A validated stability-indicating RP-HPLC method for paracetamol and lornoxicam: Application to pharmaceutical dosage forms
Texto
Imagem
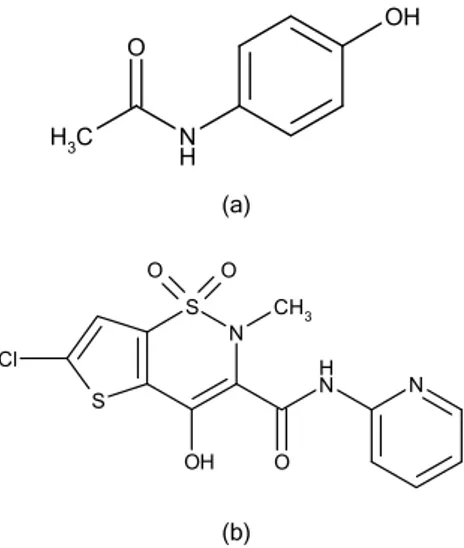


Documentos relacionados
The present study describes the development and subsequent validation of simple and accurate stability indicating RP-HPLC method for the determination of sparloxacin and
To develop a rugged and suitable stability indicating HPLC method for the quantitative determination of PNT and their im- purities, the chromatographic conditions were selected
A simple, speciic, precise, accurate, linear, rapid, economic and validated stability indicating an RP-HPLC method for the simultaneous quantiication of cefepime and tazobactam in
Simultaneous estimation of rosuvastatin and amlodipine in pharmaceutical formulations using stability indicating HPLC method.. Muhammad Ashfaq 1,* , Tazeem Akhtar 1 , Ghulam Mustafa
Hydroethanolic spray-dried extract was chosen for the further development of phytomedicines, and a stability- indicative HPLC-UV method was developed and validated for the
A validated, simple, sensitive, precise and selective stability-indicating square-wave adsorptive cathodic stripping voltammetric method was described for the trace
A novel stability-indicating high-performance liquid chromatographic assay method was developed and validated for quantitative determination of pramipexole dihydrochloride
A suitable stability-indicating method was developed for both determination of POH encapsulation efficiency in polymeric nanoparticles formulation and evaluating the presence