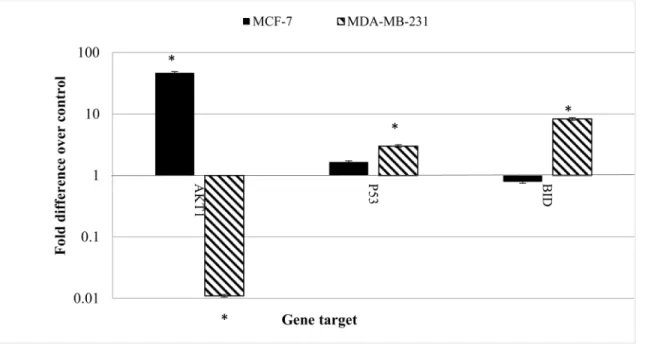
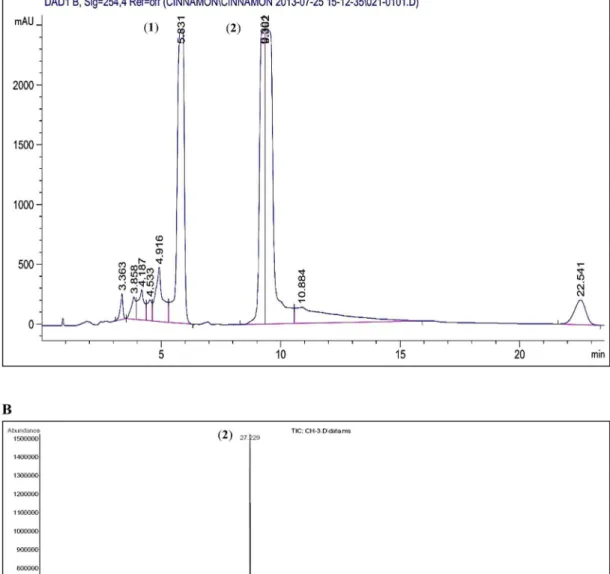
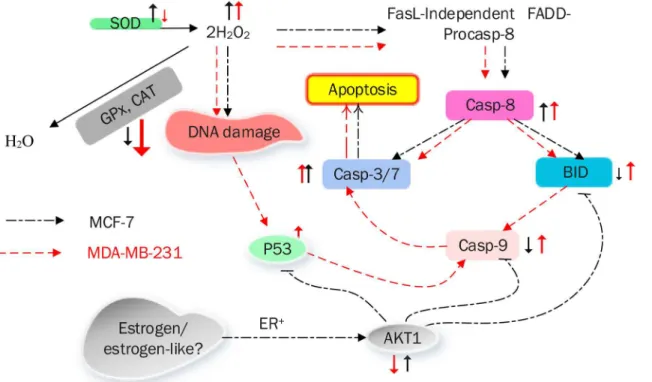
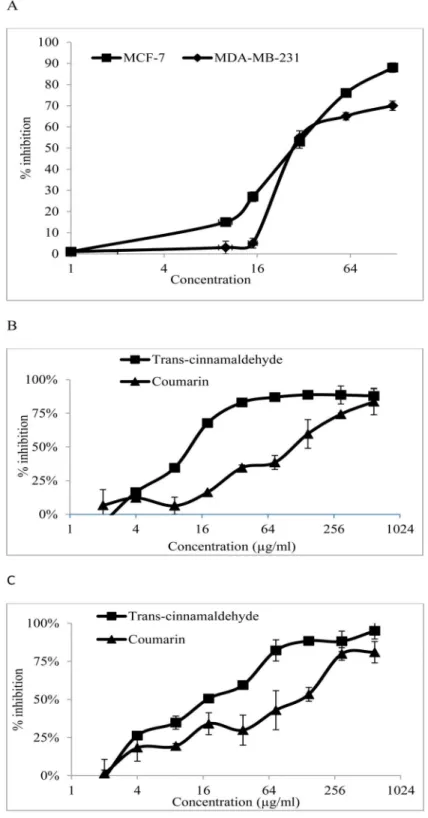
Cinnamomum cassia Suppresses Caspase-9 through Stimulation of AKT1 in MCF-7 Cells but Not in MDA-MB-231 Cells.
Texto
Imagem


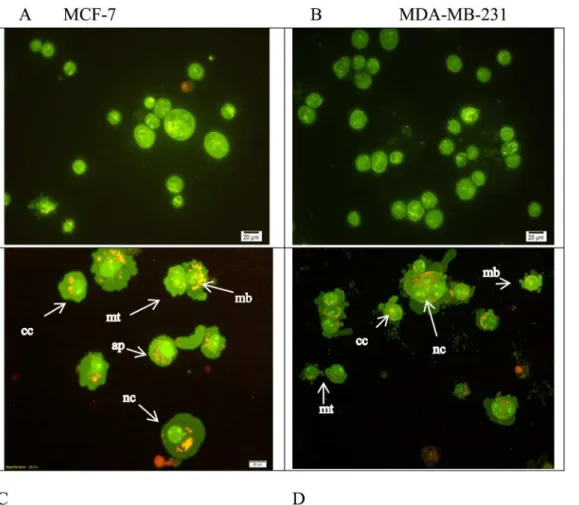
Documentos relacionados
The increased levels of caspase-3 activation suggest that silver nanoparticles induced apoptosis in MCF 7 breast cancer cells in a caspase-3-dependent manner..
Examining cell behaviors of MCF-10A, MCF- 7, and MDA-MB-231 cells with our established platform showed that (a) the highly metastatic MDA-MB-231 breast cancer cells had the
The compounds were evaluated for their in vitro cytotoxicity activities against cultured human cancer cells of PC-3 human prostate cancer, MCF-7 and MDA-MB-231 breast carcinoma,
Consistent with the effects of miR- 21 antagomir on EMT and CSC phenotype in MDA-MB-231 cells (Fig. 5C, E) in MDA-MB-231/SsiPTEN cells (SsiPTEN+miRNA antagomir negative control
Depletion of USP19 results in reduced rates of cell growth and accumulation of p27 Kip1 in MCF10A breast epithelial cells but not in MCF7 and MDA-MB-231 breast carcinoma cells.. We
Methods: We investigated the effects of PP on cell growth, migration-invasion, apoptosis and EMT-related proteins expression in MCF-7 and TNBC cell lines MDA-MB-231, MDA-MB-468
However, at higher concentra- tions (40–60 m M in MDA-MB-231 and 30–40 m M in MCF-7 cells), temporin-1CEa disrupted the membrane integrity and caused rapid peptides influx into
BP-C1 , is significantly reduced cell viability of human breast cancer cells through induction of apoptosis via activation of caspase 8 and caspase 9, increasing the expression