicos estarem atualmente a ser estudados, a utilização da cetamina tem sido limitada pelos seus potenciais efeitos secundários. Dados recentes sugerem que a cetamina pode causar a morte neuronal durante fases iniciais do desenvolvimento cerebral. Além disso, os riscos da utilização indevida da cetamina, nomeadamente a sua utilização como droga de abuso, têm sido relacionados com contaminação ambiental e potenciais riscos ecotoxicológicos. Tudo isto tem gerado preocupações sobre o uso e consumo de cetamina, que são revistas na introdução desta tese (capítulo I).
Os trabalhos desenvolvidos nesta tese têm por base a utilização de peixe-zebra (Danio rerio) que tem mostrado grande potencial em estudos sobre processos de desenvolvimento e de toxicidade de compostos. O peixe-zebra tornou-se um organismo modelo no âmbito da política dos 3Rs e uma alternativa à experimentação animal em investigação biomédica e ecotoxicologia. A presente tese apresenta uma visão geral sobre a utilização de fases iniciais de desenvolvimento de embriões de peixe-zebra, para elucidar os mecanismos subjacentes associados à toxicidade induzida por cetamina.
Com base em ocorrências farmacológicas, os embriões de peixe-zebra foram
expostos a cetamina (0,2; 0,4 e 0,8 mg mL-1), durante três fases diferentes do
desenvolvimento, por um período de 20 minutos. Os resultados deste trabalho são apresentados no capítulo II, divididos em cinco estudos. No capítulo II.1, são abordados os efeitos da exposição a cetamina durante a fase de blástula (2,5 horas após a fecundação - hpf). Verificou-se que a exposição a cetamina durante esta fase de desenvolvimento induz distúrbios nos processos normais de crescimento, induzindo malformações nos embriões e mortalidade. O capítulo II.2 prossegue o trabalho anterior e acrescenta o estudo dos efeitos da exposição a cetamina durante as fases de gástrula (5,5 hpf) e segmentação (10,5 hpf). Além dos parâmetros de desenvolvimento, a absorção de cetamina, o desenvolvimento de cartilagem e osso e a expressão de genes de desenvolvimento foram também avaliados. Em geral, este estudo mostra que o perfil de toxicidade da cetamina é dependente do momento de exposição, tendo sido identificada
molecular e bioquímica, dos parâmetros bioquímicos, como biomarcadores da toxicidade induzida por cetamina, é apresentado no capítulo II.3. A capacidade de resistência à desregulação de processos de stresse oxidativo foi avaliada nas diferentes fases de desenvolvimento. Apesar de não afetar o estado redox geral, a cetamina é capaz de interferir com diversos processos de stresse oxidativo, que foram relacionados com o desenvolvimento gradual do sistema de defesa antioxidante no embrião. Foram também analisados os efeitos da cetamina ao nível de processos de apoptose durante o desenvolvimento embrionário, resultados estes apresentados no capítulo II.4. Neste estudo, diferentes padrões de expressão de genes foram observados e relacionados com a ativação direta e indireta de apoptose durante períodos embrionários de suscetibilidade diferencial. Por fim, no capítulo II.5 são descritos os efeitos ao nível do comportamento, após exposição a cetamina. Este estudo mostrou uma tendência dependente da dose para
afetar as respostas relacionadascom medo e/ou ansiedade após exposição em fases mais
avançadas, tais como a gástrula e segmentação, indicando possíveis distúrbios neuro-comportamentais.
O capítulo III está subdividido em dois trabalhos nos quais se descrevem os impactos de concentrações de cetamina, relevantes do ponto de vista ambiental, (50; 70
e 90 mg L-1, LC50=94,4 mg L-1), após um período de exposição de 24 horas com início na
fase de blástula. No capítulo III.1 descrevem-se as malformações graves dependentes da dose à exposição da cetamina bem como os seus efeitos a longo prazo a nível comportamental, tais como um aumento da tigmotaxia. O capítulo III.2 prolonga os estudos iniciados no capítulo anterior demonstrando que além das alterações morfológicas, a exposição à cetamina foi capaz de induzir respostas de stresse oxidativo e apoptose através do envolvimento de vias dependentes do p53.
Por fim, no capítulo IV apresentam-se as conclusões gerais da tese acerca dos efeitos teratogénicos e embriotóxicos da exposição a cetamina em fases iniciais do desenvolvimento de peixe-zebra e os seus possíveis impactos. Orientações para futuros trabalhos são também discutidos.
Palavras-chave: Anestesia; Cetamina; Comportamento; Desenvolvimento; Expressão génica; Peixe-zebra; Stresse oxidativo;
Abstract
Ketamine is a non-competitive antagonist of the receptor N-methyl-D-aspartate (NMDA). This compound is a dissociative anaesthetic and analgesic, used clinically in humans and animals for over 40 years. Due to several of its clinical properties, ketamine is still widely used, when compared to other anaesthetic agents, with special emphasis on emergency situations in developing countries. However, despite new potential clinical uses are currently being studied, the use of ketamine has been limited by its potential side effects. Recent data suggest that ketamine may cause neuronal death during the early stages of brain development. Moreover, the risks of improper use of ketamine, including its use as a drug of abuse, have been linked to environmental contamination and potential ecotoxicological risks. All this has raised concerns about the use and consumption of ketamine, which are reviewed in the introduction of this thesis (chapter I).
The work in this thesis are based on the use of zebrafish (Danio rerio) that has shown to have a great potential in studies on development processes and compound toxicity. The zebrafish has become a model organism in the context of the 3Rs policy and an alternative to animal testing in biomedical research and ecotoxicology. This thesis provides an overview on the use of the early stages of zebrafish development to elucidate the mechanisms related to the toxicity induced by ketamine.
Based on pharmacological events, zebrafish embryos were exposed ketamine (0.2,
0.4 and 0.8 mg mL-1) during three different stages of development, for a period of 20
minutes. The results of this work are presented in chapter II, which is divided into five studies. In chapter II.1, the effects of ketamine exposure at blastula stage (2.5 hours after fertilization - hpf) are addressed. It was found that ketamine exposure during this development phase induces disorders in normal growth processes, inducing defects in embryos and mortality. Chapter II.2 continues the previous work and adds the study of the effects of exposure to ketamine during the stages of gastrula (5.5 hpf) and segmentation (10.5 hpf). In addition to the development parameters, the absorption of ketamine, bone and cartilage development and the expression of developmental genes were also evaluated. Overall, this study shows that the toxicity profile of ketamine is dependent on the time of exposure, being blastula identified as the most sensitive phase. The study, with resource to molecular biology and biochemistry techniques, of biochemical parameters, as biomarkers of the toxicity induced by ketamine is shown in chapter II.3. The resilience of the deregulation of oxidative stress processes was evaluated at different stages of development. Although not affecting the overall redox state,
with the progressive development of the embryo antioxidant defence system. Evaluation of ketamine effects at the level of apoptosis processes during embryonic development were also evaluated and the results presented in chapter II.4. In this study, different gene expression patterns were observed and related with the direct and indirect activation of embryonic apoptosis during periods of differential susceptibility. Finally, chapter II.5 describes the effects on behaviour after exposure to ketamine. This study showed a dose-dependent tendency to affect behavioural responses related with anxiety and fear after exposure in more developed stages, such as gastrula and segmentation, indicating possible neurobehavioural disorders.
Chapter III is divided in two studies describing the effects of environmental
relevant ketamine concentrations (50, 70 and 90 mg L-1,LC50 = 94.4 mg L-1) after a period
of exposure of 24 hours starting on blastula stage. In chapter III.1 describes the serious dose-dependent malformations of ketamine exposure and its long term effects at the behavioral level, such as increased thigmotaxis. Chapter III.2 extends the studies initiated in the previous chapter and shows that in addition to the morphological changes, exposure to ketamine was able to induce oxidative stress responses and apoptosis through engagement of the p53-dependent pathways.
Finally, chapter IV presents the general conclusions of this thesis about the embryotoxic and teratogenic effects of exposure to ketamine in the early stages of zebrafish development and its potential impacts. Guidelines for future work are also discussed.
Keywords: Anaesthesia; Behaviour; Development; Gene expression; Ketamine; Oxidative stress; Zebrafish.
Table of Contents
Acknowledgements ... i Abstract ... vii List of figures ... xv List of tables ... xix List of abbreviations………...xxi List of publications ... xxv CHAPTER I - INTRODUCTION……...………...1 1.1 A general overview ... 5 1.2 Ketamine ... 6 1.2.1 Physicochemical characterization ... 6 1.2.2 Pharmacological properties ... 7 1.2.2.1 Pharmacokinetics ... 7 1.2.2.2 Pharmacodynamics ... 9 1.2.2.2.1 Mechanism of action ... 10 1.2.3 Therapeutic applications ... 11 1.2.4 Adverse effects ... 12 1.2.4.1 Ecotoxicological impact ... 14
1.3 Zebrafish embryos as an alternative vertebrate model organism ... 15
1.3.1 Zebrafish early development ... 18
1.3.2 Zebrafish early stages for anaesthesia research ... 20
1.3.2.1 Ketamine effects in zebrafish early stages ... 21
1.4 Thesis objectives ... 22
CHAPTER II - 20-MINUTES EXPOSURES……..………...25 II.1 - Ketamine NMDA receptor-independent toxicity during zebrafish (Danio rerio) embryonic development…….………..………..27
2.1.1 Abstract ... 29 2.1.2 Introduction ... 30 2.1.3 Material and methods ... 31 2.1.3.1 Chemicals ... 31 2.1.3.2 Maintenance of zebrafish and embryo collection ... 31 2.1.3.3 Toxicant and exposure procedure ... 32 2.1.3.4 Mortality rate ... 32 2.1.3.5 Toxicological endpoints evaluation ... 33
2.1.3.7 Cellular death pattern ... 34 2.1.3.8 Reactive oxygen species generation ... 34 2.1.3.9 Statistical analysis ... 34 2.1.4 Results ... 35 2.1.4.1 Dose-dependent increase in mortality with ketamine ... 35 2.1.4.2 Ketamine-induced developmental toxicity... 35 2.1.4.3 Ketamine associated with developmental anomalies ... 40 2.1.4.4 Apoptosis is not affected by ketamine ... 40 2.1.4.5 Reactive oxygen species generation did not substantially increase with ketamine ... 40 2.1.5 Discussion ... 43 2.1.6 Conclusions ... 45
II.2 - Embryonic stage-dependent teratogenicity of ketamine in zebrafish (Danio rerio)47 2.2.1 Abstract ... 49 2.2.2 Introduction ... 50 2.2.3.1 Chemicals and other reagents ... 51 2.2.3.2 Animals and treatments ... 51 2.2.3.3 Analysis of internal ketamine accumulation by HPLC-UV ... 53 2.2.3.4 Zebrafish embryotoxicity test... 53 2.2.3.5 Development of cartilage and bones ... 54 2.2.3.6 Geometric morphometrics ... 55 2.2.3.7 Developmental-related gene expression... 55 2.2.3.8 Statistical analysis ... 57 2.2.4 Results ... 58 2.2.4.1 Concentration-dependent increase in ketamine internal concentrations ... 58 2.2.4.2 Dose-dependent increase in mortality for 50% epiboly exposure... 59 2.2.4.3 No differences observed for sub-lethal endpoints for 50% epiboly and 1-4 somites ... 59 2.2.4.4 Ketamine-induced malformations on 50% epiboly and 1-4 somites ... 60 2.2.4.5 Ketamine exposure during 256-cell induces abnormal craniofacial development ... 61 2.2.4.6 Head shape variability in 256-cell exposed embryos ... 64 2.2.4.7 Ketamine induces oscillations on developmental-related genes ... 65 2.2.5 Discussion ... 67 2.2.6 Conclusion ... 71
Appendix A: Supplementary data ... 75
II.3 - Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryo………..87
2.3.1 Abstract ... 89 2.3.2 Introduction ... 90 2.3.3 Experimental ... 91 2.3.3.1 Chemicals ... 92 2.3.3.2 Test organisms... 92 2.3.3.3 Exposure experiments ... 92 2.3.3.4 Sample collection ... 93 2.3.3.5 Reactive oxygen species accumulation and visualization ... 93 2.3.3.6 Enzymatic determinations ... 94 2.3.3.7 Glutathione levels and oxidative stress index ... 95 2.3.3.8 Oxidative damage biomarkers ... 96 2.3.3.9 Gene expression analysis ... 97 2.3.3.10 Statistics ... 98 2.3.4 Results ... 99 2.3.4.1 ROS levels and in vivo accumulation ... 99 2.3.4.2 Enzymatic activities ... 100 2.3.4.3 Glutathione levels ... 103 2.3.4.4 Oxidative damage biomarkers ... 105 2.3.4.5 Gene expression analysis ... 105 2.3.5 Discussion ... 106 Appendix B: Supplementary data ... 113
II.4 - Zebrafish embryos apoptotic pathways gene stage-expression following ketamine
exposure………115
2.4.1 Abstract ... 117 2.4.2 Introduction ... 118 2.4.3 Material and Methods ... 119 2.4.3.1 Reagents ... 119 2.4.3.2 Fish care and maintenance ... 119 2.4.3.3 Exposure experiments ... 120 2.4.3.4 In vivo detection of cell death ... 120 2.4.3.5 Gene expression analysis ... 121 2.4.3.5.1 RNA extraction and cDNA synthesis... 121
2.4.3.6 Caspase activities ... 123 2.4.3.7 Statistical analysis ... 124 2.4.4 Results ... 124 2.4.4.1 Embryonic cell death ... 124 2.4.4.2 Gene expression analysis ... 125 2.4.4.3 Caspase-3 and -9 activities ... 128 2.4.5 Discussion ... 128
II.5 - Behavioural alterations of zebrafish larvae after early embryonic exposure to ketamine………...……….133
2.5.1 Abstract ... 135 2.5.2 Introduction ... 136 2.5.3 Material and Methods ... 137 2.5.3.1 Statement of ethic on animal use... 137 2.5.3.2 Fish husbandry and eggs production ... 137 2.5.3.3 Chemicals ... 138 2.5.3.4 Experimental exposures ... 138 2.5.3.4.1 Video acquisition... 139 2.5.3.4.2 Locomotor activity and thigmotactic behaviour ... 139 2.5.3.4.3 Avoidance behaviour... 140 2.5.3.4.4 Social behaviour analysis ... 140 2.5.3.5 Statistical analyses... 141 2.5.4 Results ... 142 2.5.4.1 Effects of ketamine-exposure in exploratory behaviour ... 142 2.5.4.2 Ketamine effects in avoidance behaviour ... 142 2.5.4.3 Social behaviour ... 143 2.5.5 Discussion ... 144 2.5.6 Conclusions ... 147
CHAPTER III - 24-HOURS CONTINUOUS EXPOSURE..………...……….149
III.1 - Zebrafish larvae morphological abnormalities and thigmotaxis after 24 hours embryonic exposure to ketamine………...……….151
3.1.1 Abstract ... 153 3.1.2 Introduction ... 154 3.1.3 Methods ... 154 3.1.3.1 Ethics statement... 154
3.1.3.2 Chemicals and other reagents ... 155 3.1.3.3 Animals ... 155 3.1.3.4 24-h LC50 ... 155 3.1.3.5 Embryo assay ... 156 3.1.3.6 HPLC-UV quantification of ketamine ... 156 3.1.3.7 Developmental toxicity testing... 157 3.1.3.8 Cartilage and bone analysis ... 158 3.1.3.9 Behavioural analysis ... 158 3.1.3.10 Analysis of mRNA expression ... 160 3.1.3.13 Data analysis ... 160 3.1.4 Results ... 161 3.1.4.1 Embryo ketamine concentrations ... 161 3.1.4.2 Ketamine-induced mortality ... 161 3.1.4.3 Developmental endpoints ... 162 3.1.4.4 Morphological assessment ... 164 3.1.4.5 Cartilage and bone malformations ... 164 3.1.4.6 Craniofacial variations ... 166 3.1.4.7 Larval behaviour ... 166 3.1.4.8 Gene transcription profile... 167 3.1.5 Discussion ... 168 3.1.6 Conclusions ... 170 Appendix C: Supplementary data ... 173
III.2 - p53-dependent apoptosis and oxidative stress induced by 24 hours exposure to ketamine in zebrafish (Danio rerio) embryos………179
3.2.1 Abstract ... 181 3.2.2 Introduction ... 182 3.2.3 Methods ... 183 3.2.3.1 Ethics statement... 183 3.2.3.2 Reagents ... 183 3.2.3.3 Selection of exposure concentrations ... 183 3.2.3.4 Experimental design ... 183 3.2.3.5 Intracelullar ROS and cell death ... 184 3.2.3.6 Biochemical determinations ... 185 3.2.3.7 Analysis of mRNA expression ... 186 3.2.3.8 Statistical analysis ... 187
3.2.4.1 Embryonic cell death and ROS ... 189 3.2.4.2 Oxidative stress and apoptosis related biomarkers ... 189 3.2.4.3 Gene transcription profile... 190 3.2.5 Discussion ... 191 3.2.6 Conclusions ... 194 Appendix D – Supplementary data ... 199
CHAPTER IV - CONCLUDING REMARKS ... 203
List of figures
CHAPTER I - INTRODUCTION
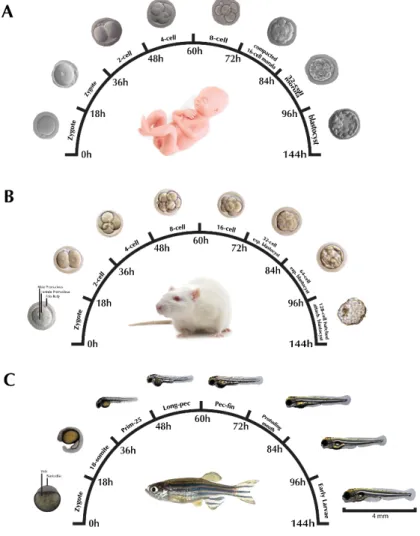
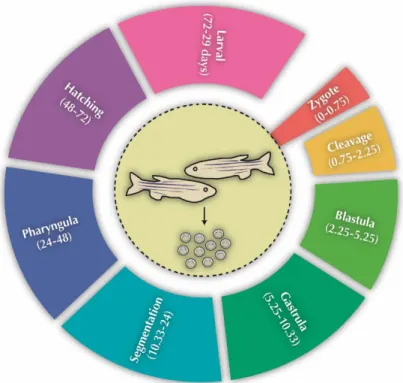
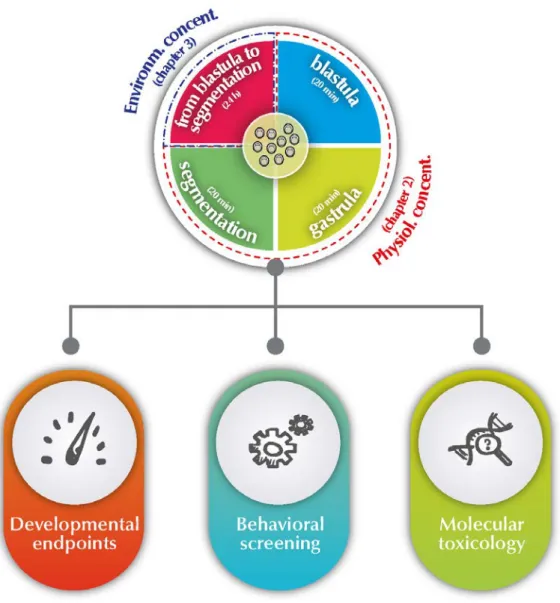
Figure 1.1 - The arylcycloalkylamine chemical structure of ketamine isomers ... 7 Figure 1.2 - Human ketamine metabolism by cytochrome P450 family. ... 9 Figure 1.3 - Representation of the NMDA receptor complex. ... 11 Figure 1.4 - Translating times across human, rodent and zebrafish early development. 17 Figure 1.5 - Early life cycle of zebrafish and its embryonic developmental stages from zygote to larval. ... 18 Figure 1.6 - Schematic overview of thesis objectives and outline. ... 23
CHAPTER II - 20-MINUTES EXPOSURES
II.1 - Ketamine NMDA receptor-independent toxicity during zebrafish (Danio rerio) embryonic development
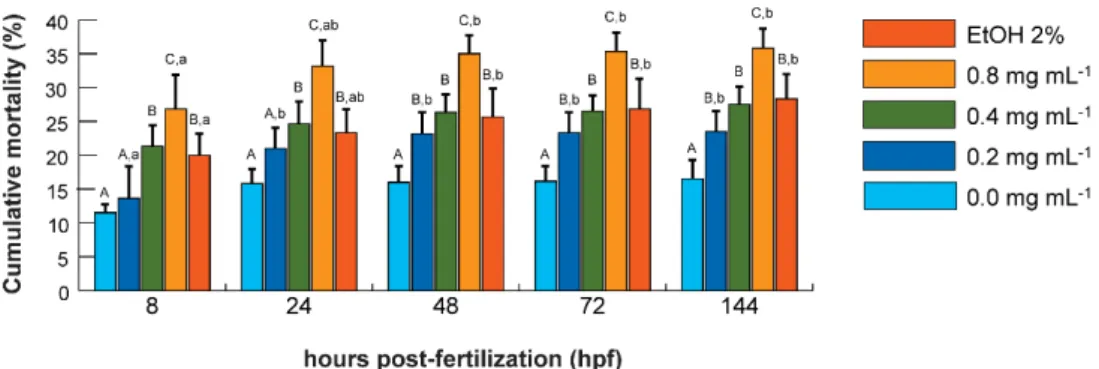
Figure 2.1.1 - Experimental design overview... 32 Figure 2.1.2 - Dose-response and time course mortality of zebrafish exposed to ketamine and ethanol. ... 36
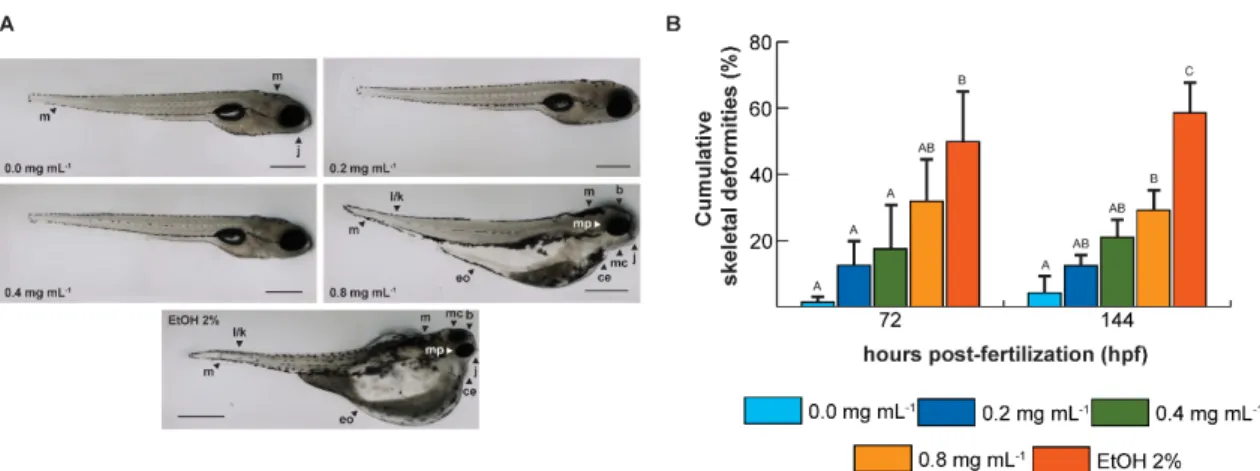
Figure 2.1.3 - Malformation in zebrafish exposed to ketamine and ethanol and cumulative
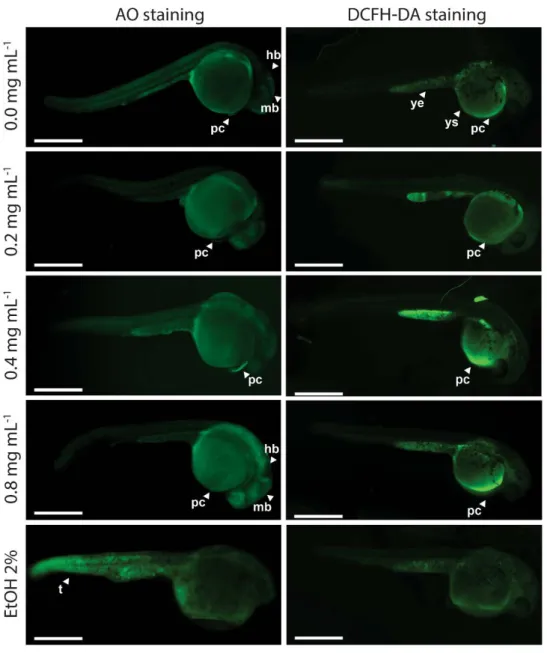
tail and spine skeletal deformities of zebrafish at 72 and 144 h post-fertilization ... 37 Figure 2.1.4 - Cellular death pattern and reactive oxygen species spatial distribution in zebrafish embryos at 24 hpf... 41
II.2 - Embryonic stage-dependent teratogenicity of ketamine in zebrafish (Danio rerio)
Figure 2.2.1 - Experimental design for ketamine exposure at different zebrafish developmental stages. ... 52 Figure 2.2.2 - Dose-response and time course mortality of zebrafish exposed to ketamine. ... 59 Figure 2.2.3 - Characteristic cartilage malformations of embryos exposed to ketamine during 256-cell. ... 63 Figure 2.2.4 - Effects of ketamine on bone development of zebrafish embryos during 256-cell. ... 64 Figure 2.2.5 - Expression profiles of shh a and nog3 following ketamine exposures at 256-cell, 50% epiboly and 1-4 somites. ... 66
(Danio rerio) embryos development
Figure 2.3.1 - Schematic diagram showing the developmental timing of ketamine exposure and time point-based analyses. ... 93 Figure 2.3.2 - Reactive oxygen species accumulation in control zebrafish embryos and exposed to ketamine in blastula, gastrula and segmentation. ... 99 Figure 2.3.3 - Representative DCF fluorescence images of 144 hpf larvae exposed to ketamine during gastrula and segmentation. ... 100 Figure 2.3.4 - Expression profile of gclc, gstp1, sod1 and cat after exposure to ketamine during blastula, gastrula and segmentation stages. ... 105
II.4 - Zebrafish embryos apoptotic pathways gene stage-expression following ketamine exposure
Figure 2.4.1 - Schematic diagrams showing the timing of ketamine exposures in zebrafish early developmental stages. ... 120 Figure 2.4.2 - Distribution of apoptotic cells in zebrafish embryos exposed to ketamine during gastrula and segmentation. ... 125 Figure 2.4.3 - RNA transcript levels of genes related to apoptosis and proliferation following exposure to ketamine during blastula, gastrula or segmentation stages. ... 127 Figure 2.4.4 - Enzymatic activities measured by colorimetric assay of caspase-3 and caspase-9 after exposure to different concentrations of ketamine in blastula, gastrula or segmentation stages. ... 128
II.5 - Behavioural alterations of zebrafish larvae after early embryonic exposure to ketamine
Figure 2.5.1 - Schematic diagram showing the timing of ketamine exposures in zebrafish early developmental stages. ... 138 Figure 2.5.2 - Ketamine early exposure effects on exploratory behaviour at 144 hpf larvae. ... 143 Figure 2.5.3 - Assessment of avoidance behaviour at 144 hpf larvae following early exposure to ketamine. ... 144 Figure 2.5.4 - Larval social behaviours of ketamine-exposed embryos. ... 144
CHAPTER III - 24-HOURS CONTINUOUS EXPOSURE
III.1 - Zebrafish larvae morphological abnormalities and thigmotaxis after 24 hours embryonic exposure to ketamine
Figure 3.1.1 - Schematic diagram showing the timing of ketamine exposures and experimental analysis in zebrafish embryos. ... 156 Figure 3.1.2 – Cumulative mortality of zebrafish embryos exposed to ketamine. ... 162 Figure 3.1.3 - Malformations in zebrafish larvae exposed to ketamine. ... 164 Figure 3.1.4 - Lateral and ventral view of 144 hpf zebrafish larvae unexposed and exposed to ketamine and stained with alcian blue to enhance cartilage. ... 165 Figure 3.1.5 - Effects of embryonic ketamine exposure on the thigmotaxis and avoidance of zebrafish larvae at 144 hpf. ... 167 Figure 3.1.6 - Relative gene expression levels of shh a and nog3 following 24 hours exposure to ketamine. ... 167
Appendix C
Figure C1 - Dose–response curve of mortality of zebrafish following 24h exposure to ketamine. ... 173 Figure C2 - Principal components analysis of zebrafish head, cartilage and bone at 144 hpf after 24-h ketamine exposure. ... 173 Figure C3 - Behavioural parameters evaluated at 144 hpf larvae following 24h embryo exposure to ketamine. ... 174
III.2 - p53-dependent apoptosis and oxidative stress induced by 24 hours exposure to ketamine in zebrafish (Danio rerio) embryos
Figure 3.2.1 - Schematic diagram showing the timing of ketamine exposure in zebrafish early development... 184 Figure 3.2.2 - Distribution of apoptotic cells and reactive oxygen species in 26 hpf zebrafish embryos after exposure to ketamine for 24 hours. ... 189 Figure 3.2.3 - Reactive oxygen species and specific activities of superoxide dismutase, catalase, oxidized glutathione, casp-3 and -9, and levels of protein carbonyl at 8 and 26 hpf. ... 190 Figure 3.2.4 - RNA transcript levels of genes related to oxidative stress (sod1 and cat) and apoptosis (casp6, casp8, casp9 and tp53) following exposure to ketamine. ... 191
... 195
List of tables
CHAPTER I - INTRODUCTION
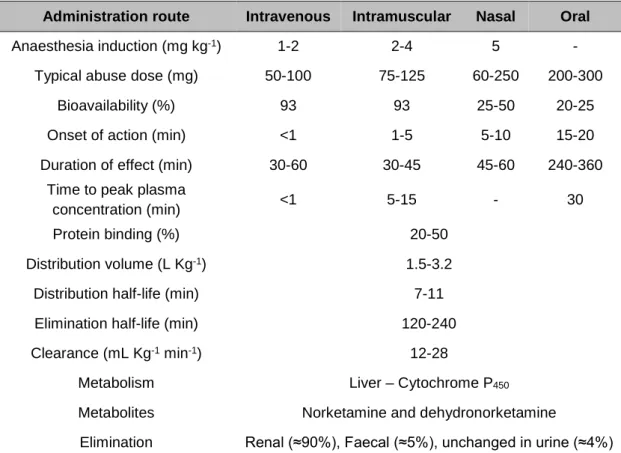
Table 1.1 - Ketamine pharmacokinetics based on different administration routes. ... 8
CHAPTER II - 20-MINUTES EXPOSURES
II.1 - Ketamine NMDA receptor-independent toxicity during zebrafish (Danio rerio) embryonic development
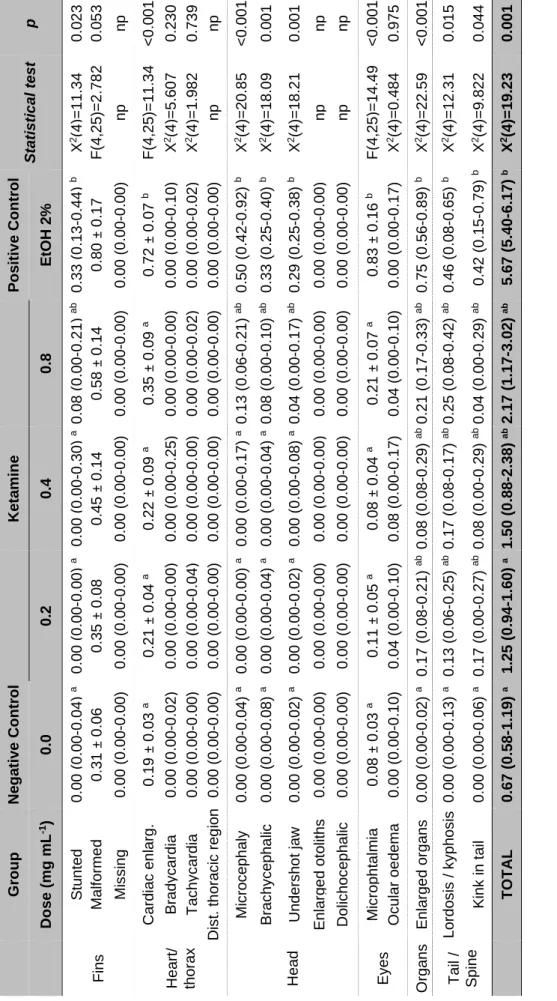
Table 2.1.1 – Frequency of morphological characteristics identified in surviving zebrafish (D. rerio) embyos at 24 and 48 hpf. ... 38 Table 2.1.2 - Quantitative analysis of zebrafish embryos/larvae developmental parameters at 48, 72 and 144 hpf ... 39 Table 2.1.3 - General outcomes of morphological analyses of zebrafish larvae at 144 hpf. ... 42
II.2 - Embryonic stage-dependent teratogenicity of ketamine in zebrafish (Danio rerio)
Table 2.2.1 - Information of specific primers used for amplification in real-time PCR 57
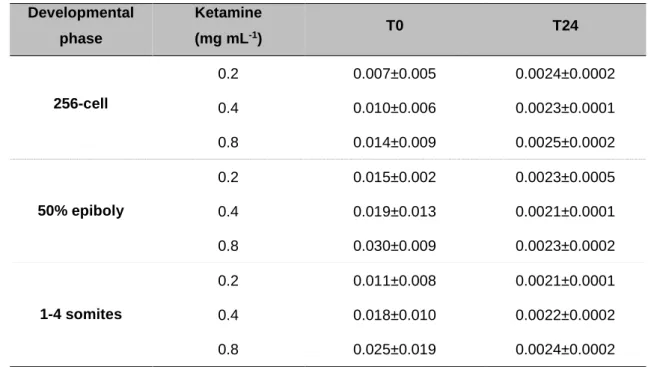
Table 2.2.2 - HPLC-UV measured concentration of ketamine (pmol embryo-1) in
zebrafish embryos immediately and 24 hours after exposures ... 58 Table 2.2.3 - Significant findings from the morphological analyses of zebrafish larvae at 144 hpf after exposure during 50% epiboly and 1-4 somites stages. ... 61
II.3 - Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos
Table 2.3.1 - Information of specific primers used for amplification in real-time PCR 98 Table 2.3.2 - Specific activities of superoxide dismutase, catalase and glutathione peroxidase at 8 and 24 hpf in control zebrafish embryos and exposed to ketamine in blastula, gastrula and segmentation stages ... 101 Table 2.3.3 - Specific activities of lactate dehydrogenase and acetylcholinesterase at 8 and 24 hpf in control zebrafish embryos and exposed to ketamine treatments in different developmental stages. ... 102
and 24 hpf in control zebrafish embryos and exposed to ketamine treatments in different developmental stages. ... 104
II.4 - Zebrafish embryos apoptotic pathways gene stage-expression following ketamine exposure
Table 2.4.1 - Information of specific primers used for amplification in real-time PCR ... 122
CHAPTER III - 24-HOURS CONTINUOUS EXPOSURE
III.1 - Zebrafish larvae morphological abnormalities and thigmotaxis after 24 hours embryonic exposure to ketamine
Table 3.1.1 - Frequency of sub-lethal endpoints identified in zebrafish embryos after exposure for 24h to ketamine. ... 163
Appendix C
Table C1 - Information of specific primers used for amplification in real-time PCR with GenBank accession numbers shown in parentheses. ... 175 Table C2 - Frequency of lethal endpoints identified in zebrafish embryos after exposure for 24h to ketamine. ... 175 Table C3 - General outcomes of morphological analyses of zebrafish larvae at 144 hpf after 24h exposure to ketamine. ... 176 Table C4 - General outcomes of cartilage and bone analyses of zebrafish larvae at 144 hpf after 24h exposure to ketamine. ... 177
III.2 - p53-dependent apoptosis and oxidative stress induced by 24 hours exposure to ketamine in zebrafish (Danio rerio) embryos
Table 3.2.1 - Information of specific primers used for amplification in real-time PCR with GenBank accession numbers shown in parenthesis. ... 188
Appendix D
Table D1 - Relative non-significant enzymatic activities in zebrafish embryos at 8 and
26 hpf after exposure to ketamine at 50, 70, and 90 mg mL-1. ... 199
Table D2 - Relative non-significant fold-changes of the tested genes at 8 and 26 hpf in
the groups exposed to 50, 70, and 90 mg mL-1. ... 200
List of abbreviations
5-HIAA 5-Hydroxyindoleacetic Acid
Ac-DEVD-CHO N-Acetyl-Asp-Glu-Val-Asp-aldehyde Ac-DEVD-pNA N-Acetyl-Asp-Glu-Val-Asp-p-Nitroaniline
AcHE Acetylcholinesterase
Ac-LEHD-CHO N-Acetyl-Ile-Glu-Thr-Asp-aldehyde
Ac-LEHD-pNA N-Acetyl-Ile-Glu-Thr-Asp-p-Nitroaniline
AIF Apoptosis-inducing factor
AO Acridine orange
APS-C Advanced photo system type-C
ATP Adenosine triphosphate
AVCHD Advanced video codec high definition
BHT Butylated hydroxytoluene
BMP Bone morphogenetic protein
CAS Chemical abstracts service
CAT Catalase
CCD Charge-coupled device
cDNA complementary DNA
CHAPS 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
CMOS Complementary metal-oxide semiconductor
CNS Central nervous system
CO Protein carbonyls
ct Cycle treshold
CYP Cytochrome P450
DCF 2,7-Dichlorofluorescein
DCFH-DA 2,7-Dichlorodihydrofluorescein diacetate
DMSO Dimethyl sulfoxide
DNPH 2,4-Dinitrophenylhydrazine
dpf days post-fertilization
DTNB 5,5'-Dithiobis(2-nitrobenzoic acid)
EDTA Ethylenediamine tetraacetic acid
EVL Envelope layer
FITC Fluorescein-isothiocyanate
GPx Glutathione peroxidase
GR Glutathione reductase
GSH Glutathione (reduced form)
GSSG Glutathione (oxidized form)
HEPES N-2-Hydroxyethylpiperazine-N'-2-Ethanesulfonic acid
hpf hours post-fertilization
HPLC High-performance liquid chromatography
IARS International anesthesia research society
IID Inter-individual distance
IUPAC International Union of Pure and Applied Chemistry
JNK Jun N-terminal kinase
LC50 Median lethal concentration
LCD Liquid crystal display
LD50 Median lethal dose
LDH Lactate dehydrogenase
LPO Lipid peroxidation
MAPK Mitogen-activated protein kinases
MBT Midblastula transition
MDA Malondialdehyde
NADH Nicotinamide adenine dinucleotide (reduced form).
NADP+ Nicotinamide adenine dinucleotide phosphate (oxidized form)
NADPH Nicotinamide adenine dinucleotide phosphate (reduced form)
NBT Nitro-blue tetrazolium
NEM N-Ethylmaleimide
NFkB Nuclear factor kappa B
NMDA N-methyl-D-aspartate
NND Nearest neighbour distance
NPS New psychoactive substances
Nrf2 Nuclear factor erythroid 2-related factor 2
OECD Organisation for Economic Co-operation and Development
OPA ortho-phthalaldehyde
OSI Oxidative stress index
OSS Optical steadyshot
PBS Phosphate-buffered saline
PC Principal component
PCA Principal component analysis
PCP Phencyclidine
PMSF Phenylmethylsulfonyl fluoride
pNA para-Nitroaniline
qRT-PCR Quantitative real time-polymerase chain reaction
RGB Red-green-blue
ROS Reactive oxygen species
SMZ Stereo microscope-zoom
SOD Superoxide dismutase
SPSS Statistical package for the social sciences
TBA Thiobarbituric acid
TCA Trichloroacetic acid
UNODC United Nations Office on Drugs and Crime
UV Ultraviolet
WWTP Wastewater treatment plant
YSL Yolk syncytial layer
ZGA Zygotic genome activation
List of publications
This thesis is based on the work contained in the following published work:
- Felix, L.M., Antunes, L.M., Coimbra, A.M. (2014). Ketamine NMDA receptor-independent toxicity during zebrafish (Danio rerio) embryonic development. Neurotoxicol. Teratol. 41:27-34. DOI: 10.1016/j.ntt.2013.11.005.
- Felix, L., Antunes, L., Campos, S., Venâncio, C., Coimbra, A.M. (2016). Chapter 62 - Recreational use of ketamine and its interaction with NMDA receptors In Preedy, Victor R. (Eds), Neuropathology of Drug Addictions and Substance Misuse. Academic Press, San Diego, pp 672-680. DOI: 10.1016/B978-0-12-800212-4.00062-5
- Félix, L.M., Vidal, A.M., Serafim,C., Valentim, A.M., Antunes, L., Campos, S., Matos, M., Monteiro, S.M., Coimbra, A.M. (2016). Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos. RSC Adv. 6:61254-61266. DOI: 10.1039/C6RA08298J.
- Felix, L.M., Serafim, C., Valentim, A.M., Antunes, L.M., Campos, S., Matos, M., Coimbra, A.M. (2016). Embryonic stage-dependent teratogenicity of ketamine in zebrafish (Danio rerio). Chem. Res. Toxicol. DOI: 10.1021/acs.chemrestox.6b00122.
Work is reproduced with the permission of the publishers.
INTRODUCTION
Recreational use of ketamine and its interaction with NMDA receptors
Partially published in Preedy, Victor R. Neuropathology of Drug
Addictions and Substance Misuse (2016). Chapter 62. Academic
1.1 A general overview
In the past decades, significant efforts have been made to understand the mechanisms of action of ketamine, a widely used anaesthetic. Gradually, a significant number of experimental reports have helped to expand the current knowledge into the biochemical and molecular aspects of ketamine. These have been accompanied with an evolving knowledge of different physiologic systems driven by advances in technology and scientific instrumentation. However, the neuro-developmental effects of early ketamine exposure remains poorly understood.
Practical outcomes from animal studies have found that ketamine anaesthesia has neurotoxic and neuronal death properties to the developing brain which contribute to cognitive and/or behavioural abnormalities. However, recent controversial evidence has shown that ketamine protects the developing central nervous system. This conflicting result supports the need of additional research to clarify the relationship between ketamine, early development and mechanisms that may be involved.
Zebrafish (Danio rerio) has emerged as a model organism to analyse vertebrate complex development cellular interactions and to study molecular developmental mechanisms of toxicity. Recent studies using zebrafish have shown that ketamine perturbations elicit mammalian-like functional cellular and behavioural interactions/events. Additionally, zebrafish also present a pattern of sensitivity similar to that observed in mammals. This has established zebrafish as a powerful model to better understand the biological activity and underlying mechanism of action of ketamine. Although a limited number of studies on possible toxic effects of ketamine on early zebrafish embryogenesis have been reported, the full range of effects of early ketamine exposure is not yet understood. Exposure to toxic agents during early developmental stages could potentially disrupt embryonic development which could be manifested as morphological, behavioural, biochemical or genetic changes and could culminate in embryonic death.
In this thesis, the use of the early developmental stages of zebrafish to study the in vivo interactions of ketamine were addressed by a combination of molecular biology and various analytical techniques that allowed the study of the molecular changes and behavioural effects induced by ketamine in a whole simple developmental organism. The results discussed were based on the most recent findings in the area.
1.2 Ketamine
Ketamine, a non-competitive antagonist of the N-methyl D-aspartate (NMDA) receptor, was first synthesized by Calvin Stevens in 1962, in Parke-Davis Laboratories in Michigan. It is a phencyclidine derivative and since being developed, it has played an important role in terms of anaesthesia (Dong and Anand 2013; Wolff and Winstock 2006). It was introduced in human anaesthetic procedures during the Vietnam War, in 1964, but was only approved for human and veterinary use by the American Food and Drug Administration (FDA) in 1970 (Rofael and Abdel-Rahman 2002). The occurrence of hallucinogenic effects, described during the volunteer trials, limited ketamine clinical use (Domino et al. 1965). When compared with other anaesthetic agents, ketamine is still widely used in medical practice due to several clinically useful properties (Hirota and Lambert 1996). This will be further described in the clinical applications section (1.2.3). Alongside, and because it induces hallucinogenic and dissociative effects, ketamine has been raised into recreational settings where it is illicitly used (Rofael and Abdel-Rahman 2002). Its illicit consumption has increased due to its low price and easy availability, and raised new concerns related to physical and psychological harms (Krystal et al. 1994). Regardless, and despite all the controversy on ketamine molecular and mechanistic actions, which still remain unclear, ketamine has been appointed as a promising therapy for other addictions and depressive disorders (Krupitsky et al. 2002; Krupitsky and Grinenko 1997; Murrough et al. 2013).
1.2.1 Physicochemical characterization
The ketamine molecule is an arylcycloalkylamine related to phencyclidine (PCP). The International Union of Pure and Applied Chemistry (IUPAC) designation is (RS)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone. Ketamine derives from the presence of a ketone and of an amine groups. As hydrochloride, it has a molecular
weight of 274.19 g mol-1 (CAS 1867-66-9), a chemical formula C13H17Cl2NO, and a
melting point of 258-261 ºC. As a free base, ketamine is characterized by a molecular
weight of 237.72 g mol-1 (CAS 6740-88-1), a chemical formula C13H16ClNO, and a
melting point around 92-93 ºC. As hydrochloride it displays a freely solubility in water and in methanol and a less solubility in ethanol. As a base, it has a pKa of 7.5 and it is
freely soluble at 200 mg mL-1 in water and methanol.
The usual formulation of ketamine (10 mg mL-1) is as a slightly acid (pH 3.2-5.5)
saline solution that can either be ingested or injected (Smith et al. 2002). Occasionally,
when in higher concentrations (50 or 100 mg mL-1), 0.1 mg mL-1 of benzethonium chloride or chlorobutanol are added as preservatives (Mion and Villevieille 2013; Wolff and Winstock 2006). It can also be evaporated to obtain a crystalline powder similar to cocaine or heroin, which is the primary method used by abusers for snorting or inhaling (Wolff and Winstock 2006). Ketamine is commercially available under several registered brands (Li et al. 2011) as a racemic mixture of equal amounts of two enantiomers (Figure 1.1) resulting from the chiral centre of the cyclohexanone (Mion and Villevieille 2013). It is commonly obtained from either diverted or stolen medical supplies, despite its industrial-scale production being emerging (Morgan et al. 2012). According to the global synthetic drugs assessment report of 2014 from the United Nations Office on Drugs and Crime (UNODC), ketamine belong to the new psychoactive substances (NPS) group and is reported to correspond to 4% of the NPS abused worldwide.
Figure 1.1 - The arylcycloalkylamine chemical structure of ketamine isomers
presented as the free base. 1.2.2 Pharmacological properties
1.2.2.1 Pharmacokinetics
Ketamine is a racemic anaesthetic that can be administrated via multiple routes such as intravenous, intramuscular, oral or nasal (Table 1.1) (White et al. 1982). This is possible due to its water and lipid solubility which emphasizes its applications (Sinner and Graf 2008). Owing to its low binding capacity to plasma proteins, ketamine is rapidly distributed into highly vascular organs, including the brain, with subsequent redistribution to less perfused ones (Aroni et al. 2009). Due to its high lipid solubility, it also quickly crosses the blood-brain barrier and placenta (Pai and Heining 2007),
which is reflected in its relative large volume of distribution (1.5 to 3.2 L Kg-1), with the
S(+) enantiomer showing a higher volume of distribution and hepatic clearance
(12-28 mL min-1 Kg-1) compared to the R(-) enantiomer (Mion and Villevieille 2013).
The bioavailability of ketamine depends on the route of administration, decreasing from about 90% in a single intravenous or intramuscular dose to about 20% when orally taken, due to a liver extensive first pass metabolism (Clements et al. 1982; Reich and Silvay 1989). The time to reach maximum plasma concentration is also dependent on the route of administration, being faster intravenous and intramuscularly (1 to 5 minutes) relatively to nasal (20 minutes) or oral route (30 minutes), whereas the time for ketamine metabolite, norketamine, to peak is around 1 hour. The half-life of ketamine in the circulatory system ranges from 1 to 3 hours, while for norketamine, it is around 12 hours. Ketamine action duration is also dependent upon the route of administration, varying from 10 to 20 minutes for intravenous administration (Aroni et al. 2009), 30 to 240 minutes for an intramuscular injection and increasing to 4 to 6 hours or more for oral intake (Mion and Villevieille 2013; Quibell et al. 2011).
Table 1.1 - Ketamine pharmacokinetics based on different administration routes. (Kalsi et al. 2011;
Pai and Heining 2007; Quibell et al. 2011)
Ketamine is metabolized in the liver by enzymes from the cytochrome P450 (CYP) family, being CYP3A4 the principal enzyme involved in this process and with CYP2C19, CYP2B6, CYP2A6, CYP2D6 and CYP2C9 presenting minor contributions (Portmann et al. 2010). The main pathway involved in ketamine metabolism is the
Administration route Intravenous Intramuscular Nasal Oral
Anaesthesia induction (mg kg-1) 1-2 2-4 5 -
Typical abuse dose (mg) 50-100 75-125 60-250 200-300
Bioavailability (%) 93 93 25-50 20-25
Onset of action (min) <1 1-5 5-10 15-20
Duration of effect (min) 30-60 30-45 45-60 240-360
Time to peak plasma
concentration (min) <1 5-15 - 30
Protein binding (%) 20-50
Distribution volume (L Kg-1) 1.5-3.2
Distribution half-life (min) 7-11
Elimination half-life (min) 120-240
Clearance (mL Kg-1 min-1) 12-28
Metabolism Liver – Cytochrome P450
Metabolites Norketamine and dehydronorketamine
Elimination Renal (≈90%), Faecal (≈5%), unchanged in urine (≈4%)
N-demethylation of the cyclohexanone ring to norketamine, the primary metabolite presenting one-third of ketamine potency. Norketamine can be either dehydrogenated into dehydronorketamine, which does not possess anaesthetic activity, or be hydroxylated to six diasteromeric norketamine metabolites (Figure 1.2), also not active at the NMDA receptor (Zhao et al. 2012). Furthermore, ketamine and its metabolites undergo hepatic conjugation and are excreted as water-soluble conjugates through urine
(Moore et al. 2001). The half‐lives of norketamine and dehydronorketamine are longer
than that of ketamine with norketamine reaching its peak level earlier and being eliminated faster than dehydronorketamine (Mion and Villevieille 2013) (Table 1.1). Ketamine can also experience a hydroxylation into two diasteromeric compounds; however it is a qualitatively insignificant process (Woolf and Adams 1987). Although the main organ of ketamine metabolism is the liver, the kidneys, the intestine and the lungs are also able to metabolize this anaesthetic agent (Mion and Villevieille 2013).
Figure 1.2 - Human ketamine metabolism by cytochrome P450 family. Ketamine is extensively N-demethyled to norketamine which is further dehydrogenated into dehydronorketamine or hydroxylated
to six diasteromeric hydroxynorketamine. Ketamine can also be directly transformed into hydroxynorketamine despite not being a significant process.
1.2.2.2 Pharmacodynamics
Ketamine is classified as an NMDA receptor antagonist, but it also acts in numerous other sites making its pharmacology complex and with a wide range of pharmacological effects. Essentially, ketamine is a rapid-acting general anaesthetic that induces a dissociative state that interferes with neuronal plasticity, learning and memory (Curran and Morgan 2000; Morgan and Curran 2006; Sinner and Graf 2008). This state
is caused by disruption of the electrophysiological synchrony between the limbic and the thalamoneocortical system, preventing the central nervous system (CNS) from receiving or processing sensory input. This is characterized by a trancelike cataleptic state with profound analgesia and amnesia, but preserving airway patency, spontaneous respirations, and cardiopulmonary stability (Domino et al. 1965). Ketamine blocks the NMDA receptor at concentrations between 2 and 50 µM interfering with N1/N2 complex in a concentration-dependent manner and making this responsible for the most important pharmacological properties exhibited by ketamine (Mion and Villevieille 2013).
1.2.2.2.1 Mechanism of action
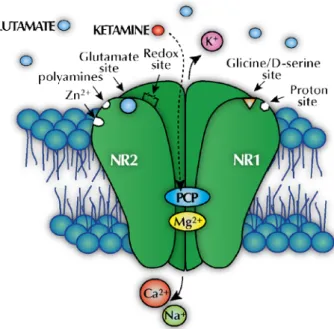
Ketamine acts on the CNS through non-competitive inhibition of the PCP recognition site of the NMDA receptor. It physically and chemically inhibits the flux of calcium and sodium through activated receptors, which are ordinarily activated by glutamate (Li et al. 2011) (Figure 1.3). While remaining trapped inside the ion channel, ketamine disrupts both the physiological and pathological functions by reducing the channel mean open time (Orser et al. 1997; Sinner and Graf 2008). Ketamine can also interact with a second membrane-associated site, decreasing the frequency of channel opening (Quibell et al. 2011). The interaction with the PCP binding site is stereoselective, with the S(+) enantiomer having a higher affinity than the R(-) enantiomer (Kalsi et al. 2011; Li et al. 2011). Likewise, norketamine displays an NMDA receptor binding affinity, less effective when compared to the parent compound, but contributing to the analgesic effect of ketamine (Mion and Villevieille 2013).
As ketamine binds to the NMDA receptor, glutamate concentration in the synaptic cleft increases being recycled by a sodium-dependent carrier located in neurons and glia cells (Greenamyre and Porter 1994) where it is enzymatically converted to glutamine by glutamine synthetase and further up-taken by neurons and hydrolysed back to glutamate (Caddy et al. 2014). Under excess conditions, glutamate acts as excitotoxin, which ultimately leads to neuronal damage and death (Greenamyre and Porter 1994). The continuous blockade of the receptor induces a compensatory up-regulation of the receptor making the receptor vulnerable to the excitotoxic effects of endogenous glutamate after ketamine withdrawal (Liu et al. 2013b).
Figure 1.3 -Representation of the NMDA receptor complex. The receptor complex is assembled as
heterotetramers of obligatory subunits: a main NR1 subunit and additional subunits of NR2 or NR3. The receptor display a variety of modulatory sites that include those for ketamine (PCP), glutamate, polyamines, zinc, magnesium, glycine, proton, and redox, among others. Calcium, sodium, and magnesium flow though the activated channel while potassium flows out. Glycine or d-serine must be bound to the receptor as well as the receptor agonist glutamate to activate the receptor. Ketamine binds to the open channel at PCP site preventing the ion flux through the channel and thus inhibiting the excitatory response to glutamate.
Ketamine also increases the activation of other glutamatergic receptors, AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid) and KA (kainate), as well as, other glutamate-independent mechanisms, such as opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors and sodium, calcium and potassium channels, resulting in intracellular protein and function changes, which mediates ketamine respiratory, cardiovascular and other pharmacological effects occurring in medical and non-medical use (Caddy et al. 2014; Kalsi et al. 2011; Kurdi et al. 2014).
1.2.3 Therapeutic applications
Due to its unique pharmacological characteristics, ketamine was found to have multiple clinical applications over a wide range of clinical situations, with special emphasis to the emergency department (Green et al. 2011; Kurdi et al. 2014). It can be used for paediatric or adult anaesthesia, veterinary anaesthesia and field medicine (Morgan et al. 2012) being best suited for short painful procedures where immobilization is required or in situations where emotional disturbances may occur (Green et al. 2011; Kurdi et al. 2014). Still, ketamine can be used for longer uncomplicated procedures with additional doses. Several other therapeutic clinical
applications have been recently evaluated without firm evidence (Kurdi et al. 2014). However, absolute and relative contraindications have further incited controversy on the clinical applications of ketamine. In fact, due to the increase of adverse effects (emergence phenomena), over the years, ketamine’s use has been limited from its conventional anaesthetic application in humans (Morgan et al. 2012).
Notwithstanding, ketamine is still the anaesthetic drug of choice in developing nations whereas its use is reserved for specific cases in developed countries (Kurdi et al. 2014; Morgan et al. 2012). In non-human animals, ketamine is the most widely used anaesthetic agent (Morgan et al. 2012). Moreover, other potential clinical uses of ketamine are being studied. Ketamine psychedelic effects have been appointed as a promising therapy for the treatment of drug addictions (Krupitsky et al. 2002) and preclinical investigations have shown that ketamine may possess antidepressant properties (Berman et al. 2000). Actually, an increasing trend in analysing the neuropsycopharmacological effects in animal models has been observed.
1.2.4 Adverse effects
As a traditional NMDA receptor antagonist, ketamine has been associated with a variety of short-term effects in humans similar to those caused by PCP (Weiner et al. 2000), such as auditory and visual hallucinations, out-of-body experiences and mystical states, which are often described as being part of the dissociative state known as “K-hole”, and a variety of neuro-behavioural effects, like cognitive impairments and neuropsychiatric alterations (Krystal et al. 1994). The psychotomimetic symptoms are
dose dependent and start to be noticed at plasma concentrations as low as 50 ng mL-1,
whereas more severe effects are observed when concentrations increase to around
500 ng mL-1 (Mion and Villevieille 2013). Ketamine can also produce weaker effects
similar to those induced by alcohol (Dillon 2003; Wolff and Winstock 2006).
Despite the wide safety margin of ketamine, it is associated with a varied range of toxicological effects, resultant from the increase of glutamate release due to the impairment of the NMDA receptor (Deakin et al. 2008). Although the median lethal dose (LD50) in humans is unknown, this dose has been determined for several species and it is approximately 100 and 20-fold higher than the average human intravenous and intramuscular doses used in medical practice, respectively (Sinner and Graf 2008).
Moreover, animal studies, namely using mice and monkey models, corroborate the toxicological adverse effects of ketamine. Indeed, the responses observed in animal
studies following ketamine exposure are similar to those observed in humans and include central nervous system (CNS), kidney and liver damage (Wai et al. 2013). Despite the accumulation of data from animal studies regarding the toxicity of ketamine, there still remains some controversy as to whether these results can be extended to embryonic stages.
In fact, experimental animal studies have clearly shown the ketamine neurotoxicity potential during early development enhancing neuronal cell death (Ikonomidou et al. 1999), which may have negative effects through dependent (Wang et al. 2005) and non-dependent NMDA mechanisms (Felix et al. 2014). Moreover, it is known that ketamine crosses the placenta and may reach the developing foetus (Ellingson et al. 1977). Animal studies have shown evidence of an increased occurrence of foetal and neonatal brain injury (Brambrink et al. 2012; Paule et al. 2011; Scallet et al. 2004; Viberg et al. 2008; Walker et al. 2010; Young et al. 2005; Zou et al. 2009a), however the teratogenicity of ketamine has not been recognized (Abdel-Rahman and Ismail 2000). Additionally, the significance of these results to humans is considered uncertain as the anaesthetic blood levels in these organisms are higher than in humans (Scallet et al. 2004). Nevertheless, changes in uterine activity (Idvall et al. 1982; Oats et al. 1979) and in reproduction function (Absalan et al. 2014) have been reported. Still, the safe use of ketamine in pregnancy and early life stages has not been established (Mellon et al. 2007).
A preclinical study has shown that ketamine has the potential to adversely affect neurodevelopment in infants (Yan et al. 2014b) and several clinical features were reported in an infant with in utero ketamine exposure (Su et al. 2010). Studies in vitro have also confirmed that ketamine induces toxicity by a dose-dependent up-regulation of NMDA receptors (Takadera et al. 2006; Vutskits et al. 2006; Wang et al. 2006). Moreover, it has been reported that ketamine disturbs the normal processes of neurogenesis and neuro proliferation and differentiation (Dong and Anand 2013).
Several mechanisms have been proposed for ketamine’s neurotoxicological effects that included an interference with functional NMDA receptors (Dong and Anand 2013; Dong et al. 2012), dysregulation of calcium homeostasis (Sinner et al. 2011), apoptosis via the mitochondrial pathway (Bosnjak et al. 2012; Liu et al. 2011b) and other cellular processes (Durga and Yalamanchili 2016; Lei et al. 2012). However, the precise cellular effects remains unclear due to opposing opinions and concerns on ketamine neurotoxicity in obstetric, paediatric clinical practices. The cases of drug abuse
have prompted ongoing investigations on the developmental effects of ketamine. Furthermore, dual neurotoxic and neuroprotective effects have been described for ketamine for the developing brain (Yan and Jiang 2014) shuffling even more the real effects of this anaesthetic drug.
1.2.4.1 Ecotoxicological impact
Besides the clinical adverse effects, there is a growing interest associated with ketamine environmental issues. Undeniably, considerable attention has been given to the prevalence of pharmaceutical drugs in the environment with special emphasis in aquatic systems (Lin et al. 2010). In the particular case of ketamine, and as it is not completely metabolized in humans and animals, about 10% is found unchanged in faeces and urine (Quibell et al. 2011) being directly discharged directly into the environment through sewage or soil from either abuse or prescribed use at clinical and veterinary settings (Khetan and Collins 2007). Despite the fact that wastewater studies are unable to differentiate ketamine sources, it is noted that the major source entering pathway is through hospital wastewaters (Lin et al. 2014; Lin et al. 2010).
Conventional wastewater treatment plants (WWTPs) only remove part of the newly emerging contaminants present in sewage waters (Kasprzyk-Hordern et al. 2009; Verlicchi et al. 2012). These can be released into aquatic systems and can reach surface and groundwater and, subsequently, drinking waters (Petrovic et al. 2009). In fact, several studies have already reported the environmental contamination by pharmaceuticals present in wastewater discharges worldwide (Pal et al. 2013; Zuccato and Castiglioni 2009). In the particular case of ketamine, it is known that it persists through conventional WWTPs (Lin et al. 2014; Lin et al. 2010) with high in-sewer stability (McCall et al. 2016). Worldwide occurrence of ketamine in wastewater, surface
and seawaters studies state concentrations ranges from 4 to 420 ng L-1 (Baker and
Kasprzyk-Hordern 2013; Bijlsma et al. 2012; Castiglioni et al. 2015; Hapeshi et al. 2015; Huerta-Fontela et al. 2008; Huerta-Fontela et al. 2007; Jiang et al. 2014; Li et al. 2016; Lin et al. 2014; Lin et al. 2010; Mackuľak et al. 2016; van der Aa et al. 2013).
As currently only limited legislation exists regarding new emerging contaminants in waters and because of its detection at low concentrations, a debate about its toxicity to humans is still ongoing as the exposure may be involuntary and, potentially, over long periods of time (WHO 2012). Moreover, the ecotoxocological effects of pharmaceuticals, including ketamine, to living organisms remains unclear. So
far, little ecotoxicological information for ketamine is available although its potential ecotoxicological impact has been recently shown in embryos of Japanese medaka (Oryzias latipes) (Liao et al. 2015). Nevertheless, the occurrence, ecotoxicological effects and risk assessment of ketamine requires more research for a better understanding of the real environmental impact of ketamine.
1.3 Zebrafish embryos as an alternative vertebrate model organism
Since the publication of George Stresinger in 1981 (Streisinger et al. 1981), zebrafish (Danio rerio) has emerged as one of the most popular vertebrate model systems for biology and biomedical studies even though the first publication using zebrafish dates back to 1951 (Kinth et al. 2013). Over the last 10 years, the number of publications listed in PubMed with this small teleost freshwater fish, native from India and south Asia, has duplicated (from 1258 in 2006 to 2799 in 2015) in part due to its applicability to study processes with relevance to human health (Barros et al. 2008). Although some important physiological differences exists relatively to mammals, the zebrafish embryo has gained an important role for drug screening (Ali et al. 2011b). Indeed, there is a growing trend to use fish and other lower vertebrate models in face of rodent models in animal testing (Badyal and Desai 2014), whose use has been declining over the years, according to worldwide statistics on the animal use for research and other scientific purposes (Anon. 2014; Inoue and Kodama 2007; Taylor et al. 2008). This is clearly in line with the current trend to implement the 3R principle to experimental animal biology supporting the humane use of animals in scientific research.
The early life stages of zebrafish, in particular, have become a model choice adopted under 3Rs policies and as an alternative to animal testing for ecotoxicology and biomedical research (Doke and Dhawale 2015). Under European legislation (Directive 2010/63/EU), zebrafish embryos fall inside this framework only once they have reached 5 days post-fertilization (dpf), the day they start to feed independently (Strahle et al. 2012). Moreover, and although the nervous system morphogenesis begins as early as 10 hours post-fertilization (hpf) (Schmitz et al. 1993), the behavioural responses that the early life stages of fish exhibit are not associated with pain perception (Key 2015). Another useful trait of zebrafish embryonic development is that the fundamental molecular processes associated with its embryogenesis are similar with higher vertebrates (Kimmel 1989; Sipes et al. 2011). Furthermore, its genome has been sequenced and published owing to recent advances in genetics and molecular biology.